Efficient and Stable Ni/SBA-15 Catalyst for Dry Reforming of Methane: Effect of Citric Acid Concentration
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fresh Catalyst Characterization
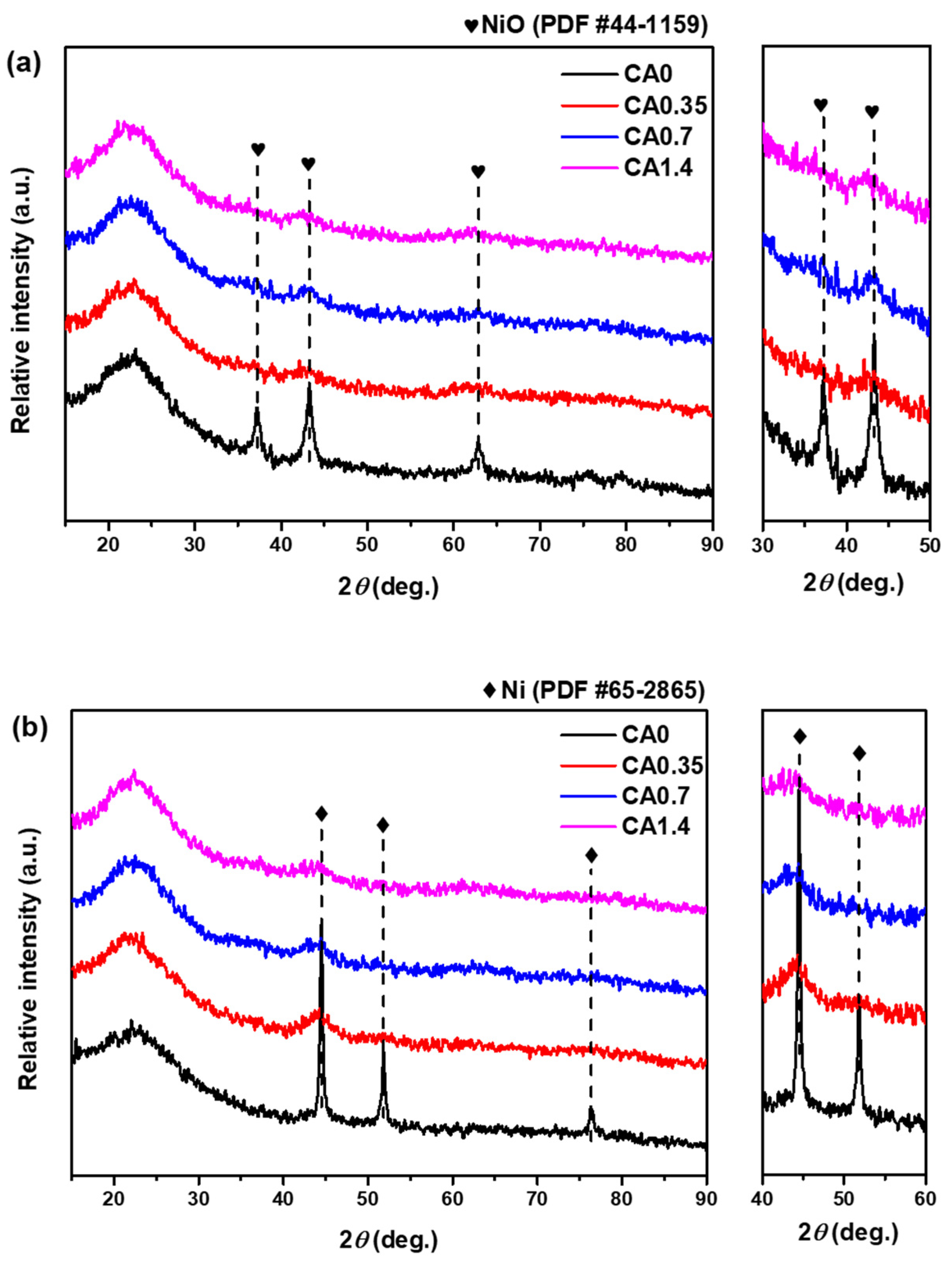
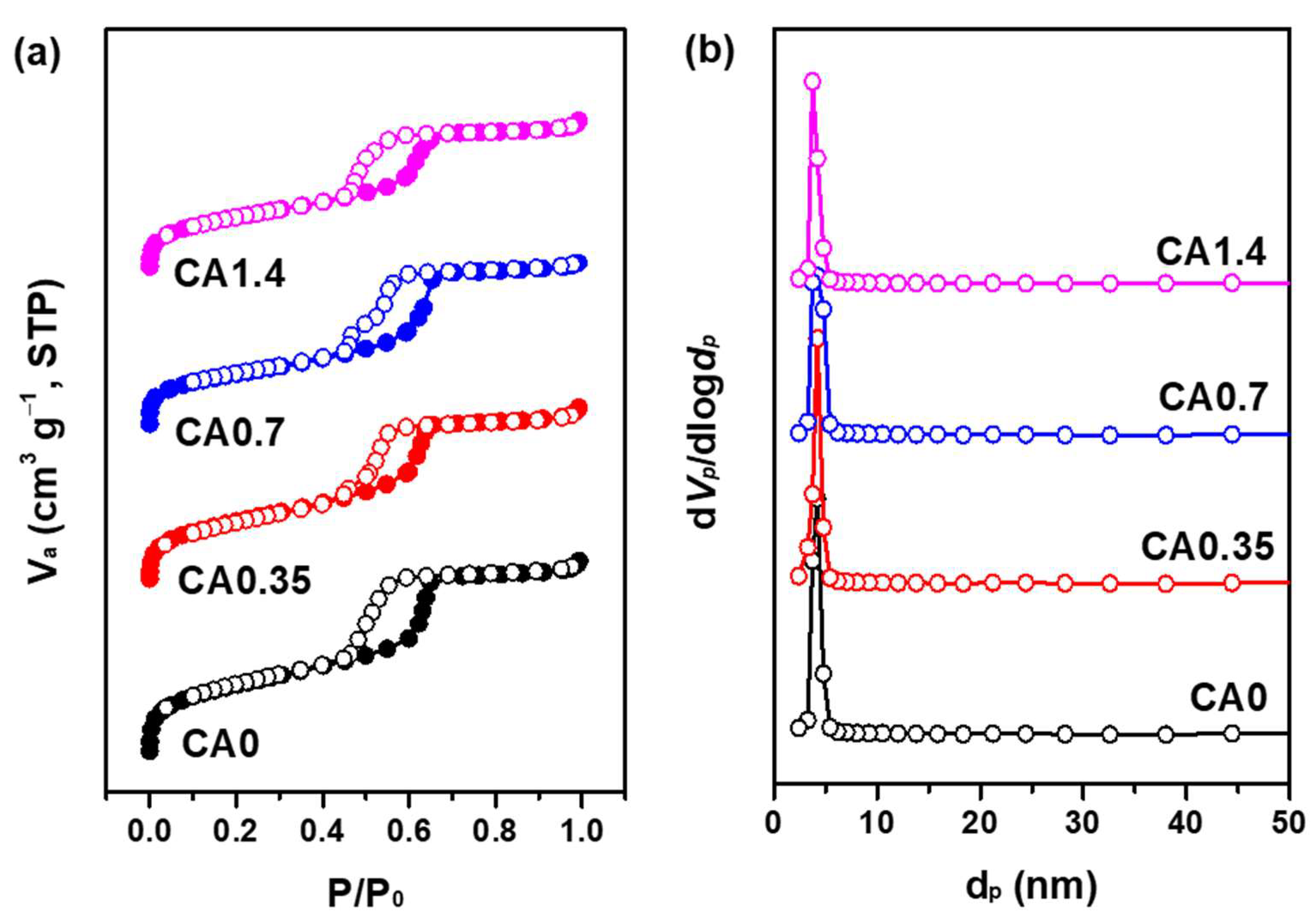
2.1.1. Textural and Structural Properties
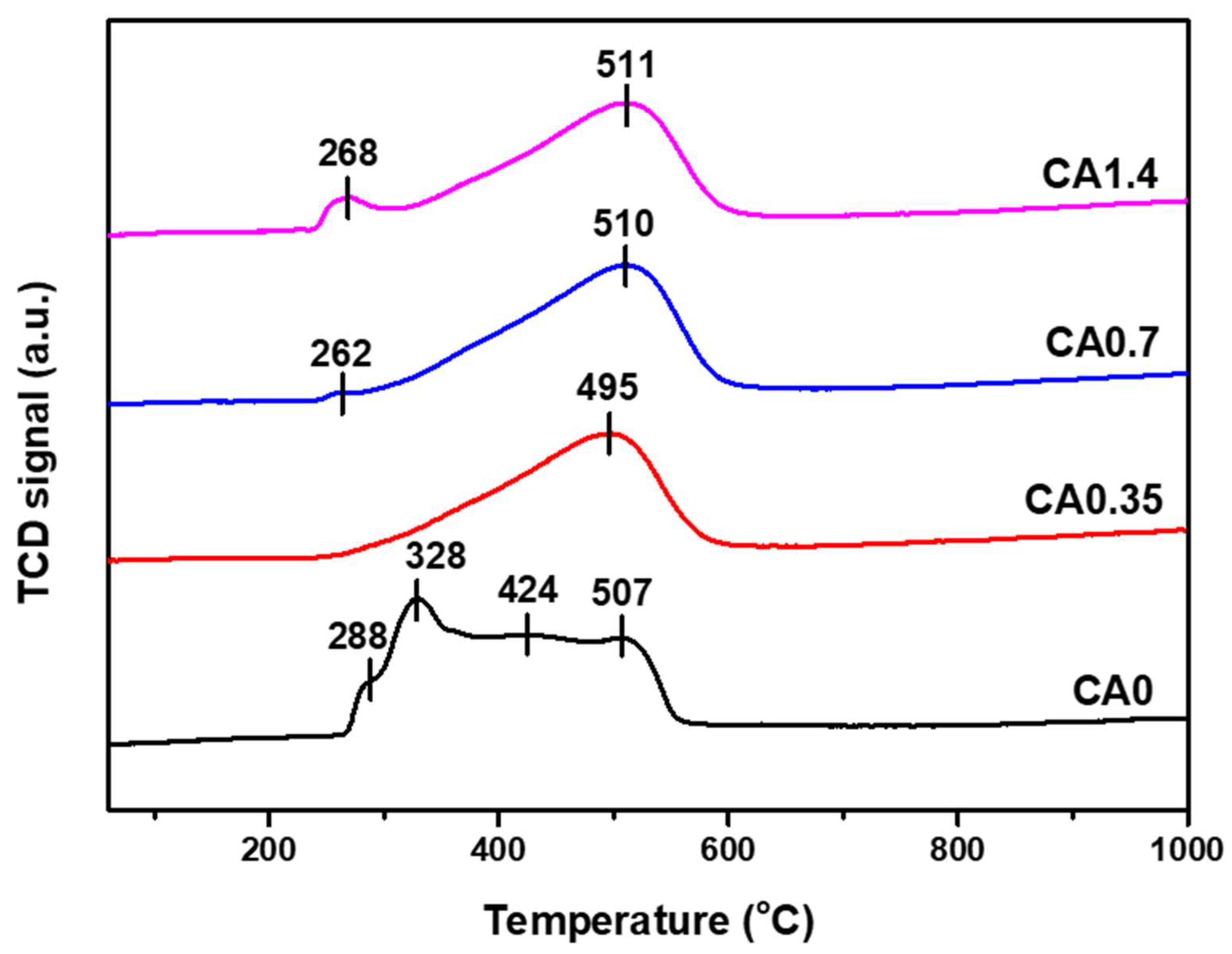
2.1.2. Reduction Behavior and Oxidation State of Catalysts
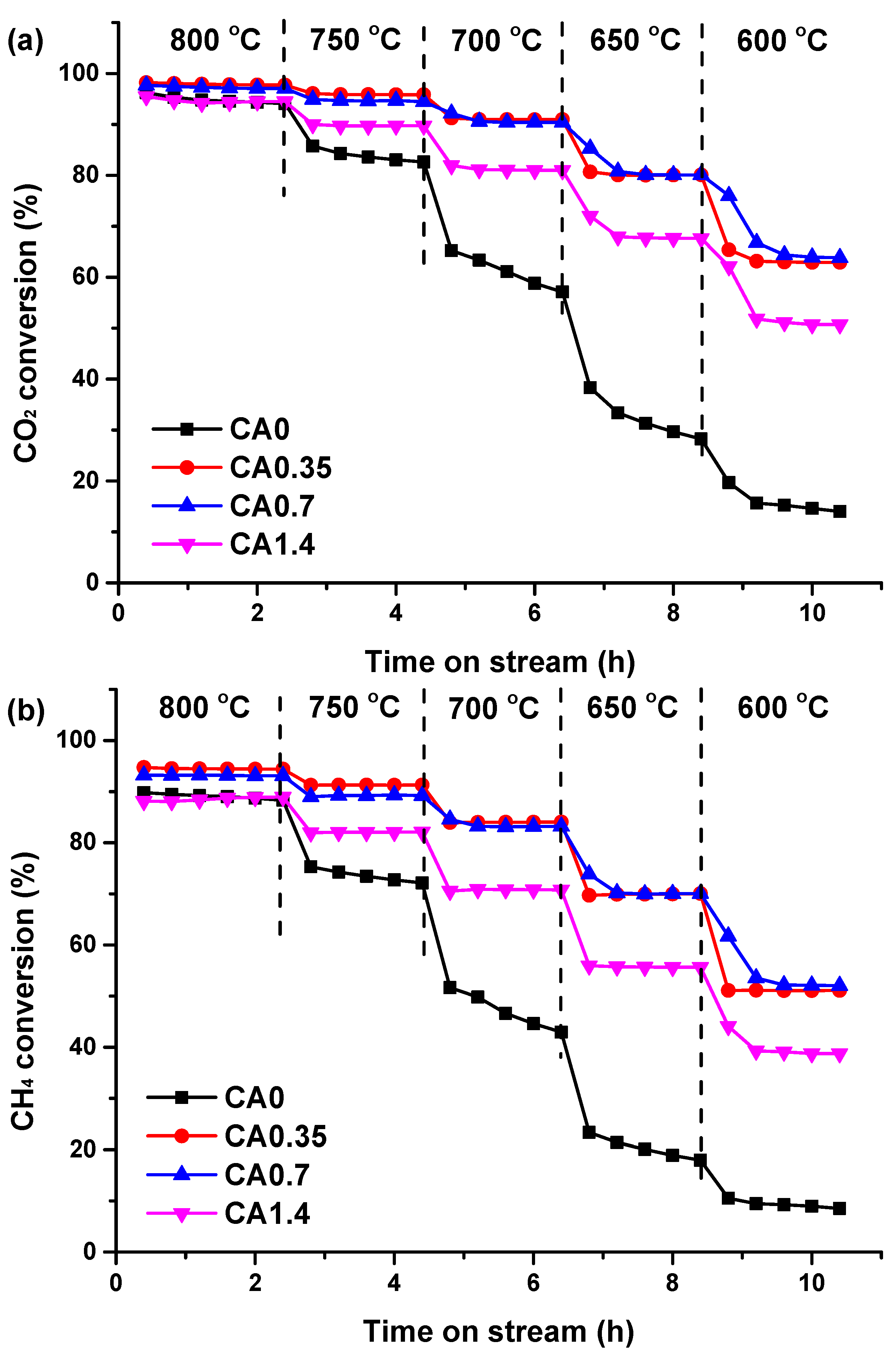
2.2. Catalytic Activity
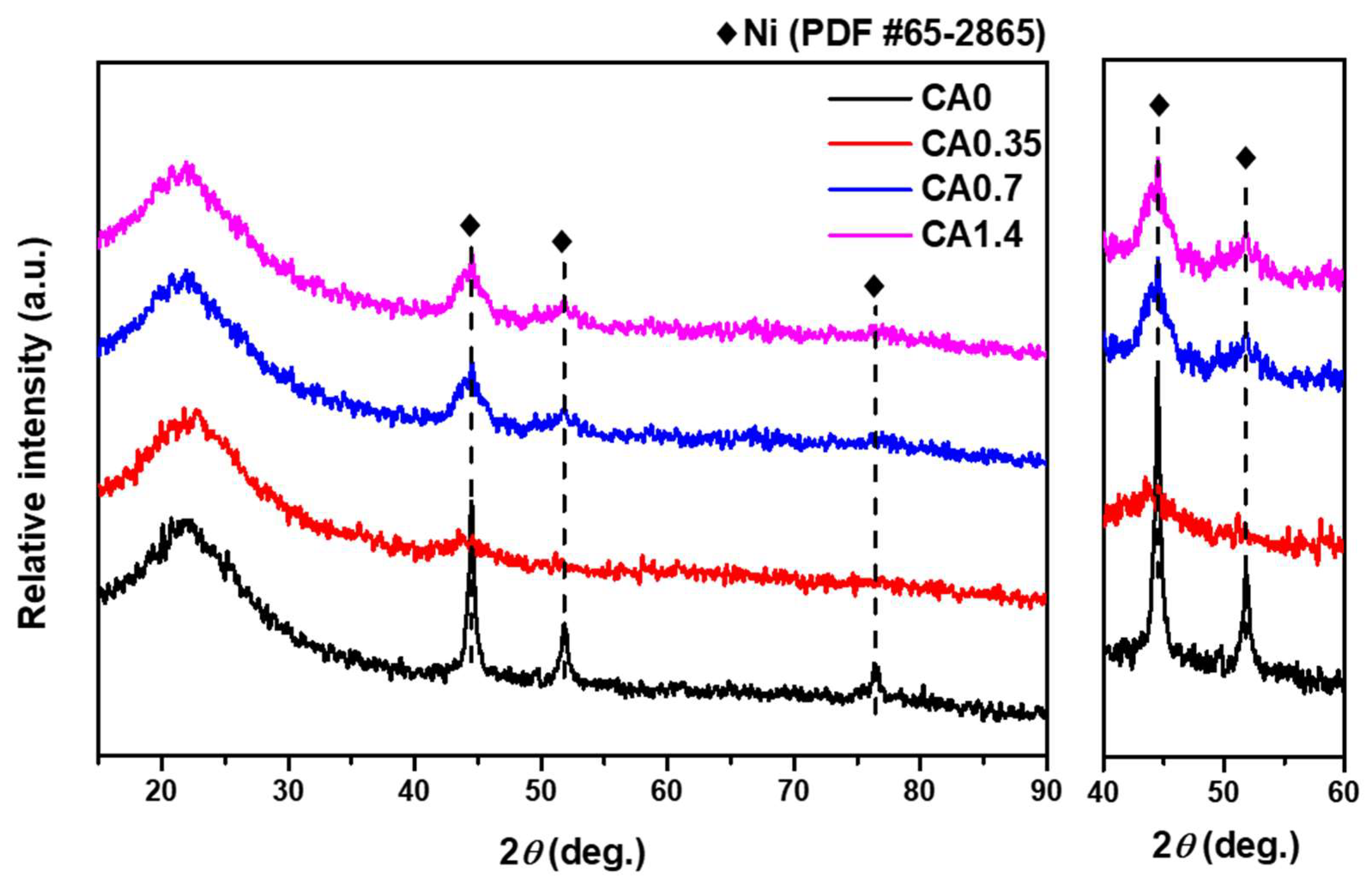
2.3. Spent-Catalyst Characterization
3. Experimental Method
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Shahul Hamid, M.Y. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Cho, H.H.; Strezov, V.; Evans, T.J. A review on global warming potential, challenges and opportunities of renewable hydrogen production technologies. Sustain. Mater. Technol. 2023, 35, e00567. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A review of recent developments in hydrogen production via biogas dry reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Jang, W.-J.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Bhattar, S.; Abedin, M.A.; Kanitkar, S.; Spivey, J.J. A review on dry reforming of methane over perovskite derived catalysts. Catal. Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- Cho, E.; Lee, Y.-H.; Kim, H.; Jang, E.J.; Kwak, J.H.; Lee, K.; Ko, C.H.; Yoon, W.L. Ni catalysts for dry methane reforming prepared by A-site exsolution on mesoporous defect spinel magnesium aluminate. Appl Catal. A Gen. 2020, 602, 135166. [Google Scholar] [CrossRef]
- Kumari, R.; Sengupta, S. Catalytic CO2 reforming of CH4 over MgAl2O4 supported Ni-Co catalysts for the syngas production. Int. J. Hydrogen Energy 2020, 45, 22775–22787. [Google Scholar] [CrossRef]
- Mizuno, S.C.M.; Braga, A.H.; Hori, C.E.; Santos, J.B.O.; Bueno, J.M.C. Steam reforming of acetic acid over MgAl2O4-supported Co and Ni catalysts: Effect of the composition of Ni/Co and reactants on reaction pathways. Catal. Today 2017, 296, 144–153. [Google Scholar] [CrossRef]
- Jalali, R.; Rezaei, M.; Nematollahi, B.; Baghalha, M. Preparation of Ni/MeAl2O4-MgAl2O4 (Me=Fe, Co, Ni, Cu, Zn, Mg) nanocatalysts for the syngas production via combined dry reforming and partial oxidation of methane. Renew. Energy 2020, 149, 1053–1067. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, S.M.; Won, J.M.; Yang, H.J.; Hong, S.C. Effect of Ce/Ti ratio on the catalytic activity and stability of Ni/CeO2-TiO2 catalyst for dry reforming of methane. Chem. Eng. J. 2015, 280, 433–440. [Google Scholar] [CrossRef]
- Sokolov, S.; Kondratenko, E.V.; Pohl, M.M.; Rodemerch, U. Effect of calcination conditions on time on-stream performance of Ni/La2O3-ZrO2 in low-temperature dry reforming of methane. Int. J. Hydrogen Energy. 2013, 38, 16121–16132. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Liu, J.; Li, T.; Wang, Y.; Zhao, Y.; Li, G.; Zhang, Y. Dry reforming of methane over Ni/SiO2 catalysts: Role of support structure properties. Fuel 2023, 340, 127490. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Lee, K.; Ko, C.H.; Roh, H.-S. Optimization of nano-catalysts for application in compact reformers. Chem. Eng. J. 2020, 431, 134299. [Google Scholar] [CrossRef]
- Ye, R.-P.; Liao, L.; Reina, T.R.; Liu, J.; Chevella, D.; Jin, Y.; Fan, M.; Liu, J. Engineering Ni/SiO2 catalysts for enhanced CO2 methanation. Fuel 2021, 285, 119151. [Google Scholar] [CrossRef]
- Ye, R.-P.; Gong, W.; Sun, Z.; Sheng, Q.; Shi, X.; Wang, T.; Yao, Y.; Razink, J.J.; Lin, L.; Zhou, Z.; et al. Enhanced stability of Ni/SiO2 catalyst for CO2 methanation: Derived from nickel phyllosilicate with strong metal-support interactions. Energy 2019, 188, 116059. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Mu, M.; Ding, F.; Liu, Z.; Guo, X.; Song, C. Organic acid-assisted preparation of highly dispersed Co/ZrO2 catalysts with superior activity for CO2 methanation. Appl. Catal. B Environ. 2019, 254, 531–540. [Google Scholar] [CrossRef]
- Li, Y.; Men, Y.; Liu, S.; Wang, J.; Wang, K.; Tang, Y.; An, W.; Pan, X.; Li, L. Remarkably efficient and stable Ni/Y2O3 catalysts for CO2 methanation: Effect of citric acid addition. Appl. Catal. B Environ. 2021, 293, 120206. [Google Scholar] [CrossRef]
- Kim, M.-J.; Youn, J.-R.; Kim, H.J.; Seo, M.W.; Lee, D.; Go, K.S.; Lee, K.B.; Jeon, S.G. Effect of surface properties controlled by Ce addition on CO2 methanation over Ni/Ce/Al2O3 catalyst. Int. J. Hydrogen Energy 2020, 45, 24595–24603. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, Y.-J.; Lee, S.-J.; Ryu, I.-S.; Kim, H.J.; Kim, Y.; Ko, C.H.; Jeon, S.G. Enhanced catalytic activity of the Rh/γ-Al2O3 pellet catalyst for N2O decomposition using high Rh dispersion induced by citric acid. Chem. Eng. Res. Des. 2019, 141, 455–463. [Google Scholar] [CrossRef]
- Kim, M.-J.; Park, S.J.; Kim, K.D.; Kim, W.; Nam, S.C.; Go, K.S.; Jeon, S.G. Fabrication of carbon nanotube with high purity and crystallinity by methane decomposition over ceria-supported catalysts. J. Ind. Eng. Chem. 2023, 119, 315–326. [Google Scholar] [CrossRef]
- Cho, E.H.; Kim, M.J.; Yoon, B.S.; Kim, Y.J.; Song, D.; Koo, K.Y.; Jung, U.; Jeon, S.-G.; Park, Y.-K.; Ko, C.H. Enhancement in nickel-silica interface generation by surfactant-assisted melt-infiltration: Surfactant selection and application in CO2 hydrogenation. Chem. Eng. J. 2022, 437, 135166. [Google Scholar]
- Cai, W.; Ye, L.; Zhang, L.; Ren, Y.; Yue, B.; Chen, X.; He, H. Highly Dispersed Nickel-Containing Mesoporous Silica with Superior Stability in Carbon Dioxide Reforming of Methane: The Effect of Anchoring. Materials 2014, 7, 2340–2355. [Google Scholar] [CrossRef]
- Han, B.; Zhao, L.; Wang, F.; Xu, L.; Yu, H.; Cui, Y.; Zhang, J.; Shi, W. Effect of Calcination Temperature on the Performance of the Ni@SiO2 Catalyst in Methane Dry Reforming. Ind. Eng. Chem. Res. 2020, 59, 13370–13379. [Google Scholar] [CrossRef]
- Bian, Z.; Zhong, W.; Yu, Y.; Wang, Z.; Jiang, B.; Kawi, S. Dry reforming of methane on Ni/mesoporous-Al2O3 catalysts: Effect of calcination temperature. Int. J. Hydrogen Energy 2021, 46, 31041–31053. [Google Scholar] [CrossRef]
- Ren, H.-P.; Hao, Q.-Q.; Wang, W.; Song, Y.-H.; Cheng, J.; Liu, Z.-W.; Liu, Z.-T.; Lu, J.; Hao, Z. High-performance Ni–SiO2 for pressurized carbon dioxide reforming of methane. Int. J. Hydrogen Energy 2014, 39, 11592–11605. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Wu, H.; Takayama, H.; Miyake, T.; He, D. Effects of β-cyclodextrin modification on properties of Ni/SBA-15 and its catalytic performance in carbon dioxide reforming of methane. Catal. Commun. 2012, 28, 168–173. [Google Scholar] [CrossRef]
- Daoura, O.; Boutros, M.; Kaydouh, M.-N.; Massiani, P.; Launay, F.; Hassan, N.E. Supported nickel nanocatalysts for the dry reforming of methane: Effect of SBA-15’s pore sizes on the catalytic performances of nickel nanoparticles. In Nanostructured Catalysts for Environmental Applications; Dosa, M., Piumetti, M., Eds.; Springer: Berlin, Germany, 2021; pp. 113–126. ISBN 978-3-030-58933-2. [Google Scholar]
- Tannous, J.; Karam, L.; Kaydouh, M.N.; El Zakhem, H.; El Hassan, N.; Casale, S. Effect of pore geometry of mesoporous supports on catalytic performances in methane reforming. MATEC Web Conf. 2018, 171, 8–12. [Google Scholar] [CrossRef]
- Karam, L.; El Hassan, N. Advantages of mesoporous silica-based catalysts in methane reforming by CO2 from kinetic perspective. J. Environ. Chem. Eng. 2018, 6, 4289–4297. [Google Scholar] [CrossRef]
- Chong, C.C.; Cheng, Y.W.; Bukhari, S.N.; Setiabudi, H.D.; Jalil, A.A. Methane dry reforming over Ni/fibrous SBA-15 catalysts: Effects of support morphology (rod-liked F-SBA-15 and dendritic DFSBA-15). Catal Today 2021, 375, 245–257. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.; Shi, Y.; Zhao, B.; Wang, M.; Liu, Q.; Wang, J.; Long, K.; Duan, Y.; Ning, P. A sintering and carbon-resistant Ni-SBA-15 catalyst prepared by solid-state grinding method for dry reforming of methane. J. CO2 Util. 2017, 17, 10–19. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Ning, P.; Zhang, T.; Wang, M.; Long, K.; Huang, J. Dry reforming of methane over Ni/SBA-15 catalysts prepared by homogeneous precipitation method. Korean J. Chem. Eng. 2017, 34, 2823–2831. [Google Scholar] [CrossRef]
- Kaydouh, M.N.; El Hassan, N.; Davidson, A.; Massiani, P. Optimization of Synthesis Conditions of Ni/SbA-15 Catalysts: Confined Nanoparticles and Improved Stability in Dry Reforming of Methane. Catalysts 2021, 11, 44. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, Q.; Qiu, W.; Zhang, Q.; Lv, W.; Wang, T.; Zhang, Q.; Ma, L. Simply packaging Ni nanoparticles inside SBA-15 channels by co-impregnation for dry reforming of methane. RSC Adv. 2017, 7, 24551–24560. [Google Scholar] [CrossRef]
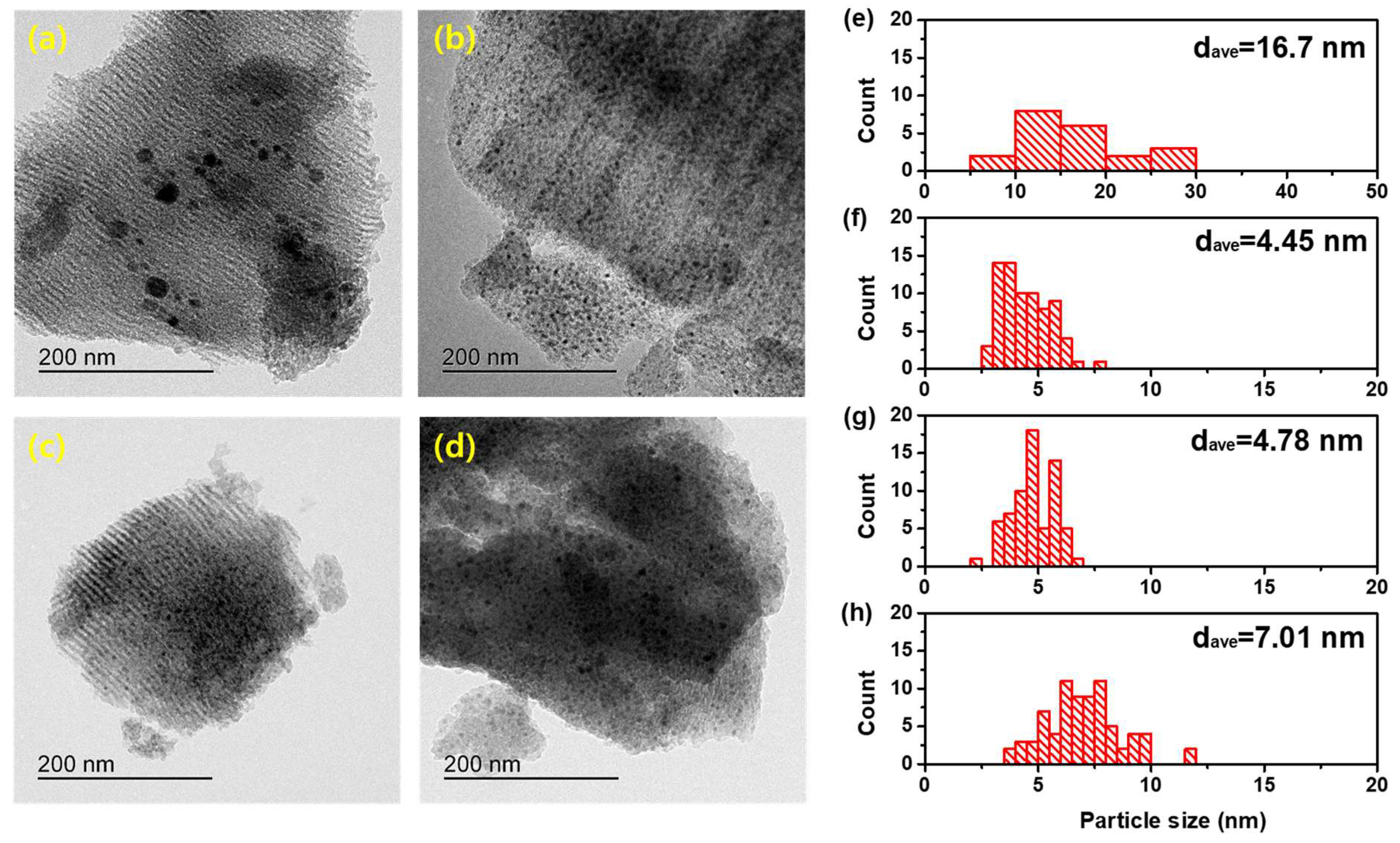



| Samples | Average Particle Size of Ni a (nm) | Ni Dispersion a (%) | BET Surface Area b (m2 g−1) | Pore Volume b (cm3 g−1) | Ni contents c (wt.%) |
|---|---|---|---|---|---|
| SBA-15 | - | - | 824.03 | 0.9029 | |
| CA0 | 49.3 | 2.05 | 584.54 | 0.6449 | 7.28 |
| CA0.35 | 8.2 | 12.23 | 517.81 | 0.5837 | 6.02 |
| CA0.7 | 7.6 | 13.29 | 449.34 | 0.5545 | 7.7 |
| CA1.4 | 7.3 | 13.83 | 455.26 | 0.5028 | 8.76 |
| Samples | Ni 2p3/2 Peak Position (eV) | ||
|---|---|---|---|
| Sat. | Ni2+ | Ni0 | |
| CA0 | 860.5 | 854.9 | 851.8 |
| CA0.35 | 861.7 | 856.2 | 853.0 |
| CA0.7 | 859.1 | 854.9 | 852.2 |
| CA1.4 | 860.4 | 855.6 | 853.0 |
| Samples | Coke Formation (mgcoke gcat−1 h−1) |
|---|---|
| CA0 | 21.9 |
| CA0.35 | 2.4 |
| CA0.7 | 4.8 |
| CA1.4 | 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waris, M.; Ra, H.; Yoon, S.; Kim, M.-J.; Lee, K. Efficient and Stable Ni/SBA-15 Catalyst for Dry Reforming of Methane: Effect of Citric Acid Concentration. Catalysts 2023, 13, 916. https://doi.org/10.3390/catal13060916
Waris M, Ra H, Yoon S, Kim M-J, Lee K. Efficient and Stable Ni/SBA-15 Catalyst for Dry Reforming of Methane: Effect of Citric Acid Concentration. Catalysts. 2023; 13(6):916. https://doi.org/10.3390/catal13060916
Chicago/Turabian StyleWaris, Mamoona, Howon Ra, Sungmin Yoon, Min-Jae Kim, and Kyubock Lee. 2023. "Efficient and Stable Ni/SBA-15 Catalyst for Dry Reforming of Methane: Effect of Citric Acid Concentration" Catalysts 13, no. 6: 916. https://doi.org/10.3390/catal13060916





