Improving the Stability of Ru-Doped Ni-Based Catalysts for Steam Methane Reforming during Daily Startup and Shutdown Operation
Abstract
:1. Introduction
2. Results and Discussion
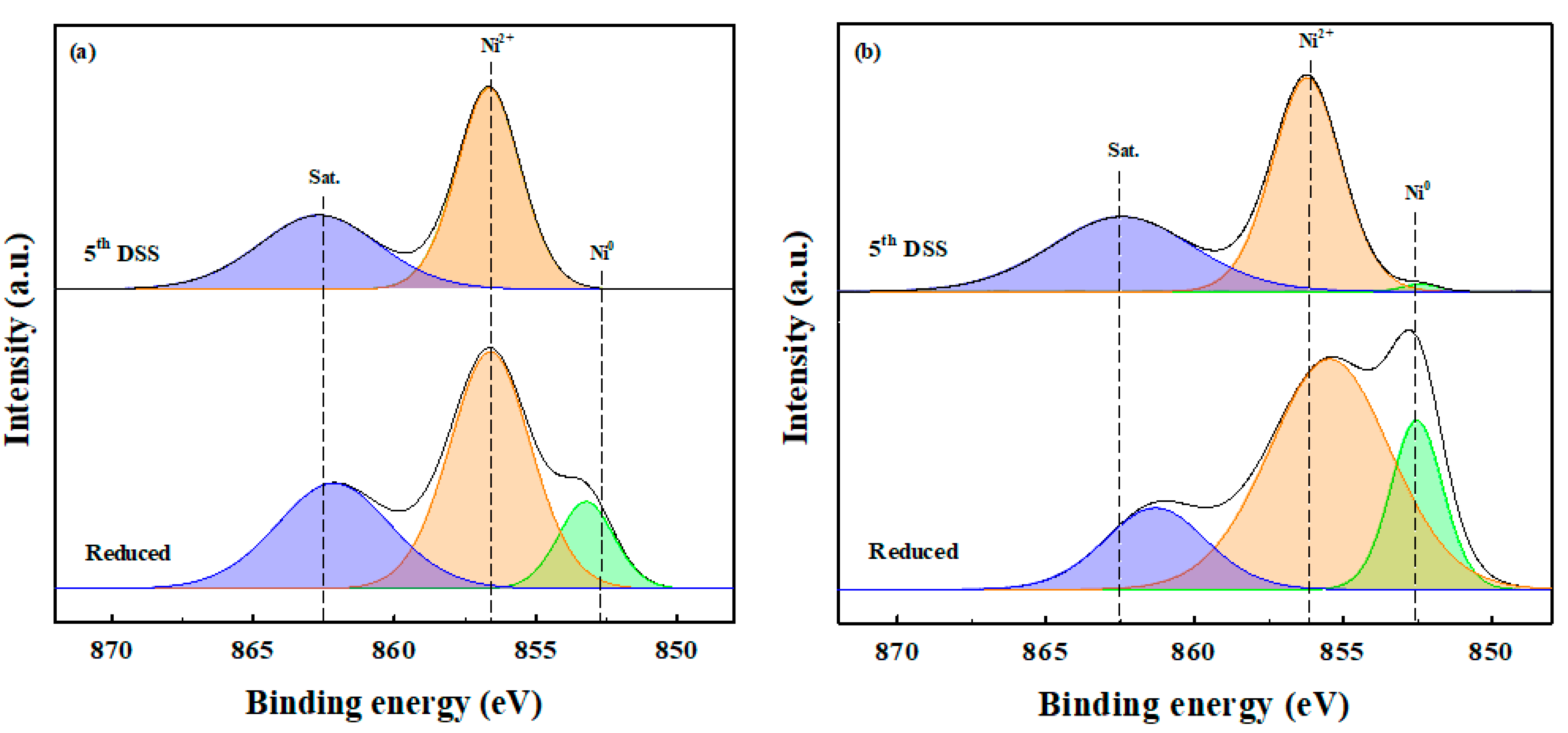
2.1. Characterization of the Ni-Based Catalysts
2.2. Steam Reforming Reaction with Ni-Based Catalysts
3. Materials and Methods
3.1. Materials
3.2. Catalyst Synthesis
3.3. Characterization
3.4. Steam–Methane Reforming Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carapellucci, R.; Giordano, L. Steam, dry and autothermal methane reforming for hydrogen production: A thermodynamic equilibrium analysis. J. Power Sources 2020, 469, 228391. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, Z.; Jiang, B.; Hongmanorom, P.; Zhong, W.; Kawi, S. A review on perovskite catalysts for reforming of methane to hydrogen production. Renew. Sustain. Energy Rev. 2020, 134, 110291. [Google Scholar] [CrossRef]
- Summa, P.; Samojeden, B.; Motak, M. Dry and steam reforming of methane. Comparison and analysis of recently investigated catalytic materials. A short review. Pol. J. Chem. Technol. 2019, 21, 31–37. [Google Scholar] [CrossRef]
- Torimoto, M.; Ogo, S.; Hisai, Y.; Nakano, N.; Takahashi, A.; Ma, Q.; Seo, J.G.; Tsuneki, H.; Norby, T.; Sekine, Y. Support effects on catalysis of low temperature methane steam reforming. RSC Adv. 2020, 10, 26418–26424. [Google Scholar] [CrossRef]
- Cho, E.; Yu, Y.J.; Kim, Y.; Phan, T.N.; Park, D.; Ko, C.H. Egg-shell-type Ni supported on MgAl2O4 pellets as catalyst for steam methane reforming: Enhanced coke-resistance and pellet stability. Catal. Today 2020, 352, 157–165. [Google Scholar] [CrossRef]
- Cho, E.H.; Koo, K.Y.; Lee, H.W.; Park, Y.-K.; Yoon, W.L.; Ko, C.H. Preparation of egg-shell-type Ni/Ru bimetal alumina pellet catalysts: Steam methane reforming for hydrogen production. Int. J. Hydrog. Energy 2017, 42, 18350–18357. [Google Scholar] [CrossRef]
- Khani, Y.; Shariatinia, Z.; Bahadoran, F. High catalytic activity and stability of ZnLaAlO4 supported Ni, Pt and Ru nanocatalysts applied in the dry, steam and combined dry-steam reforming of methane. Chem. Eng. J. 2016, 299, 353–366. [Google Scholar] [CrossRef]
- Jones, G.; Jakobsen, J.G.; Shim, S.S.; Kleis, J.; Andersson, M.P.; Rossmeisl, J.; Abild-Pedersen, F.; Bligaard, T.; Helveg, S.; Hinnemann, B. First principles calculations and experimental insight into methane steam reforming over transition metal catalysts. J. Catal. 2008, 259, 147–160. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D. Synthesis Chemistry and Properties of Ni Catalysts Fabricated on SiC@Al2O3 Core-Shell Microstructure for Methane Steam Reforming. Catalysts 2020, 10, 391. [Google Scholar] [CrossRef]
- Iglesias, I.; Forti, M.; Baronetti, G.; Mariño, F. Zr-enhanced stability of ceria based supports for methane steam reforming at severe reaction conditions. Int. J. Hydrog. Energy 2019, 44, 8121–8132. [Google Scholar] [CrossRef]
- Morales-Cano, F.; Lundegaard, L.F.; Tiruvalam, R.R.; Falsig, H.; Skjøth-Rasmussen, M.S. Improving the sintering resistance of Ni/Al2O3 steam-reforming catalysts by promotion with noble metals. Appl. Catal. A Gen. 2015, 498, 117–125. [Google Scholar] [CrossRef]
- Dehghan-Niri, R.; Walmsley, J.C.; Holmen, A.; Midgley, P.A.; Rytter, E.; Dam, A.H.; Hungria, A.B.; Hernandez-Garrido, J.C.; Chen, D. Nanoconfinement of Ni clusters towards a high sintering resistance of steam methane reforming catalysts. Catal. Sci. Technol. 2012, 2, 2476–2484. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, L.; Kameyama, H. Steam reforming reactions over a metal-monolithic anodic alumina-supported Ni catalyst with trace amounts of noble metal. Int. J. Hydrog. Energy 2011, 36, 5321–5333. [Google Scholar] [CrossRef]
- Li, D.; Nakagawa, Y.; Tomishige, K. Methane reforming to synthesis gas over Ni catalysts modified with noble metals. Appl. Catal. A Gen. 2011, 408, 1–24. [Google Scholar] [CrossRef]
- Payne, B.; Biesinger, M.; McIntyre, N. The study of polycrystalline nickel metal oxidation by water vapour. J. Electron Spectrosc. Relat. Phenom. 2009, 175, 55–65. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Watson, R.B.; Wang, X.; Ozkan, U.S. Deactivation characteristics of lanthanide-promoted sol–gel Ni/Al2O3 catalysts in propane steam reforming. J. Catal. 2005, 234, 496–508. [Google Scholar] [CrossRef]
- Zolghadri, S.; Honarvar, B.; Rahimpour, M.R. Synthesis, application, and characteristics of mesoporous alumina as a support of promoted Ni-Co bimetallic catalysts in steam reforming of methane. Fuel 2023, 335, 127005. [Google Scholar] [CrossRef]
- He, L.; Ren, Y.; Yue, B.; Tsang, S.C.E.; He, H. Tuning metal–support interactions on Ni/Al2O3 catalysts to improve catalytic activity and stability for dry reforming of methane. Processes 2021, 9, 706. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, K.; Choudary, N.; Pant, K. Effect of support materials on the performance of Ni-based catalysts in tri-reforming of methane. Fuel Process. Technol 2019, 186, 40–52. [Google Scholar] [CrossRef]
- Zhou, L.; Guo, Y.; Zhang, Q.; Yagi, M.; Li, H.; Chen, J.; Sakurai, M.; Kameyama, H. Self-activation and self-regenerative activity of trace Ru-doped plate-type anodic alumina supported nickel catalysts in steam reforming of methane. Catal. Commun. 2008, 10, 325–329. [Google Scholar] [CrossRef]
- Li, D.; Nishida, K.; Zhan, Y.; Shishido, T.; Oumi, Y.; Sano, T.; Takehira, K. Sustainable Ru-doped Ni catalyst derived from hydrotalcite in propane reforming. Appl. Clay Sci. 2009, 43, 49–56. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Jia, L.; Kameyama, H. Trace Ru-doped anodic alumina-supported Ni catalysts for steam reforming of kerosene: Activity performance and electrical-heating possibility. Fuel Process. Technol. 2011, 92, 2341–2347. [Google Scholar] [CrossRef]
- Jeong, J.H.; Lee, J.W.; Seo, D.J.; Seo, Y.; Yoon, W.L.; Lee, D.K.; Kim, D.H. Ru-doped Ni catalysts effective for the steam reforming of methane without the pre-reduction treatment with H2. Appl. Catal. A Gen. 2006, 302, 151–156. [Google Scholar] [CrossRef]
- Matus, E.; Sukhova, O.; Ismagilov, I.; Kerzhentsev, M.; Stonkus, O.; Ismagilov, Z. Hydrogen Production through Autothermal Reforming of Ethanol: Enhancement of Ni Catalyst Performance via Promotion. Energies 2021, 14, 5176. [Google Scholar] [CrossRef]
- Takehira, K.; Ohi, T.; Miyata, T.; Shiraga, M.; Sano, T. Steam reforming of CH4 over Ni-Ru catalysts supported on Mg-Al mixed oxide. Top. Catal. 2007, 42, 471–474. [Google Scholar] [CrossRef]
- Yoon, Y.; Kim, H.; Lee, J. Enhanced catalytic behavior of Ni alloys in steam methane reforming. J. Power Sources 2017, 359, 450–457. [Google Scholar] [CrossRef]
- Pinna, F. Supported metal catalysts preparation. Catal. Today 1998, 41, 129–137. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jo, S.; Kim, T.-Y.; Woo, J.-H.; Lee, Y.; Kim, M.-S.; Park, H.-O.; Lee, S.-C.; Kim, J.-C. Preparation of Eggshell-Type Ru/Al2O3 Catalysts for Hydrogen Production Using Steam-Methane Reforming on PEMFC. Catalysts 2021, 11, 951. [Google Scholar] [CrossRef]
- Jang, M.-S.; Cho, E.H.; Koo, K.Y.; Yoon, W.L.; Ko, C.H. Facile preparation of egg-shell-type pellet catalysts using immiscibility between hydrophobic solvent and hydrophilic solution: Enhancement of catalytic activity due to position control of metallic nickel inside alumina pellet. Appl. Catal. A Gen. 2017, 530, 211–216. [Google Scholar] [CrossRef]
- Zhuang, Y.; Claeys, M.; Van Steen, E. Novel synthesis route for egg-shell, egg-white and egg-yolk type of cobalt on silica catalysts. Appl. Catal. A Gen. 2006, 301, 138–142. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Methane steam reforming over Ni/Ce–ZrO2 catalyst: Influences of Ce–ZrO2 support on reactivity, resistance toward carbon formation, and intrinsic reaction kinetics. Appl. Catal. A Gen. 2005, 290, 200–211. [Google Scholar] [CrossRef]
- Lv, Z.; Ruan, J.; Tu, W.; Hu, X.; He, D.; Huang, X.; Qin, C. Integrated CO2 capture and In-Situ methanation by efficient dual functional Li4SiO4@Ni/CeO2. Sep. Purif. Technol. 2023, 309, 123044. [Google Scholar] [CrossRef]
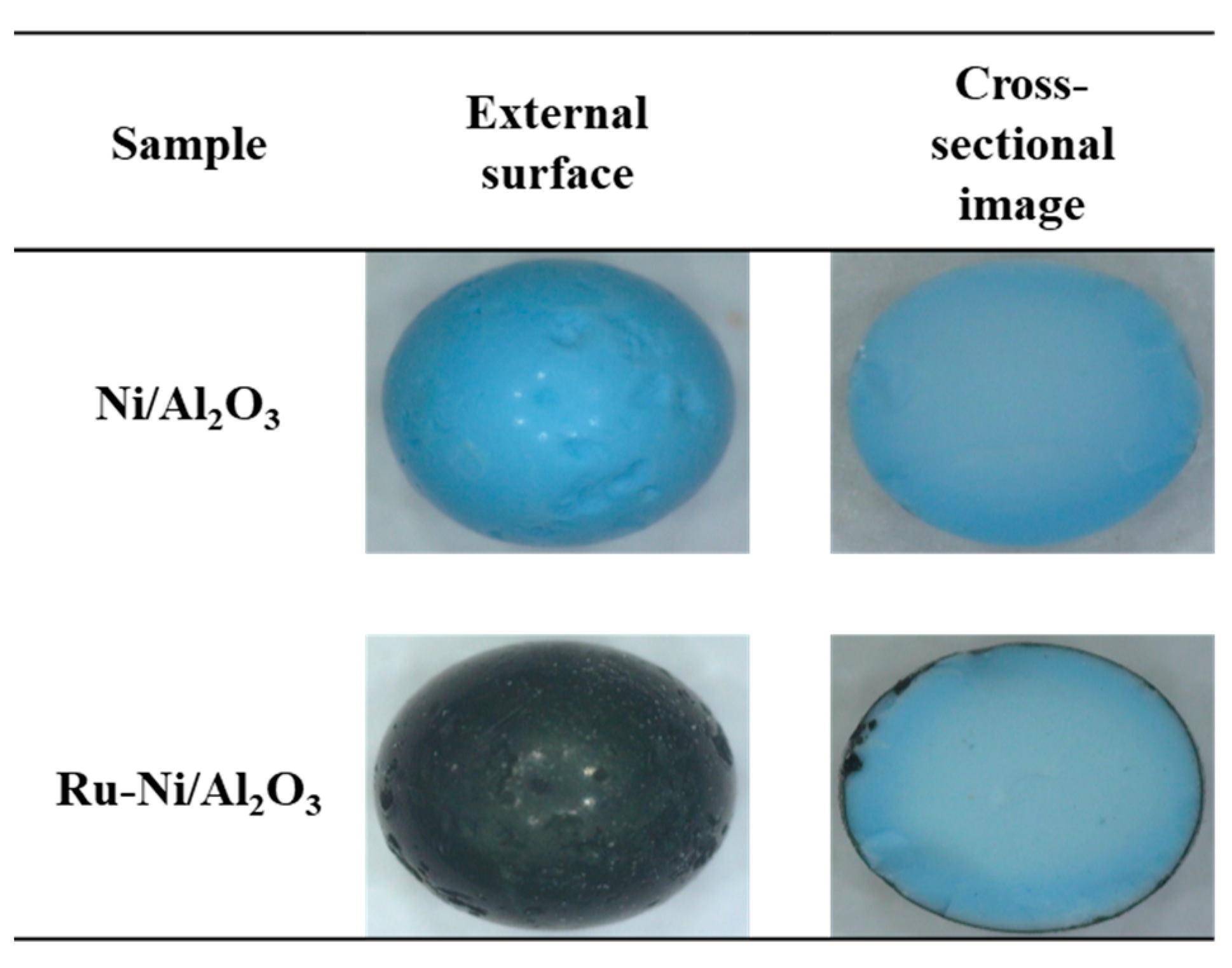

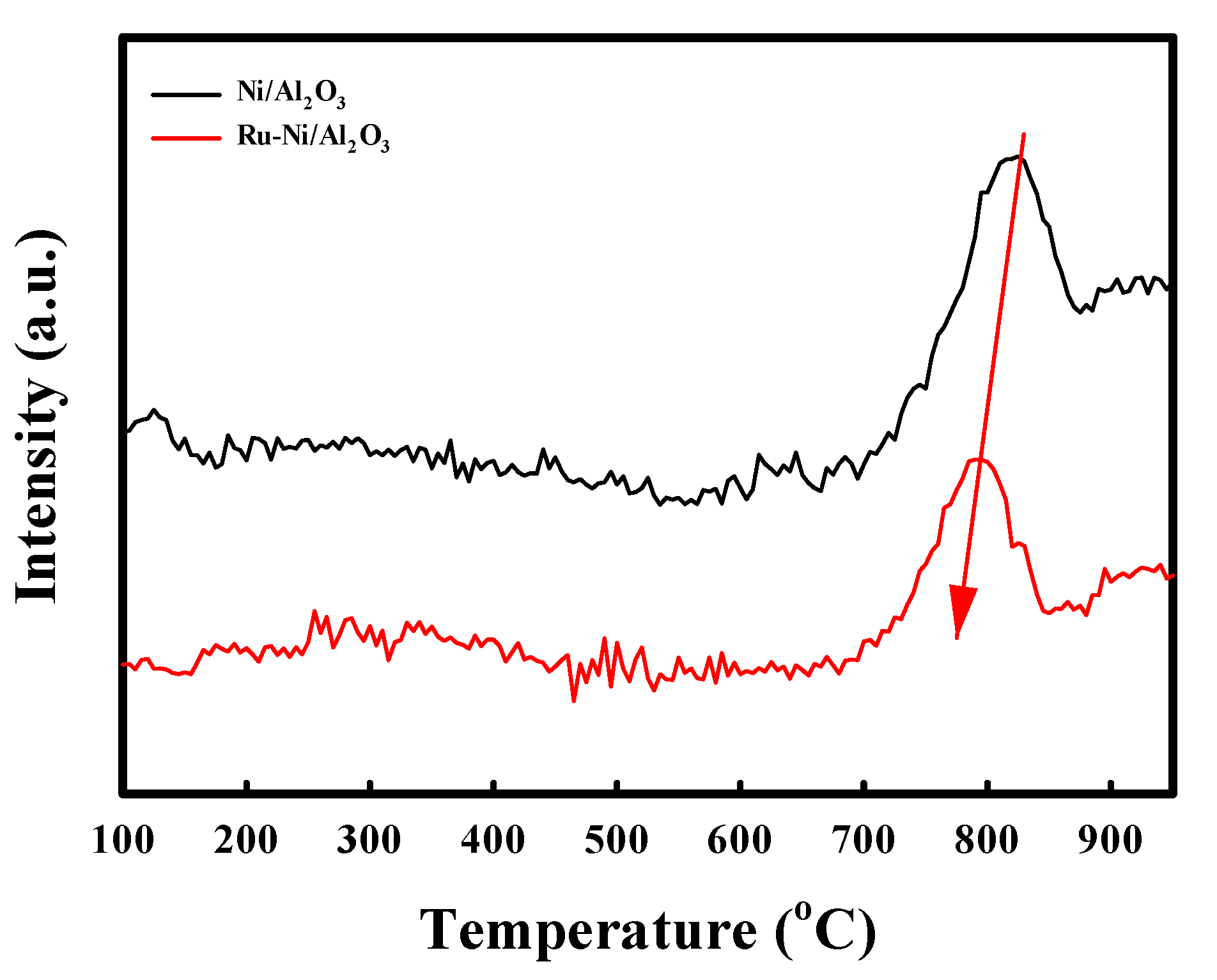
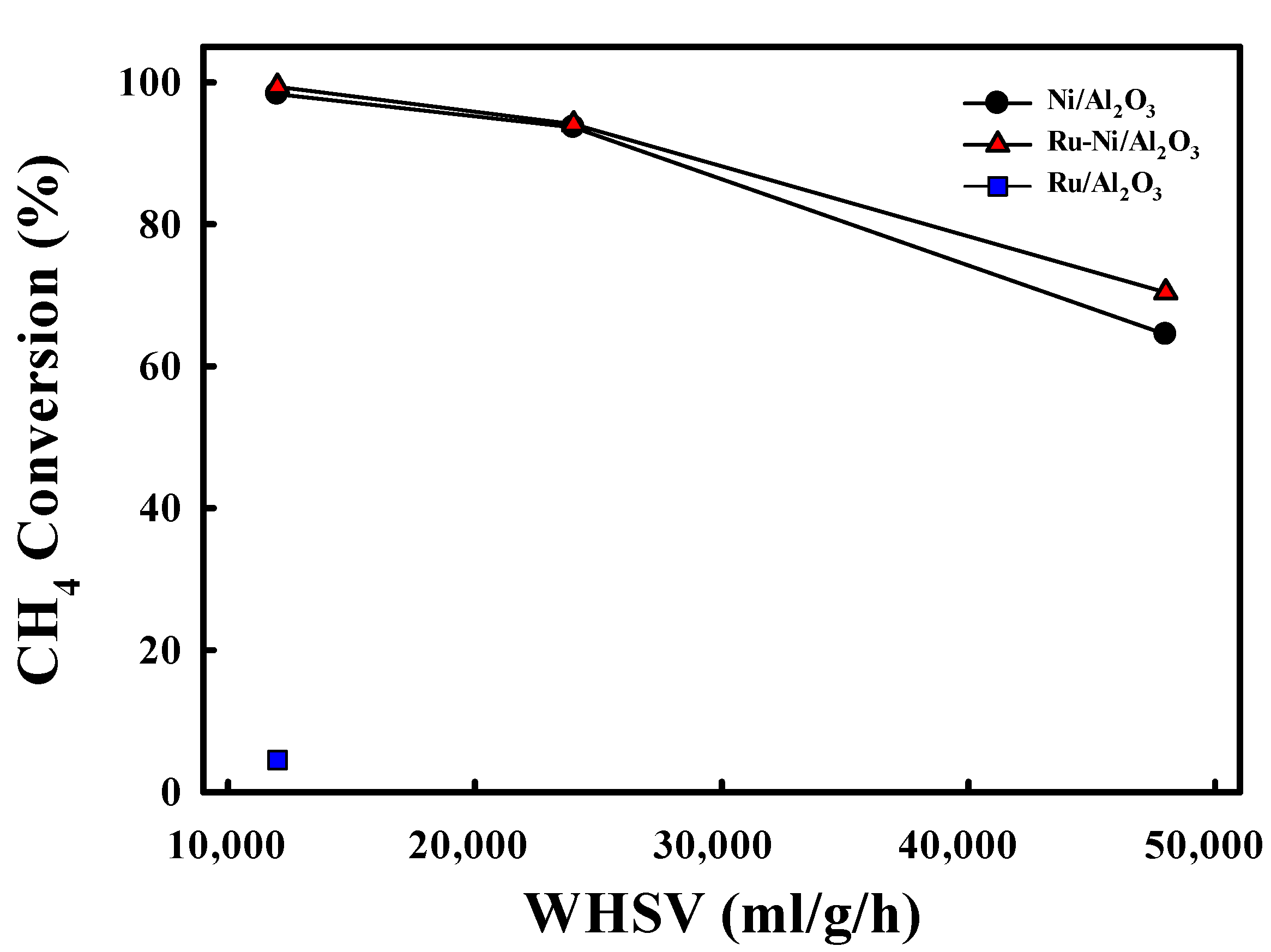
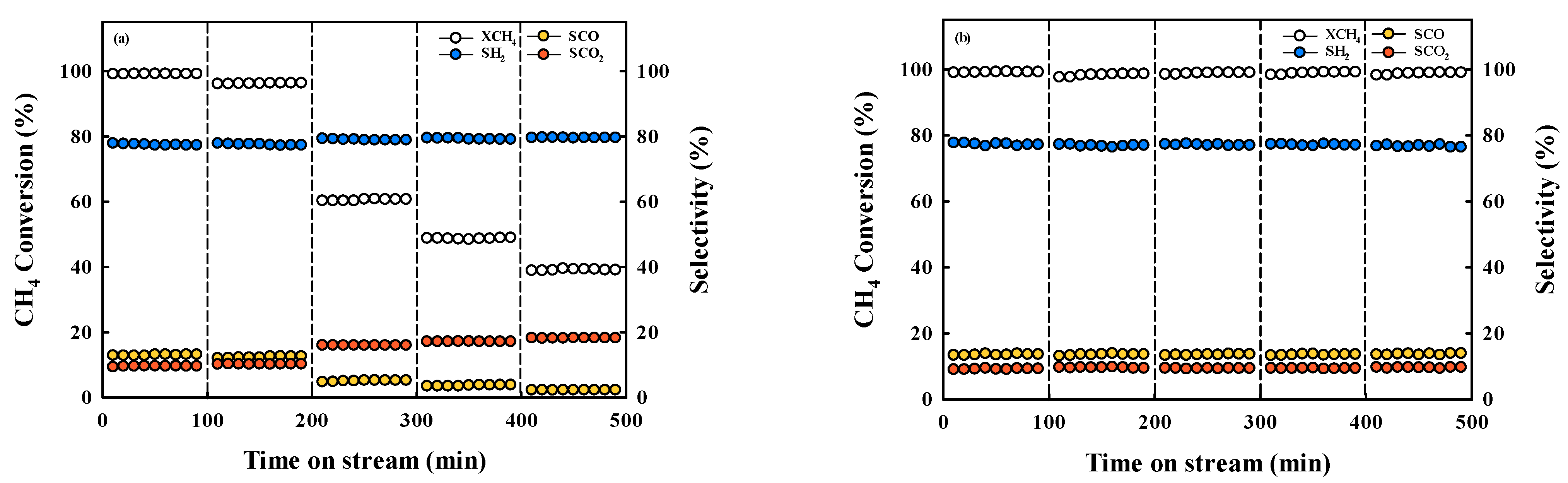


| Catalyst | Reaction Condition | Cycle | CH4 Converison (%) | Ref |
|---|---|---|---|---|
| Ni/Al2O3 | SV: 12,000 mL/g/h 700 °C | 1 | 99.0 | This study |
| 3 | 60.2 | |||
| 5 | 38.8 | |||
| Ru–Ni/Al2O3 | 1 | 99.1 | This study | |
| 3 | 99.0 | |||
| 5 | 99.0 | |||
| 2%Ru/Al2O3 | SV: 12,000 mL/g/h 700 °C | 1 | 99.0 | [28] |
| 3 | 99.0 | |||
| 5 | 99.0 |
| Catalyst | State | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | H2 Consumption (mmol/g) |
|---|---|---|---|---|---|
| Ni/Al2O3 | Fresh | 127.2 | 0.39 | 8.1 | 1.515 |
| After the 1st DSS | 124.3 | 0.43 | 9.3 | 0.189 | |
| After the 5th DSS | 105.2 | 0.38 | 9.4 | 0.250 | |
| Ru–Ni/Al2O3 | Fresh | 137.0 | 0.43 | 8.7 | 1.578 |
| After the 1st DSS | 130.9 | 0.43 | 9.4 | 0.252 | |
| After the 5th DSS | 106.5 | 0.38 | 11.6 | 0.884 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-Y.; Lee, J.-H.; Jo, S.; Kim, J.; Woo, J.-H.; Dhanusuraman, R.; Kim, J.-C.; Lee, S.-C. Improving the Stability of Ru-Doped Ni-Based Catalysts for Steam Methane Reforming during Daily Startup and Shutdown Operation. Catalysts 2023, 13, 949. https://doi.org/10.3390/catal13060949
Kim T-Y, Lee J-H, Jo S, Kim J, Woo J-H, Dhanusuraman R, Kim J-C, Lee S-C. Improving the Stability of Ru-Doped Ni-Based Catalysts for Steam Methane Reforming during Daily Startup and Shutdown Operation. Catalysts. 2023; 13(6):949. https://doi.org/10.3390/catal13060949
Chicago/Turabian StyleKim, Tae-Young, Jong-Heon Lee, Seongbin Jo, Jueon Kim, Jin-Hyeok Woo, Ragupathy Dhanusuraman, Jae-Chang Kim, and Soo-Chool Lee. 2023. "Improving the Stability of Ru-Doped Ni-Based Catalysts for Steam Methane Reforming during Daily Startup and Shutdown Operation" Catalysts 13, no. 6: 949. https://doi.org/10.3390/catal13060949







