Removal of Hexamethyldisiloxane by NaOH–Activated Porous Carbons Produced from Coconut Shells
Abstract
:1. Introduction
2. Results
2.1. Yield of APCs
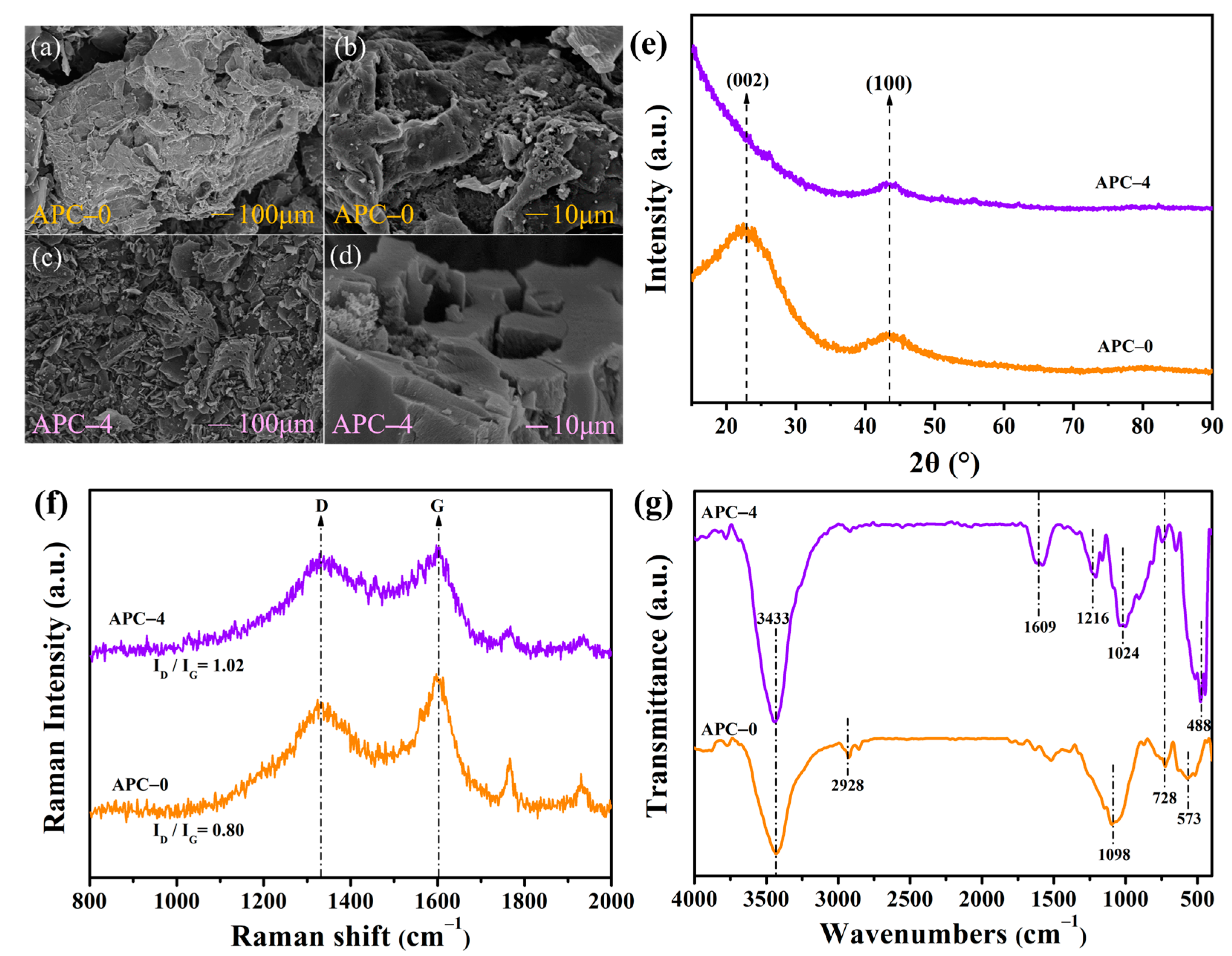
2.2. Basic Characterisation of APC–0 and APC–4
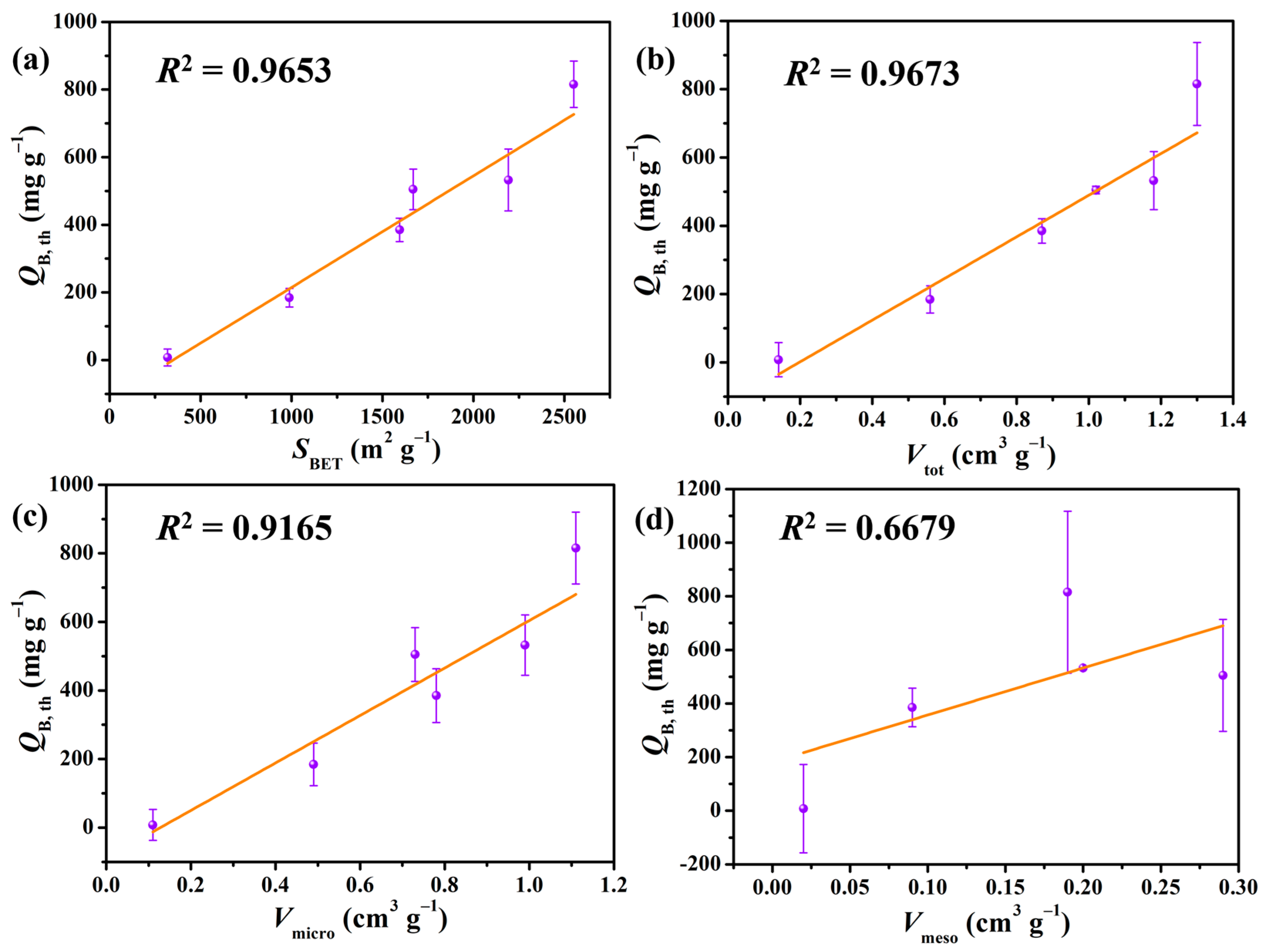
2.3. Effect of NaOH on the Textural Properties of APCs
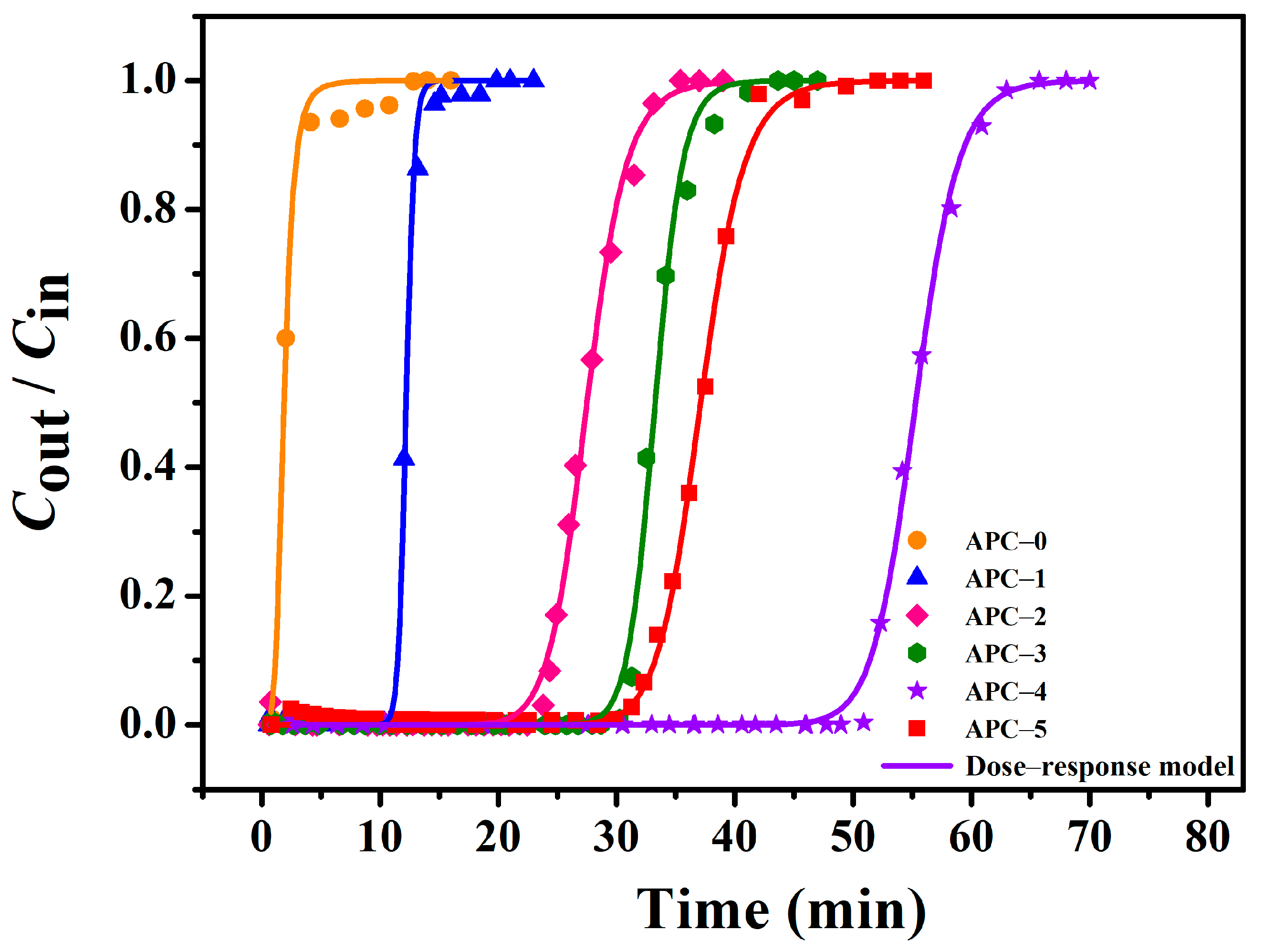
2.4. The Dynamic Adsorption Behaviour of APCs
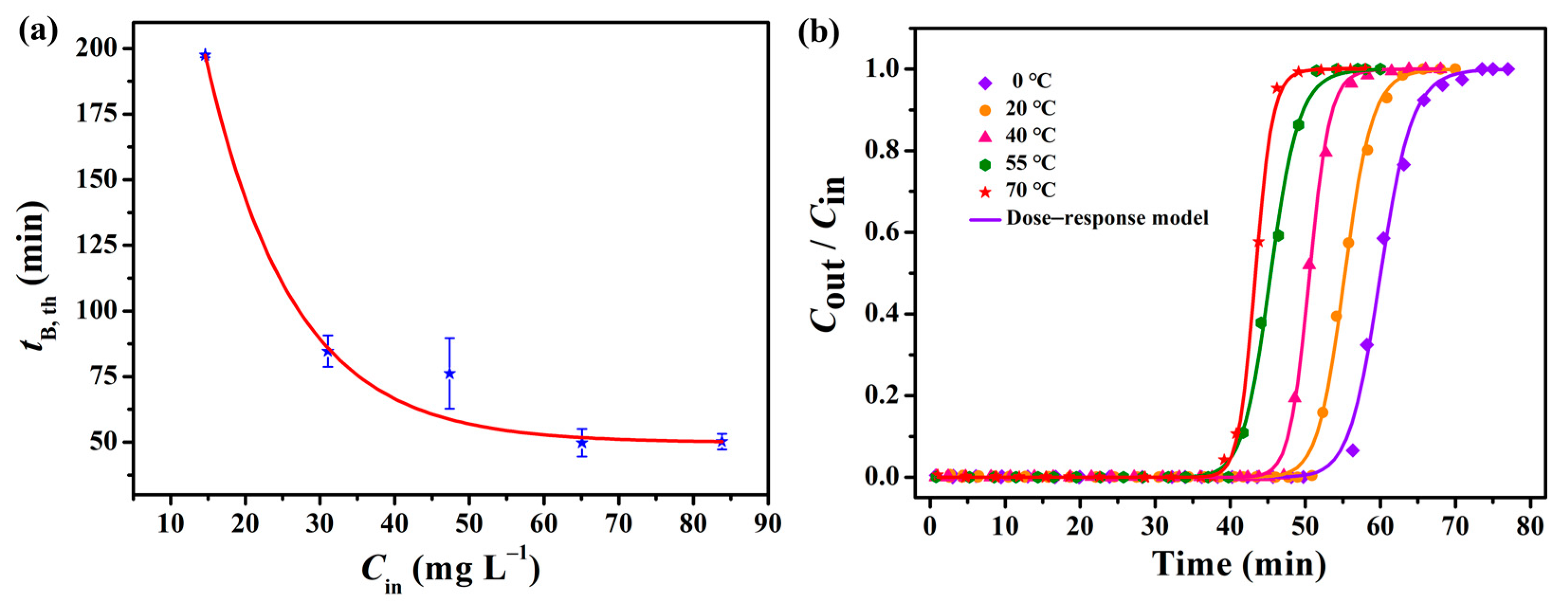
2.5. Effect of Process Conditions on Adsorption
2.6. Assessment of Regeneration Capacity
2.7. Performance Comparison between APC and AC
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of APCs
3.3. Experiments on the Adsorption of L2
3.4. Breakthrough Curve Model
3.5. Regeneration of Spent APCs
3.6. Characterisation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bak, C.U.; Lim, C.J.; Kim, Y.D.; Kim, W.S. Multi-stage adsorptive purification process for improving desulfurization performance of biogas. Sep. Purif. Technol. 2019, 227, 115702. [Google Scholar] [CrossRef]
- Hou, X.; Zheng, Y.; Lv, S.; Ma, Z.; Ma, X. Effective removal of hexamethyldisiloxane using a citric acid modified three-dimensional graphene aerogel. Renew. Energy 2022, 199, 62–70. [Google Scholar] [CrossRef]
- Zheng, Y.; Hou, X.; Liu, Y.; Ma, Z. Hexamethyldisiloxane removal from biogas using reduced graphene-oxide aerogels as adsorbents. Renew. Energy 2021, 178, 153–161. [Google Scholar] [CrossRef]
- Jiang, T.; Zhong, W.; Jafari, T.; Du, S.; He, J.; Fu, Y.J.; Singh, P.; Suib, S.L. Siloxane D4 adsorption by mesoporous aluminosilicates. Chem. Eng. J. 2016, 289, 356–364. [Google Scholar] [CrossRef]
- Zhang, Y.; Kawasaki, Y.; Oshita, K.; Takaoka, M.; Minami, D.; Inoue, G.; Tanaka, T. Economic assessment of biogas purification systems for removal of both H2S and siloxane from biogas. Renew. Energy 2021, 168, 119–130. [Google Scholar] [CrossRef]
- Gaj, K. Adsorptive biogas purification from siloxanes—A critical review. Energies 2020, 13, 2605. [Google Scholar] [CrossRef]
- Hou, X.; Zheng, Y.; Ma, X.; Liu, Y.; Ma, Z. The effects of hydrophobicity and textural properties on hexamethyldisiloxane adsorption in reduced graphene oxide aerogels. Molecules 2021, 26, 1130. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hou, X.; Lv, S.; Ma, Z.; Ma, X. Efficient removal of siloxane from biogas by using β-cyclodextrin-modified reduced graphene oxide aerogels. Nanomaterials 2022, 12, 2643. [Google Scholar] [CrossRef]
- Zheng, Y.; Hou, X.; Ma, X.; Hao, Z.; Ma, Z. Vitamin C-assisted fabrication of aerogels from industrial graphene oxide for gaseous hexamethyldisiloxane adsorption. Appl. Sci. 2021, 11, 8486. [Google Scholar] [CrossRef]
- Haider, J.; Abdul Qyyum, M.; Riaz, A.; Naquash, A.; Kazmi, B.; Yasin, M.; Nizami, A.S.; Byun, M.; Lee, M.; Lim, H. State-of-the-art process simulations and techno-economic assessments of ionic liquid-based biogas upgrading techniques: Challenges and prospects. Fuel 2022, 314, 123064. [Google Scholar] [CrossRef]
- Piechota, G. Removal of siloxanes from biogas upgraded to biomethane by Cryogenic Temperature Condensation System. J. Cleaner Prod. 2021, 308, 127404. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Hu, D.; Fan, J.; Zeng, G. A review on removal of siloxanes from biogas: With a special focus on volatile methylsiloxanes. Environ. Sci. Pollut. Res. Int. 2018, 25, 30847–30862. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gong, H.; Chen, Z.; Zhang, M. Adsorption characteristics of activated carbon for siloxanes. J. Environ. Chem. Eng. 2013, 1, 1182–1187. [Google Scholar] [CrossRef]
- Sigot, L.; Ducom, G.; Benadda, B.; Labouré, C. Adsorption of octamethylcyclotetrasiloxane on silica gel for biogas purification. Fuel 2014, 135, 205–209. [Google Scholar] [CrossRef]
- Tu, J.; Qiao, Z.; Wang, Y.; Li, G.; Zhang, X.; Li, G.; Ruan, D. American ginseng biowaste-derived activated carbon for high-performance supercapacitors. Int. J. Electrochem. Sci. 2023, 18, 16–24. [Google Scholar] [CrossRef]
- Gislon, P.; Galli, S.; Monteleone, G. Siloxanes removal from biogas by high surface area adsorbents. Waste Manag. 2013, 33, 2687–2693. [Google Scholar] [CrossRef]
- Wang, G.; Li, N.; Xing, X.; Sun, Y.; Zhang, Z.; Hao, Z. Gaseous adsorption of hexamethyldisiloxane on carbons: Isotherms, isosteric heats and kinetics. Chemosphere 2020, 247, 125862. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, Z.; Xia, S.; Lu, Q.; Walters, K. Catalytic pyrolysis of biomass and polymer wastes. Catalysts 2018, 8, 659. [Google Scholar] [CrossRef]
- Shetty, A.; Molahalli, V.; Sharma, A.; Hegde, G. Biomass-derived carbon materials in heterogeneous catalysis: A step towards sustainable future. Catalysts 2022, 13, 20. [Google Scholar] [CrossRef]
- Lu, H.; Gan, L. Catalytic degradation of bisphenol A in water by poplar wood powder waste Derived biochar via peroxymonosulfate activation. Catalysts 2022, 12, 1164. [Google Scholar] [CrossRef]
- Rethinasabapathy, M.; Lee, J.H.; Roh, K.C.; Kang, S.M.; Oh, S.Y.; Park, B.; Lee, G.W.; Cha, Y.L.; Huh, Y.S. Silver grass-derived activated carbon with coexisting micro-, meso- and macropores as excellent bioanodes for microbial colonization and power generation in sustainable microbial fuel cells. Bioresour. Technol. 2020, 300, 122646. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Koziel, J.A.; Białowiec, A.; Lee, M.; Ma, H.; Li, P.; Meiirkhanuly, Z.; Brown, R.C. The impact of surficial biochar treatment on acute H2S emissions during swine manure agitation before pump-out: Proof-of-the-concept. Catalysts 2020, 10, 940. [Google Scholar] [CrossRef]
- Magioglou, E.; Frontistis, Z.; Vakros, J.; Manariotis, I.; Mantzavinos, D. Activation of persulfate by biochars from valorized olive stones for the degradation of sulfamethoxazole. Catalysts 2019, 9, 419. [Google Scholar] [CrossRef]
- Li, S.; Ma, X.; Ma, Z.; Dong, X.; Wei, Z.; Liu, X.; Zhu, L. Mg/Al-layered double hydroxide modified biochar for simultaneous removal phosphate and nitrate from aqueous solution. Environ. Technol. Innov. 2021, 23, 101771. [Google Scholar] [CrossRef]
- Ma, X.; Li, S.; Ren, H.; Zhang, Y.; Ma, Z. Egg white-mediated fabrication of Mg/Al-LDH-hard biochar composite for phosphate adsorption. Molecules 2022, 27, 8951. [Google Scholar] [CrossRef] [PubMed]
- Cazetta, A.L.; Vargas, A.M.M.; Nogami, E.M.; Kunita, M.H.; Guilherme, M.R.; Martins, A.C.; Silva, T.L.; Moraes, J.C.G.; Almeida, V.C. NaOH-activated carbon of high surface area produced from coconut shell: Kinetics and equilibrium studies from the methylene blue adsorption. Chem. Eng. J. 2011, 174, 117–125. [Google Scholar] [CrossRef]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.S.; Bandoch, G.F.G.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, C.; Li, D.; Lei, Y.; Yao, H.; Zhou, G.; Wang, K.; Rao, Y.; Liu, W.; Xu, C.; et al. Micro-mesoporous activated carbon simultaneously possessing large surface area and ultra-high pore volume for efficiently adsorbing various VOCs. Carbon 2020, 170, 567–579. [Google Scholar] [CrossRef]
- Rajasekaran, S.J.; Grace, A.N.; Jacob, G.; Alodhayb, A.; Pandiaraj, S.; Raghavan, V. Investigation of different aqueous electrolytes for biomass-derived activated carbon-based supercapacitors. Catalysts 2023, 13, 286. [Google Scholar] [CrossRef]
- Pezoti Junior, O.; Cazetta, A.L.; Gomes, R.C.; Barizão, É.O.; Souza, I.P.A.F.; Martins, A.C.; Asefa, T.; Almeida, V.C. Synthesis of ZnCl2-activated carbon from macadamia nut endocarp (Macadamia integrifolia) by microwave-assisted pyrolysis: Optimization using RSM and methylene blue adsorption. J. Anal. Appl. Pyrolysis 2014, 105, 166–176. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, Y.; Li, X.; Ma, Z. Removal of siloxane (L2) from biogas using methyl-functionalised silica gel as adsorbent. Chem. Eng. J. 2020, 389, 124440. [Google Scholar] [CrossRef]
- Meng, Z.; Hou, X.; Liu, Y.; Ma, Z.; Shen, H. Facile fabrication of iron-modified biochar as a renewable adsorbent for efficient siloxane (L2) removal. J. Environ. Chem. Eng. 2021, 9, 105799. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Azaïs, P.; Cacciaguerra, T.; Cazorla-Amorós, D.; Linares-Solano, A.; Béguin, F. KOH and NaOH activation mechanisms of multiwalled carbon nanotubes with different structural organisation. Carbon 2005, 43, 786–795. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Faye, M.C.A.S.; Zhang, Y. Characterization of biochar derived from rice husks and its potential in chlorobenzene degradation. Carbon 2018, 130, 730–740. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.; Shao, Q.; Wang, G. Physicochemical properties evolution of chars from palm kernel shell pyrolysis. J. Therm. Anal. Calorim. 2018, 133, 1271–1280. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Zeng, Q.; Liang, Z.; Ye, X.; Lv, Y.; Liu, M. Preparation of Eucommia ulmoides lignin-based high-performance biochar containing sulfonic group: Synergistic pyrolysis mechanism and tetracycline hydrochloride adsorption. Bioresour. Technol. 2021, 329, 124856. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ma, L.Q.; Gress, J.; Harris, W.; Li, Y. Enhanced Cr(VI) reduction and As(III) oxidation in ice phase: Important role of dissolved organic matter from biochar. J. Hazard. Mater. 2014, 267, 62–70. [Google Scholar] [CrossRef]
- Liu, Y.H.; Meng, Z.Y.; Wang, J.Y.; Dong, Y.F.; Ma, Z.C. Removal of siloxanes from biogas using acetylated silica gel as adsorbent. Pet. Sci. 2019, 16, 920–928. [Google Scholar] [CrossRef]
- Finocchio, E.; Montanari, T.; Garuti, G.; Pistarino, C.; Federici, F.; Cugino, M.; Busca, G. Purification of biogases from siloxanes by adsorption: On the regenerability of activated carbon sorbents. Energy Fuel 2009, 23, 4156–4159. [Google Scholar] [CrossRef]
- Cabrera-Codony, A.; Gonzalez-Olmos, R.; Martin, M.J. Regeneration of siloxane-exhausted activated carbon by advanced oxidation processes. J. Hazard. Mater. 2015, 285, 501–508. [Google Scholar] [CrossRef]


| Samples | APC–0 | APC–1 | APC–2 | APC–3 | APC–4 | APC–5 |
|---|---|---|---|---|---|---|
| Yield (%) | 90.00 | 85.05 | 57.85 | 40.19 | 38.76 | 34.95 |
| Samples | APC–0 | APC–4 | |
|---|---|---|---|
| Content, wt.% | C | 76.82 | 82.82 |
| O | 21.30 | 16.06 | |
| H | 1.50 | 1.01 | |
| N | 0.35 | 0.08 | |
| S | 0.03 | 0.03 | |
| Samples | SBET (m2 g−1) | Vtot (cm3 g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) a | Daver (nm) |
|---|---|---|---|---|---|
| APC–0 | 319 | 0.14 | 0.12 | 0.02 | 0.88 |
| APC–1 | 988 | 0.56 | 0.56 | / | 1.12 |
| APC–2 | 1595 | 0.87 | 0.78 | 0.09 | 1.09 |
| APC–3 | 1669 | 1.02 | 0.73 | 0.29 | 1.22 |
| APC–4 | 2551 | 1.30 | 1.11 | 0.19 | 1.02 |
| APC–5 | 2192 | 1.18 | 0.98 | 0.2 | 1.08 |
| Adsorbents | Experimental | Model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| tB/ min | QB/ mg g−1 | Qm/ mg g−1 | tB,th/ min | QB,th/ mg g−1 | Qm,th/ mg g−1 | q0 | a | R2 | |
| APC–0 | 0.95 | 9.0 | 20.7 | 0.94 | 7.9 | 16.5 | 0.0158 | 4.48 | 0.9933 |
| APC–1 | 11.44 | 186.1 | 208.2 | 11.25 | 184.4 | 199.1 | 0.2078 | 35.96 | 0.9967 |
| APC–2 | 24.05 | 399.9 | 467.6 | 23.11 | 385.1 | 459.1 | 0.4675 | 16.50 | 0.9967 |
| APC–3 | 30.85 | 513.8 | 589.1 | 30.18 | 504.9 | 555.0 | 0.5655 | 28.41 | 0.9958 |
| APC–4 | 51.22 | 823.3 | 907.1 | 50.26 | 815.5 | 894.7 | 0.9397 | 30.26 | 0.9981 |
| APC–5 | 31.88 | 527.6 | 603.4 | 32.02 | 532.5 | 616.6 | 0.6309 | 20.04 | 0.9985 |
| Term | Value | tB,th/ min | QB,th/ mg g−1 | Qm,th/ mg g−1 | q0 | a | R2 |
|---|---|---|---|---|---|---|---|
| T/°C | 0 | 53.90 | 898.6 | 998.0 | 1.0205 | 28.52 | 0.9979 |
| 20 | 50.26 | 815.5 | 894.7 | 0.9397 | 30.26 | 0.9981 | |
| 40 | 46.93 | 782.4 | 839.8 | 0.8596 | 37.55 | 0.9987 | |
| 55 | 40.26 | 671.4 | 755.9 | 0.7717 | 24.02 | 0.9982 | |
| 70 | 40.16 | 671.9 | 722.5 | 0.7371 | 37.99 | 0.9994 |
| Adsorbent | Origin | Impregnant | SBET, m2 g−1 | Adsorbed Gas | Qm, mg g−1 | Regeneration Method | RE a, % | Reference |
|---|---|---|---|---|---|---|---|---|
| Activated carbons | NORIT RGM1 | CuII and CrVI salts | / | D3 | 878 | Heating at 100–200 °C | 50 | [39] |
| Activated carbons | Commercial | Virgin, acid | 1100 | L2 | 100 | Four-step heating treatment at 160 °C | 70–80 | [16] |
| Activated carbons | Wood | H3PO4 | 2142 | D4 | 526 | By the oxidation with H2O2 and O3 | 40–92 | [40] |
| APC–4 | Coconut shells | NaOH | 2551 | L2 | 894.7 | Heating at 100 °C | 94 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Ma, X.; Fu, Q.; Zheng, Y.; Ma, Z. Removal of Hexamethyldisiloxane by NaOH–Activated Porous Carbons Produced from Coconut Shells. Catalysts 2023, 13, 918. https://doi.org/10.3390/catal13060918
Lv S, Ma X, Fu Q, Zheng Y, Ma Z. Removal of Hexamethyldisiloxane by NaOH–Activated Porous Carbons Produced from Coconut Shells. Catalysts. 2023; 13(6):918. https://doi.org/10.3390/catal13060918
Chicago/Turabian StyleLv, Siqi, Xiaolong Ma, Qingling Fu, Yanhui Zheng, and Zichuan Ma. 2023. "Removal of Hexamethyldisiloxane by NaOH–Activated Porous Carbons Produced from Coconut Shells" Catalysts 13, no. 6: 918. https://doi.org/10.3390/catal13060918





