Role of Nanocellulose in Light Harvesting and Artificial Photosynthesis
Abstract
:1. Introduction
2. Processing of Nanocellulose Structures for Artificial Photosynthesis
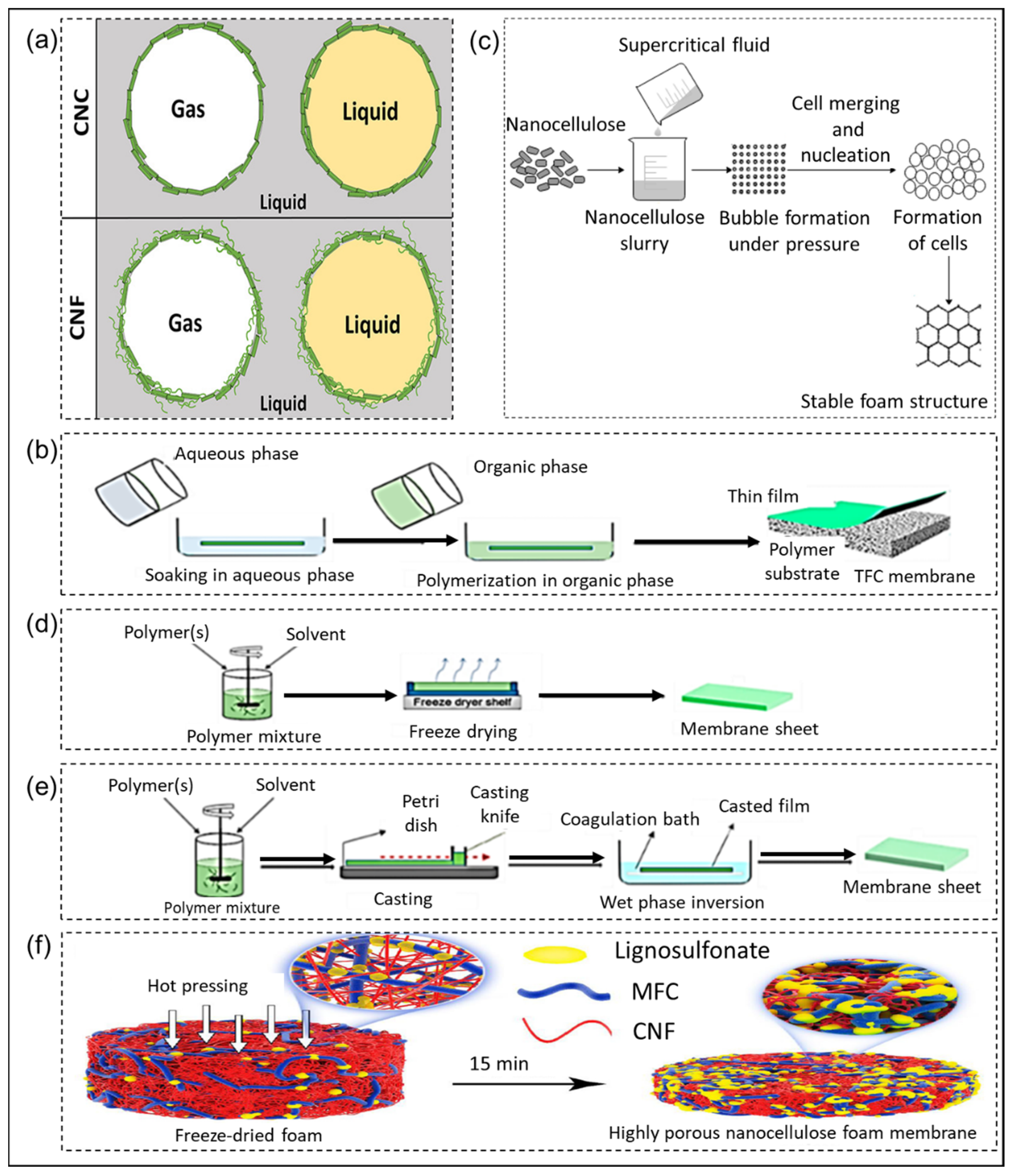
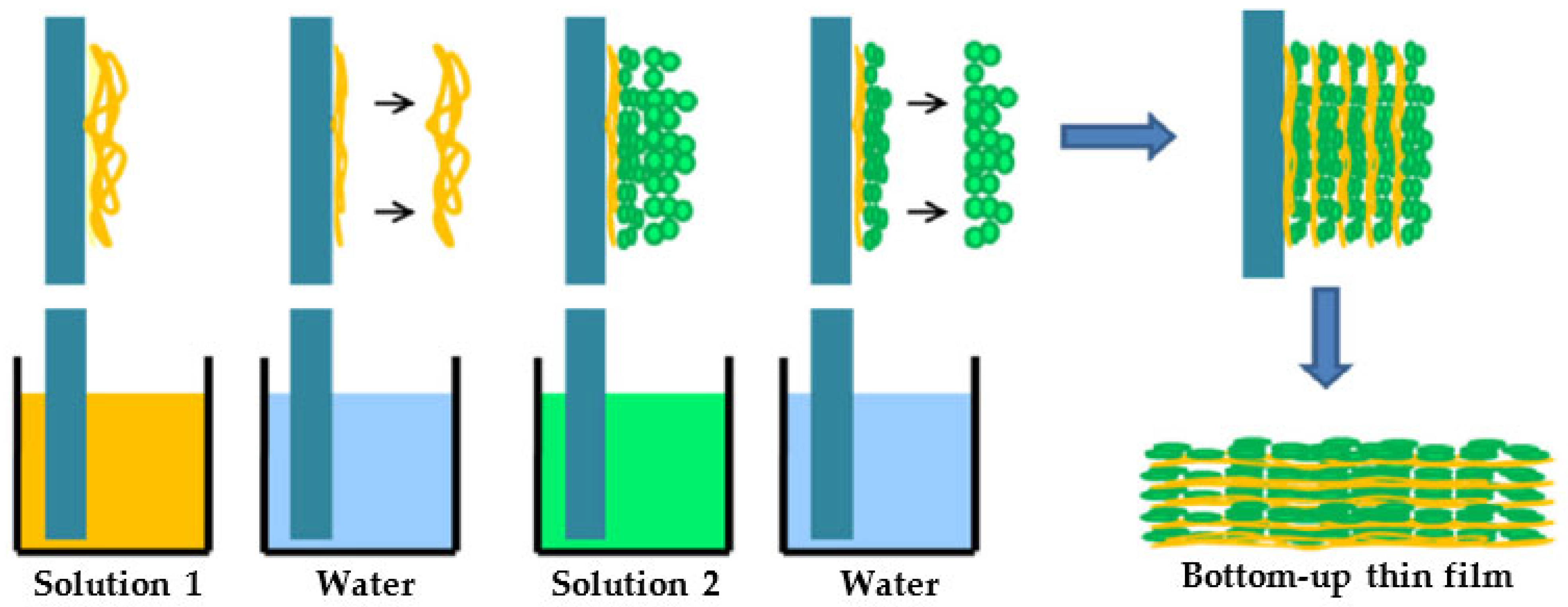
2.1. Nanocellulose Films

2.2. Nanocellulose Hydrogels

2.3. Nanocellulose Foams and Membranes
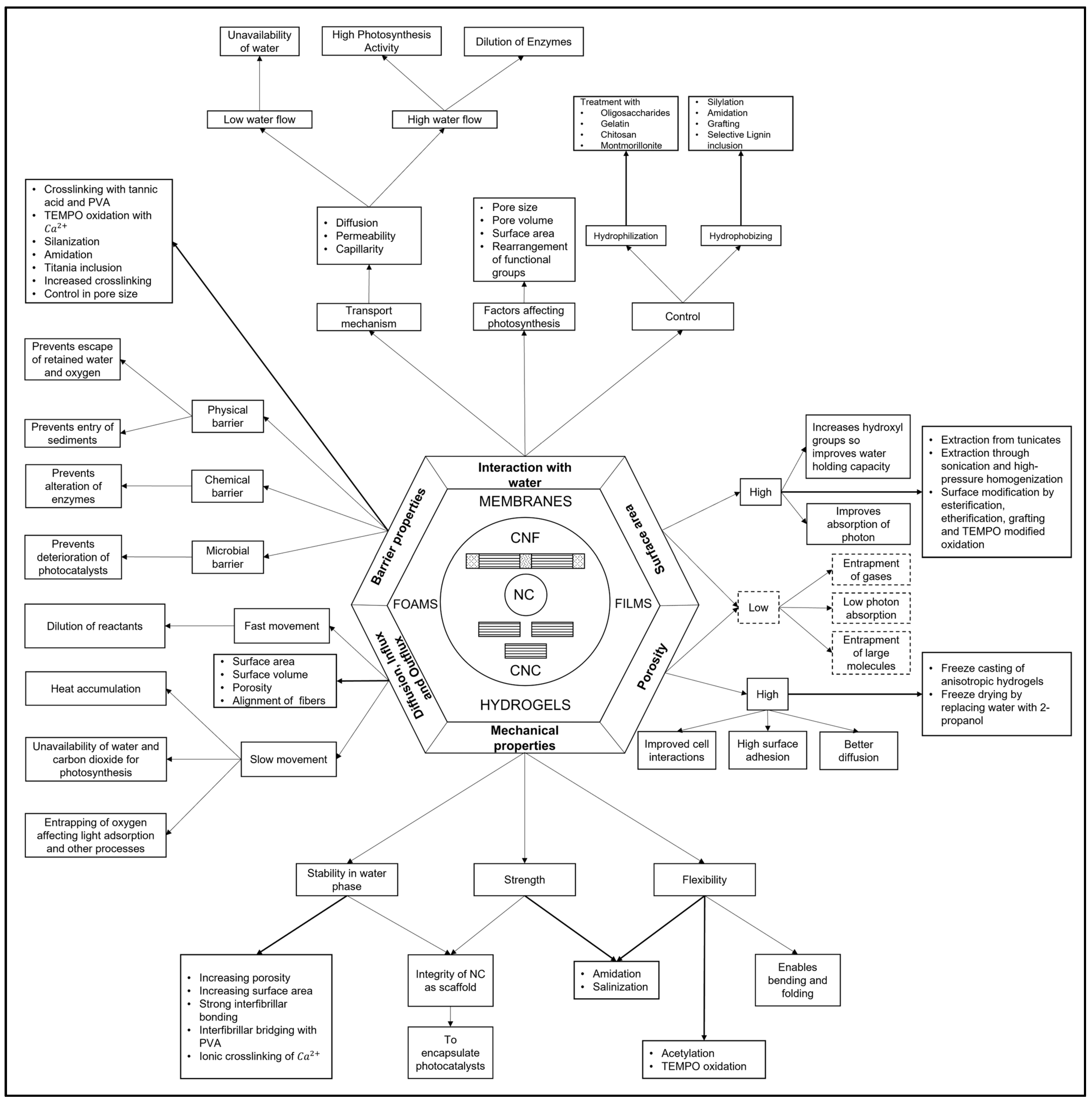
3. Nanocellulose Properties for Artificial Photosynthesis
4. Nanocellulose for Light Harvesting and Light Interactions
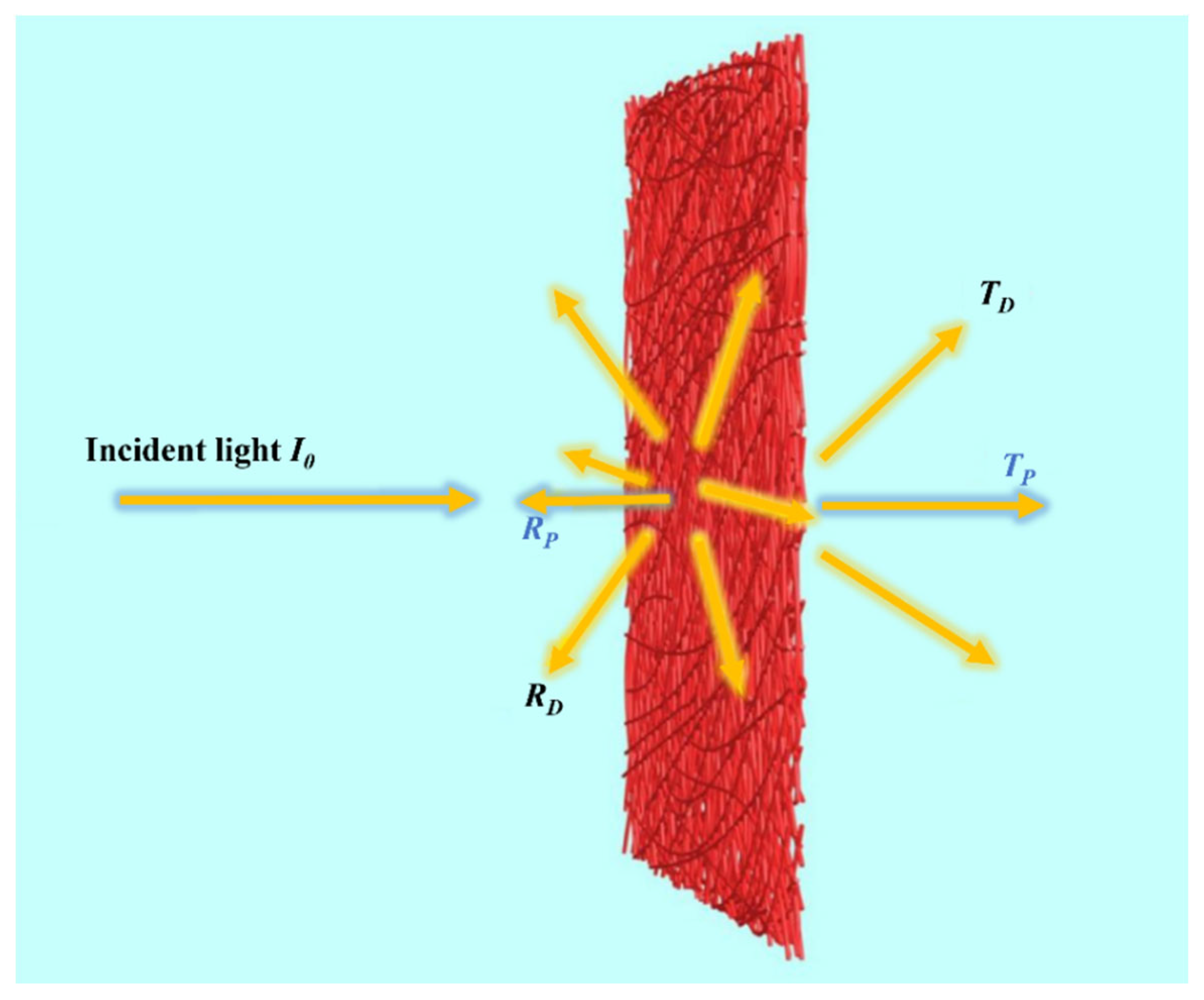
4.1. Efficiency of Light Interactions in Nanofiber Structures
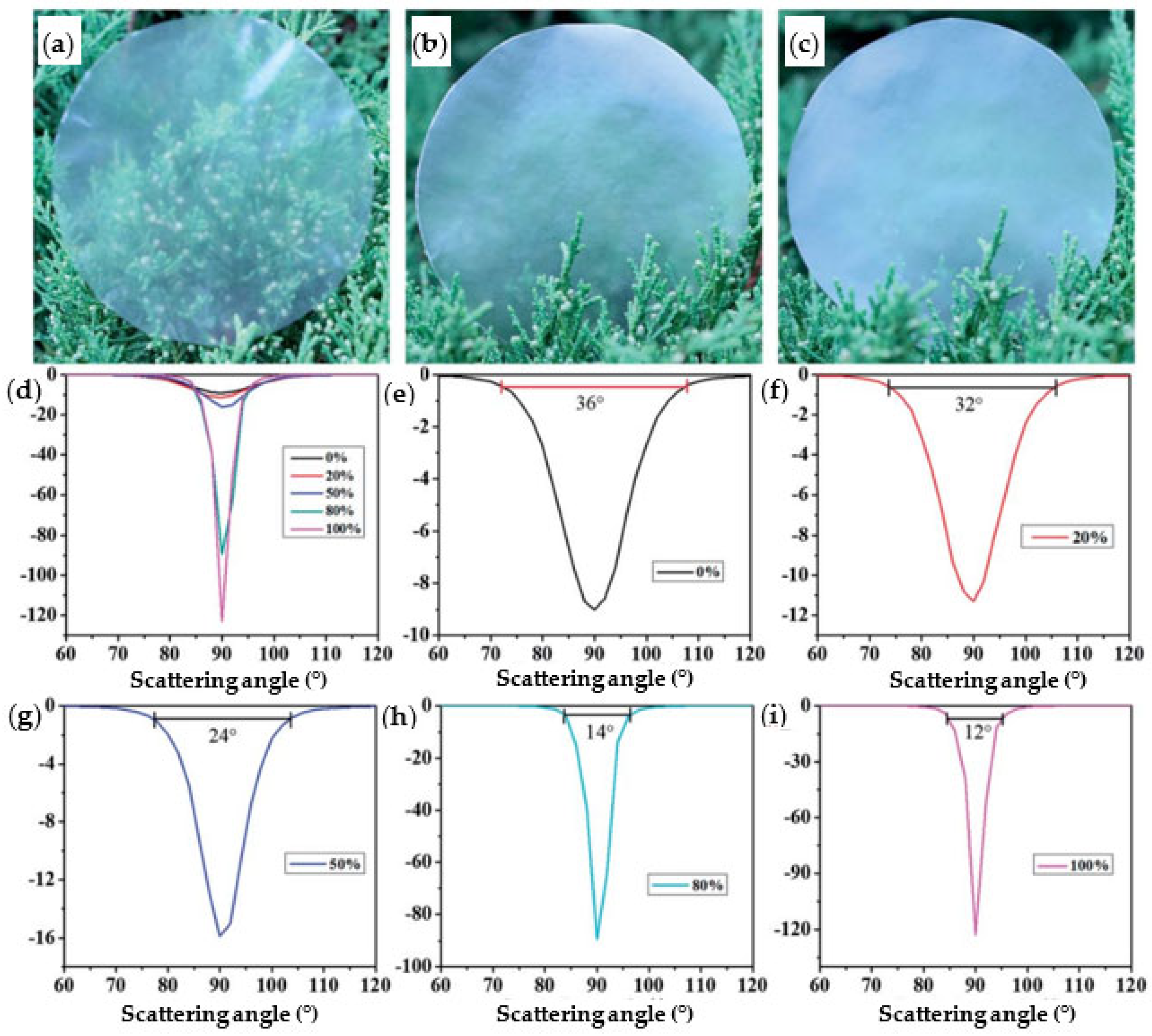
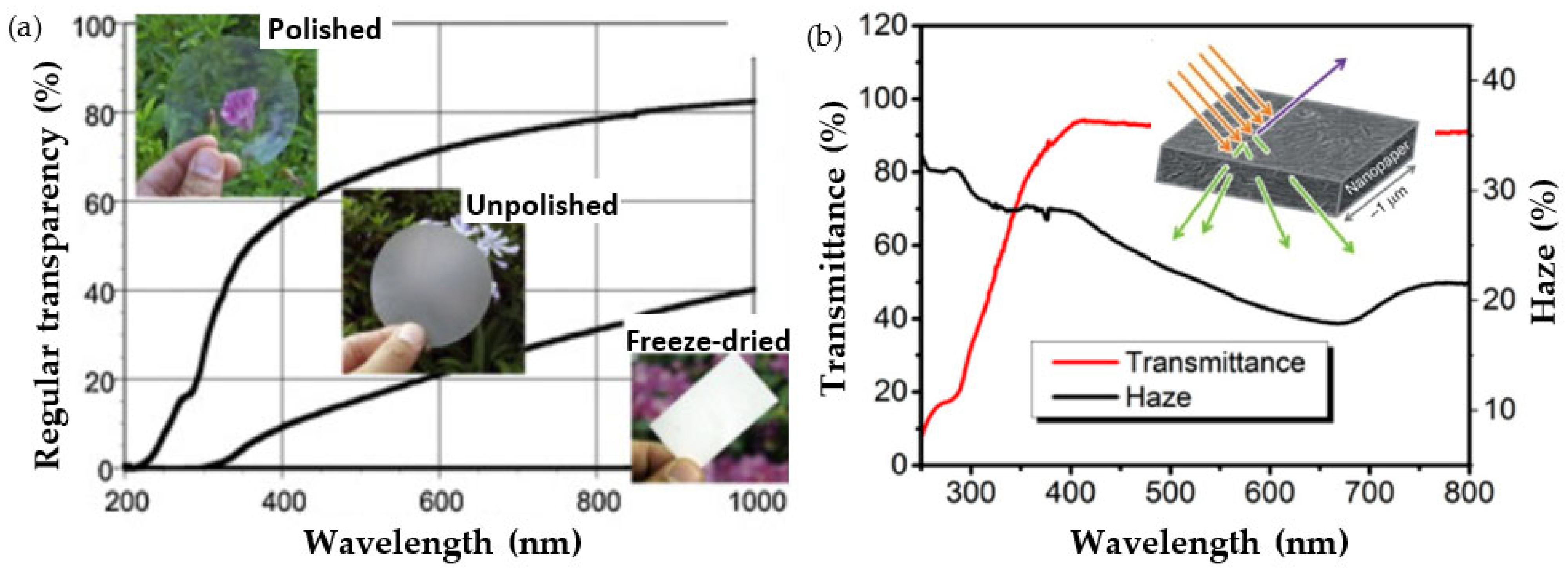
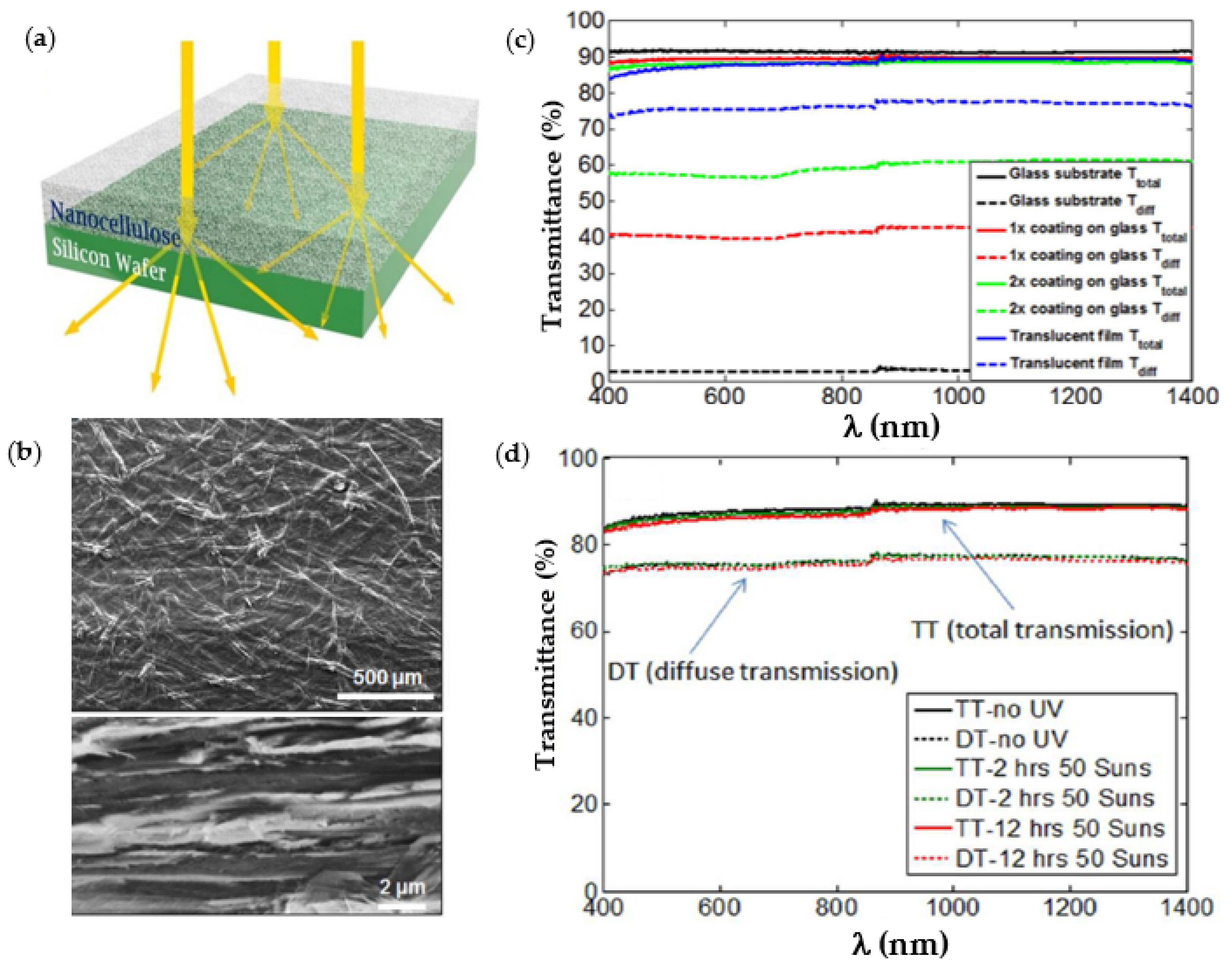
4.2. Optical Properties in Nanofiber Structures: Transparency, Translucence, Haze
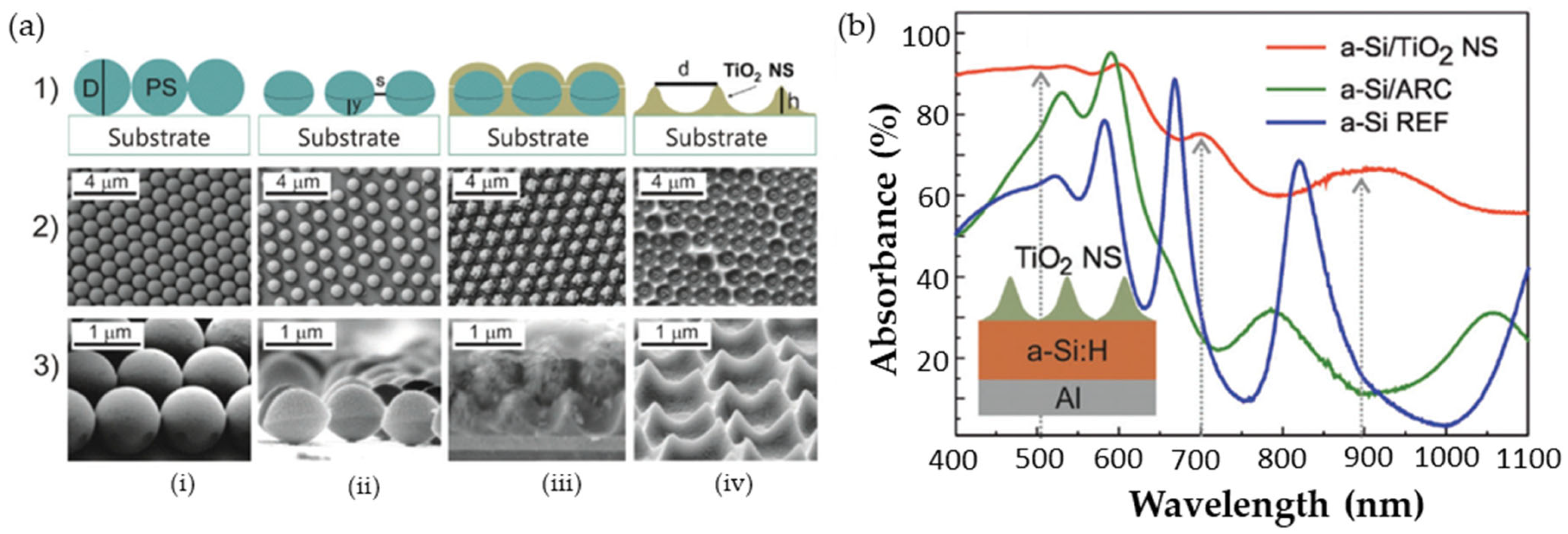
4.3. Optimizing Light Harvesting in Nanostructures
5. Nanocellulose in Artificial Photosynthesis
5.1. Mimicking Nature for Artificial Photosynthesis Platforms
5.2. Assembled Nanocellulose Composites in Artificial Photosynthetic Systems
5.3. Nanocellulose as Support for Artificial Leave Structures and Assemblies
5.3.1. Basic Artificial Leaf Structures
5.3.2. Biomimetic Artificial Photosynthesis
6. Nanocellulose as Template for Photosynthetic Cell Factories
6.1. Incorporation of Photosynthetic Active Cells
6.2. Photosynthetic Cell Factories on Paper
6.3. Photosynthetic Cell Factories on Nanopaper
6.3.1. Bacterial Nanocellulose (BNC)
6.3.2. Cellulose Nanofibrils (CNF)
6.3.3. Electrospun Cellulose Nanoyarns (CNY)
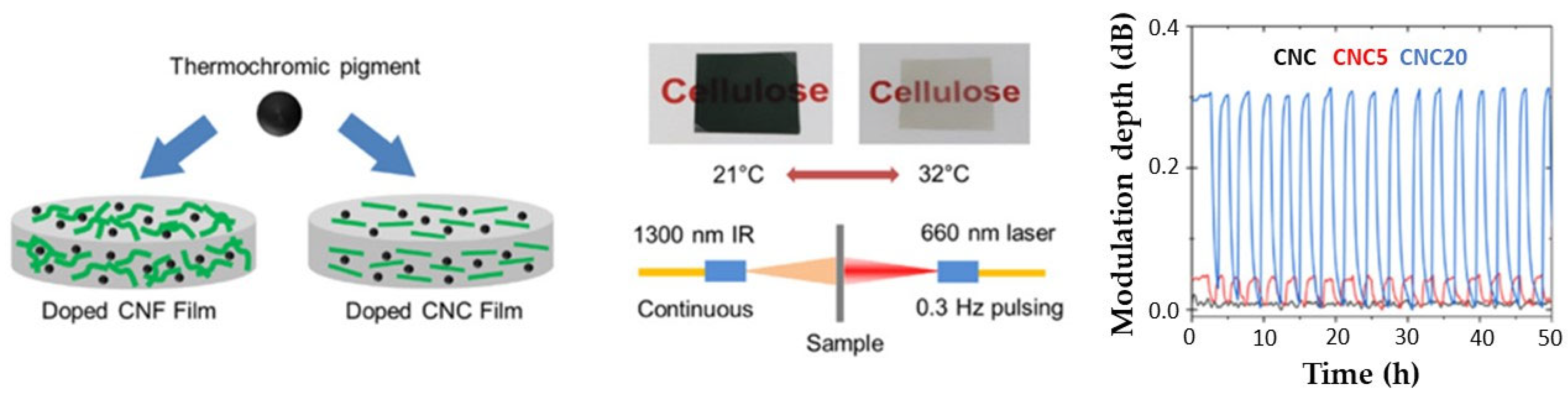
6.4. Photosynthetic Nanocellulose Cell Factories with Improved Light Interactions
6.5. Advanced Artificial Leaf Structures with Controlled Morphologies
7. Challenges and Outlook
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Archer, M.D.; Barber, J. Photosynthesis and Photoconversion. In Molecular to Global Photosynthesis; Barber, J., Ed.; Imperial College Press: London, UK, 2004; Volume 2, pp. 1–41. [Google Scholar]
- Kumaravel, V.; Barlett, J.; Pillai, S.C. Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products. ACS Energy Lett. 2020, 5, 486–519. [Google Scholar] [CrossRef] [Green Version]
- Barber, J.; Tran, P.D. From Natural to Artificial Photosynthesis. J. R. Soc. Interface 2013, 10, 20120984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, T.; Emmerson, R.; Battle, M.; Pullin, J.; Wall, S.; Hosmann, T.A. Carbon Fixation. In Photosynthesis in Action; Ruban, A., Foyer, C., Muchie, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 31–58. [Google Scholar]
- Kornienko, N.; Zhang, J.Z.; Sakimoto, K.K.; Yang, P.; Reisner, E. Interfacing Nature’s Catalytic Machinery with Synthetic Materials for Semi-Artificial Photosynthesis. Nat. Nanotechnol. 2018, 13, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Fleming, G.R.; Schlau-Cohen, G.S.; Amarnath, K.; Zaks, J. Design Principles of Photosynthetic Light-Harvesting. Faraday Discuss. 2012, 155, 27–41. [Google Scholar] [CrossRef]
- Li, G.; Fei, J.; Xu, Y.; Li, Y.; Li, J. Bioinspired Assembly of Hierarchical Light-Harvesting Architectures for Improved Photophosphorylation. Adv. Funct. Mater. 2018, 28, 1706557. [Google Scholar] [CrossRef]
- McConnell, I.; Li, G.; Brudvig, G.W. Energy Conversion in Natural and Artificial Photosynthesis. Chem. Biol. 2010, 17, 434–447. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.R.; Clark, E.L.; Bell, A.T. Thermodynamic and achievable efficiencies for solar-driven electrochemical reduction of carbon dioxide to transportation fuels. Proc. Natl. Acad. Sci. USA 2015, 112, E5111–E6118. [Google Scholar] [CrossRef] [Green Version]
- Ghirardi, M.L.; Dubini, A.; Yu, J.; Maness, P.C. Photobiological hydrogen-producing systems. Chem. Soc. Rev. 2009, 38, 52–61. [Google Scholar] [CrossRef]
- Guan, X.; Chowdhury, F.A.; Wang, Y.; Pant, N.; Vanka, S.; Trudeau, M.L.; Guo, L.; Vayssieres, L.; Mi, Z. Making of an Industry-Friendly Artificial Photosynthesis Device. ACS Energy Lett. 2018, 3, 2230–2231. [Google Scholar] [CrossRef]
- Bonke, S.A.; Wiechen, M.; MacFarlane, D.R.; Spiccia, L. Renewable fuels from concentrated solar power: Towards practical artificial photosynthesis. Energy Environ. Sci. 2015, 8, 2791–2796. [Google Scholar] [CrossRef]
- Chen, S.; Qi, L.; Li, C.; Domen, K.; Zhang, F. Surface Strategies for Particulate Photocatalysts toward Artificial Photosynthesis. Joule 2018, 2, 2260–2288. [Google Scholar] [CrossRef] [Green Version]
- Ikram, M.; Qumar, U.; Ali, S.; Ul-Hamid, A. Impact of Metal Oxide Nanoparticles on Adsorptive and Photocatalytic Schemes. In Advanced Materials for Wastewater Treatment and Desalination; CRC Press: Boca Raton, FL, USA, 2022; pp. 53–77. [Google Scholar]
- Spillmann, C.M.; Medintz, I.L. Use of biomolecular scaffolds for assembling multistep light harvesting and energy transfer devices. J. Photochem. Photobiol. C Photochem. Rev. 2015, 23, 1–24. [Google Scholar] [CrossRef]
- Lewis, N.S. Developing a scalable artificial photosynthesis technology through nanomaterials by design. Nat. Nanotechnol. 2016, 11, 1010–1019. [Google Scholar] [CrossRef]
- Liu, G.; Gao, F.; Gao, V.; Xiong, Y. Bioinspiration toward efficient photosynthetic systems: From biohybrids to biomimetics. Chem. Catal. 2021, 1, 1367–1377. [Google Scholar] [CrossRef]
- Saxena, I.M.; Brown, J.R.M. Biosynthesis of Cellulose. Prog. Biotechnol. 2001, 18, 69–76. [Google Scholar]
- Barhoum, A.; Rastogi, V.K.; Mahur, B.K.; Rastogi, A.; Abdel-Haleem, F.M.; Samyn, P. Nanocelluloses as New Generation Materials: Natural Resources, Structure-Related Properties, Engineering Nanostructures, and Technical Challenges. Mater. Today Chem. 2022, 26, 101247. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [Green Version]
- Nechyporchuk, O.; Belgacem, M.H.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops. Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Samyn, P.; Meftahi, A.; Geravand, S.A.; Heravi, M.E.; Najarzadeh, H.; Sabery, M.S.K.; Barhoum, A. Opportunities for bacterial nanocellulose in biomedical applications: Review on biosynthesis, modification and challenges. Int. J. Biol. Macromol. 2023, 15, 123316. [Google Scholar] [CrossRef]
- Meftahi, A.; Samyn, P.; Geravand, S.A.; Khajavi, R.; Alibkshi, S.; Bechelany, M.; Barhoum, A. Nanocelluloses as skin biocompatible materials for skincare, cosmetics, and healthcare: Formulations, regulations, and emerging applications. Carbohydr. Polym. 2021, 278, 118956. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 1–49. [Google Scholar]
- Thomas, P.; Duolikun, T.; Rumjit, N.P.; Moosavi, S.; Lai, C.W.; Bin Johan, M.R.; Fen, L.B. Comprehensive Review on Nanocellulose: Recent Developments, Challenges and Future Prospects. J. Mech. Behav. Biomed. Mater. 2020, 110, 103884. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A. Emerging Nanocellulose Technologies: Recent Developments. Adv. Mater. 2021, 33, 2000630. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yao, C.; Wang, F.; Li, Z. Cellulose-Based Nanomaterials for Energy Applications. Small 2017, 13, 1702240. [Google Scholar] [CrossRef] [PubMed]
- Lasrado, D.; Ahankari, S.S.; Kar, K. Nanocellulose-based polymer composites for energy applications—A review. J. Appl. Polym. Sci. 2020, 137, 48959. [Google Scholar] [CrossRef]
- Varghese, R.T.; Cherian, R.M.; Antony, T.; Tharayil, A.; Das, H.; Kargarzadeh, H.; Chirayil, C.J.; Thomas, S. A Review on the Best Bioadsorbent Membrane- Nanocellulose for Effective Removal of Pollutants from Aqueous Solutions. Carbohydr. Polym. Technol. Appl. 2022, 3, 100209. [Google Scholar] [CrossRef]
- Fang, Z.; Hou, G.; Chen, C.; Hu, L. Nanocellulose-Based Films and Their Emerging Applications. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100764. [Google Scholar] [CrossRef]
- Wang, D.; Yao, Q.; Liu, J.; Sun, J.; Zhu, Q.; Chen, H. Processing nanocellulose to bulk materials: A review. Cellulose 2019, 26, 7585–7617. [Google Scholar] [CrossRef]
- Canham, R. Practical Techniques for Creating Nanocellulose Film. J. Paper Conserv. 2022, 23, 142–143. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Ong, K.K.; Noor, S.A.M.; Halim, N.A.; Shah, N.A.A.; Jamal, S.H.; Janudin, N.; Misenan, M.S.M.; et al. Emerging Developments Regarding Nanocellulose-Based Membrane Filtration Material against Microbes. Polymers 2021, 13, 3249. [Google Scholar] [CrossRef]
- Kawano, T.; Iikubo, S.; Andou, Y. The Relationship between Crystal Structure and Mechanical Performance for Fabrication of Regenerated Cellulose Film through Coagulation Conditions. Polymers 2021, 13, 4450. [Google Scholar] [CrossRef]
- Shanmugam, K.; Varanasi, S.; Garnier, G.; Batchelor, W. Rapid preparation of smooth nanocellulose films using spray coating. Cellulose 2017, 24, 2669–2676. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-Based Hydrogel Materials: Chemistry, Properties and Their Prospective Applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [Green Version]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef] [Green Version]
- Ishak, W.H.W.; Jia, O.Y.; Akmad, I. pH-Responsive Gamma-Irradiated Poly (Acrylic Acid)-Cellulose-Nanocrystal-Reinforced Hydrogels. Polymers 2020, 12, 1932. [Google Scholar] [CrossRef]
- Blazic, R.; Marusic, K.; Vidovic, E. Swelling and Viscoelastic Properties of Cellulose-Based Hydrogels Prepared by Free Radical Polymerization of Dimethylaminoethyl Methacrylate in Cellulose Solution. Gels 2023, 9, 94. [Google Scholar] [CrossRef]
- Lavoine, N.; Bergström, L. Nanocellulose-Based Foams and Aerogels: Processing, Properties, and Applications. J. Mater. Chem. A Mater. 2017, 5, 16105–16117. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Liu, R.; Huang, Y. Cellulose-Based Gels. Macromol. Chem. Phys. 2016, 217, 1322–1334. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.; Hyun, J. Free-Form Three-Dimensional Nanocellulose Structure Reinforced with Poly (Vinyl Alcohol) Using Freeze-Thaw Process. Carbohydr. Polym. 2022, 298, 120055. [Google Scholar] [CrossRef]
- Lin, J.; Jiao, G.; Kermanshahi-Pour, A. Algal Polysaccharides-Based Hydrogels: Extraction, Synthesis, Characterization, and Applications. Mar. Drugs 2022, 20, 306. [Google Scholar] [CrossRef]
- Abitbol, T.; Johnstone, T.; Quinn, T.M.; Gray, D.G. Reinforcement with cellulose nanocrystals of poly (vinyl alcohol) hydrogels prepared by cyclic freezing and thawing. Soft Matter 2011, 7, 2373–2376. [Google Scholar] [CrossRef]
- Lin, P.; Ma, S.; Wang, X.; Zhou, F. Molecularly Engineered Dual-Crosslinked Hydrogel with Ultrahigh Mechanical Strength, Toughness, and Good Self-Recovery. Adv. Mater. 2015, 27, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Otoni, C.G.; De France, K.J.; Barud, H.S.; Lona, L.M.F.; Cranston, E.D.; Rojas, O.J. Porous Nanocellulose Gels and Foams: Breakthrough Status in the Development of Scaffolds for Tissue Engineering. Mater. Today 2020, 37, 126–141. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Zheng, Z.; Liu, H.; Zhu, L.; Yang, M.; Chen, Y. Nanocellulose-Based Membranes for Highly Efficient Molecular Separation. Chem. Eng. J. 2023, 451, 138711. [Google Scholar] [CrossRef]
- Karim, Z.; Claudpierre, S.; Grahn, M.; Oksman, K.; Mathew, A.P. Nanocellulose Based Functional Membranes for Water Cleaning: Tailoring of Mechanical Properties, Porosity and Metal Ion Capture. J. Membr. Sci. 2016, 514, 418–428. [Google Scholar] [CrossRef]
- Parajuli, S.; Ureña-Benavides, E.E. Fundamental Aspects of Nanocellulose Stabilized Pickering Emulsions and Foams. Adv. Colloid Interface Sci. 2022, 299, 102530. [Google Scholar] [CrossRef]
- Mbakop, S.; Nthunya, L.N.; Onyango, M.S. Recent Advances in the Synthesis of Nanocellulose Functionalized-Hybrid Membranes and Application in Water Quality Improvement. Processes 2021, 9, 611. [Google Scholar] [CrossRef]
- Jin, F.L.; Zhao, M.; Park, M.; Park, S.-J. Recent Trends of Foaming in Polymer Processing: A Review. Polymers 2019, 11, 953. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Li, Y.; Cai, H.; Liu, Q.; Tong, G. Processing nanocellulose foam into high-performance membranes for harvesting energy from nature. Carbohydr. Polym. 2020, 241, 116253. [Google Scholar] [CrossRef]
- Torres-Rendon, J.G.; Femmer, T.; De Laporte, L.; Tigges, T.; Rahimi, K.; Gremse, F.; Zafarnia, S.; Lederle, W.; Ifuku, S.; Wessling, M.; et al. Bioactive Gyroid Scaffolds Formed by Sacrificial Templating of Nanocellulose and Nanochitin Hydrogels as Instructive Platforms for Biomimetic Tissue Engineering. Adv. Mater. 2015, 27, 2989–2995. [Google Scholar] [CrossRef] [Green Version]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose Properties and Applications in Colloids and Interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Wei, B.; Chen, S.; Tian, Q.; Lu, J.; Xu, X. In-Situ Generation and Propagation of Well-Defined Nanocellulose Strengthened Gaseous and Supercritical Carbon Dioxide Foams in Tight Formation Fractures for Conformance Control. SPE Reserv. Eval. Eng. 2020, 23, 1219–1232. [Google Scholar] [CrossRef]
- Solhi, L.; Guccini, V.; Heise, K.; Solala, I.; Niinivaara, E.; Xu, W.; Mihhels, K.; Kröger, M.; Meng, Z.; Wohlert, J.; et al. Understanding Nanocellulose-Water Interactions: Turning a Detriment into an Asset. Chem. Rev. 2023, 123, 1925–2015. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, T.; Park, J.K. Water Holding and Release Properties of Bacterial Cellulose Obtained by in Situ and Ex Situ Modification. Carbohydr. Polym. 2012, 88, 596–603. [Google Scholar] [CrossRef]
- Ansaloni, L.; Salas-Gay, J.; Ligi, S.; Baschetti, M.G. Nanocellulose-based membranes for CO2 capture. J. Membr. Sci. 2017, 522, 216–225. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Huayu, L.; Liu, K.; Du, H.; Kumar, A.; Sharma, G.; Chuanling, S. Recent Advances in Hydrophobic Modification of Nanocellulose. Curr. Org. Chem. 2020, 25, 417–436. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Shen, Z. Nanocellulose Based Filtration Membrane in Industrial Waste Water Treatment: A Review. Materials 2021, 14, 5398. [Google Scholar] [CrossRef]
- Bukowski, B.C.; Keil, F.J.; Ravikovitch, P.I.; Sastre, G.; Snurr, R.Q.; Coppens, M.-O. Connecting Theory and Simulation with Experiment for the Study of Diffusion in Nanoporous Solids. Adsorption 2021, 27, 683–760. [Google Scholar] [CrossRef]
- Kosourov, S.; Murukesan, G.; Seibert, M.; Allahverdiyeva, Y. Evaluation of Light Energy to H2 Energy Conversion Efficiency in Thin Films of Cyanobacteria and Green Alga under Photoautotrophic Conditions. Algal Res. 2017, 28, 253–263. [Google Scholar] [CrossRef]
- Kärkäs, M.D.; Verho, O.; Johnston, E.V.; Åkermark, B. Artificial Photosynthesis: Molecular Systems for Catalytic Water Oxidation. Chem. Rev. 2014, 114, 11863–12001. [Google Scholar] [CrossRef]
- Abas, N.; Kalair, E.; Kalair, A.; Ul Hasan, Q.; Khan, N. Nature Inspired Artificial Photosynthesis Technologies for Hydrogen Production: Barriers and Challenges. Int. J. Hydrogen Energy 2020, 45, 20787–20799. [Google Scholar] [CrossRef]
- Dilamian, M.; Noroozi, B. A combined homogenization-high intensity ultrasonication process for individualizaion of cellulose micro-nano fibers from rice straw. Cellulose 2019, 26, 5831–5849. [Google Scholar] [CrossRef]
- Ashrafi, Z.; Lucia, L.; Krause, W. Bioengineering Tunable Porosity in Bacterial Nanocellulose Matrices. Soft Matter 2019, 15, 9359–9367. [Google Scholar] [CrossRef] [PubMed]
- Dias, O.A.T.; Konar, S.; Leao, A.L.; Yang, W.; Tjong, J.; Sain, M. Current state of applications of nanocellulose in flexible energy and electronic devices. Front. Chem. 2020, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Zhang, L.; Yong, K.T.; Li, J.; Wu, Y. Recent progress in flexible nanocellulosic structures for wearable piezoresistive strain sensors. J. Mater. Chem. 2021, 9, 11001–11029. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, C.; Sun, J.; Zhang, C.; Wei, S.; Tian, W.; Liu, X.; Cheng, W.; Li, X.; Li, X.; et al. A dual-crosslinked self-healing and antibacterial nanocellulose hydrogel for monitoring of human motions. Mater. Des. 2022, 215, 110464. [Google Scholar] [CrossRef]
- Heidarian, P.; Gharaie, S.; Yousefi, H.; Paulino, M.; Kaynak, A.; Varley, R.; Kouzani, A.Z. A 3D printable dynamic nanocellulose/nanochitin self-healing hydrogel and soft strain sensor. Carbohydr. Polym. 2022, 291, 119545. [Google Scholar] [CrossRef]
- Levanic, J.; Gericke, M.; Heinze, T.; Poljanski, I.; Oven, P. Stable nanocellulose gels prepared by crosslinking of surface charged cellulose nanofibrils with di- and triiodoalkanes. Cellulose 2020, 27, 2053–2068. [Google Scholar] [CrossRef]
- Liu, Y.; Schütz, C.; Dalazar-Alvares, G.; Bergström, L. Assembly, Gelation, and Helicoidal Consolidation of Nanocellulose Dispersions. Langmuir 2019, 35, 3600–3606. [Google Scholar] [CrossRef] [Green Version]
- Hossain, L.; Ledesma, R.M.B.; Tanner, J.; Garnier, G. Effect of crosslinking on nanocellulose superabsorbent biodegradability. Carbohydr. Polym. Technol. Appl. 2022, 3, 100199. [Google Scholar] [CrossRef]
- Curvello, R.; Garnier, G. Cationic Cross-Linked Nanocellulose-Based Matrices for the Growth and Recovery of Intestinal Organoids. Biomacromolecules 2021, 11, 701–709. [Google Scholar] [CrossRef]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and Oxygen Barrier Properties of Cellulose Nanomaterial-Based Films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- Evdokimova, O.; Svensson, F.; Agafonov, A.; Håkansson, S.; Seisenbaeva, G.; Kessler, V. Hybrid Drug Delivery Patches Based on Spherical Cellulose Nanocrystals and Colloid Titania—Synthesis and Antibacterial Properties. Nanomaterials 2018, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- Tayeb, A.H.; Tajvedi, M.; Bousfield, D. Enhancing the Oxygen Barrier Properties of Nanocellulose at High Humidity: Numerical and Experimental Assessment. Sustain. Chem. 2020, 1, 198–208. [Google Scholar] [CrossRef]
- Norrahim, M.N.F.; Nurazzi, N.M.; Jenol, M.A.; Farid, M.A.A.; Janudin, N.; Ujang, F.A.; Yasim-Anuar, T.A.; Najmuddin, S.U.; Ilyas, R.A. Emerging development of nanocellulose as an antimicrobial material: An overview. Mater. Adv. 2021, 2, 3538–3551. [Google Scholar] [CrossRef]
- Kim, J.; Agumba, D.O.; Kim, H.C.; Hoa, P.D. Optical Properties of Nanocellulose Film and Its Applications. In Nano-, Bio-, Info-Tech Sensors and Wearable Systems; SPIE: Bellingham, WA, USA, 2021; Volume 11590, p. 115900S. [Google Scholar] [CrossRef]
- Hu, L.; Zheng, G.; Yao, J.; Liu, N.; Weil, B.; Eskilsson, M.; Karabulut, E.; Ruan, Z.; Fan, S.; Bloking, J.T.; et al. Transparent and conductive paper from nanocellulose fibers. Energy Environ. Sci. 2013, 6, 513–518. [Google Scholar] [CrossRef]
- Fang, Z.; Zhu, H.; Bao, W.; Preston, C.; Liu, Z.; Dai, J.; Li, Y.; Hu, L. Highly transparent paper with tunable haze for green electronics. Energy Environ. Sci. 2014, 7, 3313–3319. [Google Scholar] [CrossRef]
- Fang, Z.; Zhu, H.; Yuan, Y.; Ha, D.; Zhu, S.; Preston, C.; Chen, Q.; Li, Y.; Han, X.; Lee, S.; et al. Novel nanostructured paper with ultrahigh transparency and ultrahigh haze for solar cells. Nano Lett. 2014, 14, 765–773. [Google Scholar] [CrossRef]
- Zhu, H.; Parvinian, S.; Preston, C.; Vaaland, O.; Ruan, Z.; Hu, L. Transparent Nanopaper with Tailored Optical Properties. Nanoscale 2013, 5, 3787–3792. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhou, J.; Jiang, L.; Lubineau, G.; Ng, T.; Ooi, B.S.; Liao, H.Y.; Shen, C.; Chen, L.; Zhu, J.Y. Highly transparent, low-haze, hybrid cellulose nanopaper as electrodes for flexible electronics. Nanoscale 2016, 8, 12294–12306. [Google Scholar] [CrossRef]
- Isobe, N.; Kasuga, T.; Nogi, M. Clear transparent cellulose nanopaper prepared from a concentrated dispersion by high-humidity drying. RSV Adv. 2018, 8, 1833–1837. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Jia, C.; Wang, Y.; Fang, Z.; Dai, J.; Xu, L.; Huang, D.; Wu, J.; Li, Y.; Song, J.; et al. Isotropic Paper Directly from Anisotropic Wood: Top-Down Green Transparent Substrate Toward Biodegradable Electronics. ACS Appl. Mater. Interfaces 2018, 10, 28566–28571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Song, F.; Zhang, M.; Chang, H.; Zhang, X.; Li, X.; Zhu, X.; Lü, X.; Wang, Y.; Li, K. Cellulose nanopaper with controllable optical haze and high efficiency ultraviolet blocking for flexible optoelectronics. Cellulose 2019, 26, 2201–2208. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, G.; Cheng, H.; Li, X.; Zhang, Z. Optical Haze Nanopaper Enhanced Ultraviolet Harvesting for Direct Soft-Fluorescent Emission Based on Lanthanide Complex Assembly and Oxidized Cellulose Nanofibrils. ACS Sustain. Chem. Eng. 2019, 7, 9966–9975. [Google Scholar] [CrossRef]
- Miao, M.; Zhao, J.; Feng, X.; Cao, Y.; Cao, S.; Zhao, Y.; Ge, X.; Sun, L.; Shi, L.; Fang, J. Fast fabrication of transparent and multi-luminescent TEMPO-oxidized nanofibrillated cellulose nanopaper functionalized with lanthanide complexes. J. Mater. Chem. C 2015, 3, 2511–2517. [Google Scholar] [CrossRef]
- Kulpinski, P.; Erdman, A.; Namyslak, M.; Fidelus, J.D. Cellulose fibers modified by Eu3+-doped yttria-stabilized zirconia nanoparticles. Cellulose 2012, 19, 1259–1269. [Google Scholar] [CrossRef] [Green Version]
- Tatsumi, M.; Teramoto, Y.; Nishio, Y. Different orientation patterns of cellulose nanocrystal films prepared from aqueous suspensions by shearing under evaporation. Cellulose 2015, 22, 2983–2992. [Google Scholar] [CrossRef]
- Mendoza-Galvan, A.; Tejada-Galan, T.; Dominguez-Gomez, A.B.; Mauricio-Sanchez, R.A.; Järrendahl, K.; Arwin, H. Linear Birefringent Films of Cellulose Nanocrystals Produced by Dip-Coating. Nanomaterials 2019, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Moud, A.A.; Moud, A.A. Cellulose Nanocrystals (CNC) Liquid Crystalline State in Suspension: An Overview. Appl. Biosci. 2022, 13, 244–278. [Google Scholar] [CrossRef]
- Li, S.; Lee, P.S. Development and applications of transparent conductive nanocellulose paper. Sci. Technol. Adv. Mater. 2017, 18, 620–633. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.; He, L.; Xiang, L.; Tang, K.; Li, X.; Qi, J.; Xie, J. Transparent and UV-absorbing nanocellulose films prepared by directly dissolving microwave liquified bamboo in TBAA/DMSO co-solvent system. Ind. Crops Prod. 2021, 171, 113899. [Google Scholar] [CrossRef]
- Schütz, C.; Sort, J.; Bacsik, Z.; Oliynyk, V.; Pellicer, E.; Fall, A.; Wagberg, L.; Berglund, L.; Bergström, L.; Salazar-Alvarez, G. Hard and transparent films formed by nanocellulose-TiO2 nanoparticle hybrids. PLoS ONE 2012, 7, e45828. [Google Scholar] [CrossRef] [Green Version]
- Honorato, C.; Kumar, V.; Liu, J.; Koivula, H.; Xu, C.; Toivakka, M. Transparent nanocellulose-pigment composite films. J. Mater. Sci. 2015, 50, 7343–7352. [Google Scholar] [CrossRef]
- Roy, Z.; Ghosh, B.D.; Goh, K.L.; Muthoka, R.M.; Kim, J. Modulation of interfacial interactions toward strong and tough cellulose nanofiber-based transparent thin films with antifogging feature. Carbohydr. Polym. 2022, 278, 118974. [Google Scholar] [CrossRef]
- Wakabayashi, M.; Fujisawa, S.; Saito, T.; Isogai, A. Nanocellulose Film Properties Tunable by Controlling Degree of Fibrillation of TEMPO-Oxidized Cellulose. Front. Chem. 2020, 8, 37. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2009, 10, 162–165. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.; Guan, F.; Li, G.; Song, Z.; Yu, D.; Wang, H.; Liu, H. Using cellulose fibers to fabricate transparent paper by microfibrillation. Carbohydr. Polym. 2019, 214, 26–33. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Xu, D.; He, Z.; Pan, X.; Gui, S.; Dai, X.; Fan, J.; Dong, X.; Li, Y. Screening of Nanocellulose from Different Biomass Resources and Its Integration for Hydrophobic Transparent Nanopaper. Molecules 2020, 25, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Z.; Zhu, H.; Preston, C.; Han, X.; Li, Y.; Lee, S.; Chai, X.; Chen, G.; Hu, L. Highly transparent and writable wood all-cellulose hybrid nanostructured paper. J. Mater. Sci. C 2013, 1, 6191–6197. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Hokkanen, A.; Kumar, V.; Makela, T.; Harlin, A.; Orelma, H. Thermoresponsive Nanocellulose Films as an Optical Modulation Device: Proof-of-Concept. ACS Appl. Mater. Interfaces 2021, 13, 25346–25356. [Google Scholar] [CrossRef]
- Wu, W.; Tassi, N.G.; Zhu, H.; Fang, Z.; Hu, L. Nanocellulose-based translucent diffuser for optoelectronic device applications with dramatic improvement in light coupling. ACS Appl. Mater. Interfaces 2015, 7, 26860–26864. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Tao, J.; Zou, J.; Zhang, B.; Li, T.; Dai, J.; Zhu, M.; Wang, S.; Fu, K.K.; Henderson, D.; et al. Light management in plastic-paper hybrid substrate towards high-performance optoelectronics. Energy Environ. Sci. 2016, 9, 2278–2285. [Google Scholar] [CrossRef]
- Moriwaki, S.; Hanasaki, I. Swelling-based gelation of wet cellulose nanopaper evaluated by single particle tracking. Sci. Technol. Adv. Mater. 2023, 24, 2153622. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, G.; Liu, Y.; Liu, Y.; Li, B.; Fang, Z. Transparent and hazy all-cellulose composite films with superior mechanical properties. ACS Sustain. Chem. Eng. 2018, 6, 6974–6980. [Google Scholar] [CrossRef]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically transparent nanofiber paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Operamolla, A. Recent Advances on Renewable and Biodegradable Cellulose Nanopaper Substrates for Transparent Light-Harvesting Devices: Interaction with Humid Environment. Int. J. Photoeng. 2019, 2019, 3057929. [Google Scholar] [CrossRef] [Green Version]
- Maier, S.A.; Brongersma, M.L.; Kik, P.G.; Meltzer, S.; Requicha, A.A.G.; Atwater, H.A. Plasmonics-A Route to Nanoscale Optical Devices. Adv. Mater. 2001, 13, 1501–1505. [Google Scholar] [CrossRef]
- Mendes, M.J.; Morawiec, S.; Mateus, T.; Lyubchyk, A.; Aguas, H.; Ferreira, I.; Fortunato, E.; Martin, R.; Priolo, F.; Crupi, I. Broadband light trapping in thin film solar cells with self-organized plasmonic nano-colloids. Nanotechnology 2015, 26, 135202. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Chen, S.; Lu, H.; Chen, K.; Cao, Y.; Miroshnichenko, A.E.; Gu, M.; Li, X. Ultra-Broadband Directional Scattering by Colloidally Lithographed High-Index Mie Resonant Oligomers and Their Energy-Harvesting Application. ACS Appl. Mater. Interfaces 2018, 10, 16776–16782. [Google Scholar] [CrossRef]
- Mendes, M.J.; Morawiec, S.; Simone, F.; Priolo, F.; Crupi, I. Colloidal plasmonic back reflectors for light trapping in solar cells. Nanoscale 2014, 6, 4796–4805. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Sobrado, O.; Mendes, M.J.; Haque, S.; Mateus, T.; Araujo, A.; Aguas, H.; Fortunato, E.; Martins, R. Colloidal-lithographed TiO2 photonic nanostructures for solar cell light trapping. J. Mater. Chem. C 2017, 5, 6852–6861. [Google Scholar] [CrossRef]
- Tarnowicz-Staniak, N.; Vazquez-Diaz, S.; Pavlov, V.; Matczyszyn, K.; Grzelczak, M. Cellulose as an inert scaffold in plasmon-assisted photoregeneration of cofactor molecules. ACS Appl. Mater. Interfaces 2020, 12, 19377–19383. [Google Scholar] [CrossRef]
- Vicente, A.T.; Araujo, A.; Mendes, M.J.; Nunes, D.; Oliveira, M.J.; Sanchez-Sobrado, O.; Ferreira, M.P.; Aguas, H.; Fortunato, E.; Martins, R. Multifunctional cellulose-paper for light harvesting and smart sensing applications. J. Mater. Chem. C 2018, 6, 3143–3181. [Google Scholar] [CrossRef]
- Järvi, S.; Gollan, P.J.; Aro, E.M. Understanding the roles of the thylakoid lumen in photosynthesis regulation. Front. Plant Sci. 2013, 4, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, X.; Kim, E.J.; Lee, J. Sustainability of in vitro light-dependent NADPH generation by the thylakoid membrane of Synechocystis sp. PCC6803. Microbiol. Cell Fact. 2022, 21, 94. [Google Scholar] [CrossRef]
- Koochak, H.; Puthiyaveetil, S.; Mullendore, D.; Li, M.; Kirchhoff, H. The structural and functional domains of plant thylakoid membranes. Plant J. 2019, 97, 412–429. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.S.; Wang, P.; Li, K.; Zhang, Q.B.; He, F.Y.; Li, C.Y.; Su, H.N.; Chen, X.L.; Liu, L.N.; Zhang, Y.Z. Structural basis and evolution of the photosystem I-light-harvesting supercomplex of cryptophyte algae. Plant Cell 2023, koad087. [Google Scholar] [CrossRef]
- Lokstein, H.; Renger, G.; Götze, J.P. Photosynthetic Light-Harvesting (Antenna) Complexes—Structures and Functions. Molecules 2021, 26, 3378. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazar, D.; Guo, Y.; Govindjee, G. Photosynthesis: Basics, history and modelling. Ann. Bot. 2020, 126, 511–537. [Google Scholar] [CrossRef] [Green Version]
- Capretti, A.; Ringsmuth, A.K.; van Velzen, J.F.; Rosnik, A.M.; Croce, R.; Gregorkiewicz, T. Nanophotonics of higher-plant photosynthetic membranes. Light Sci. Appl. 2019, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Kirchhoff, H.; Haase, W.; Haferkamp, S.; Schott, T.; Borinski, M.; Kubitscheck, U.; Rönger, M. Structural and functional self-organization of Photosystem II in grana thylakoids. Biochim. Biophys. Acta Bioenenerg. 2007, 1767, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.P.; Boekema, E.J. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta Bioenerg. 2005, 1706, 12–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.; Liggins, J.R.; Chow, W.S. Entropy and biological systems: Experimentally-investigated entropy-driven stacking of plant photosynthetic membranes. Sci. Rep. 2014, 4, 4142. [Google Scholar] [CrossRef] [Green Version]
- Mullineaux, C.W. Factors Controlling the Mobility of Photosynthetic Proteins. Photochem. Photobiol. 2008, 84, 1310–1316. [Google Scholar] [CrossRef]
- Zsiros, O.; Unnep, R.; Nagy, G.; Almasy, L.; Patai, R.; Szekely, N.K.; Kohlbrecher, J.; Garab, G.; Der, A.; Kovacs, L. Role of Protein-Water Interface in the Stacking Interactions of Granum Thylakoid Membranes—As Revealed by the Effects of Hofmeister Salts. Front. Plant Sci. 2020, 11, 1257. [Google Scholar] [CrossRef]
- Asher, A.; Ganesan, I.; Klasek, L.; Theg, S.M. Isolation of Physiologically Active Thylakoids and Their Use in Energy-Dependent Protein Transport Assays. J. Vis. Exp. 2018, 139, 58393. [Google Scholar]
- Nelson, N.; Ben-Shem, A. The complex architecture of oxygenic photosynthesis. Nat. Rev. Mol. Cell Biol. 2005, 5, 971–982. [Google Scholar] [CrossRef]
- Hornyak, M.; Dziurka, M.; Kula-Maximenko, M.; Pastuszak, J.; Szczerba, A.; Szklarczyk, M.; Plazek, A. Photosynthetic efficiency, growth and secondary metabolism of common buckwheat (Fagopyrum esculentum Moench) in different controlled-environment production systems. Sci. Rep. 2022, 12, 257. [Google Scholar] [CrossRef]
- Zhu, X.G.; Long, S.P.; Ort, D.R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass. Curr. Opin. Biotechnol. 2008, 19, 153–159. [Google Scholar] [CrossRef]
- Garab, G. Hierarchical organization and structural flexibility of thylakoid membranes. Biochim. Boiphys. Acta Bioenerg. 2014, 1837, 481–494. [Google Scholar] [CrossRef] [Green Version]
- Gannt, E. Pigment protein complexes and the concept of the photosynthetic unit: Chlorophyll complexes and phycobilisomes. Photosynth. Res. 1996, 48, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, Z.; Huang, X. Whole-Cell-Based Photosynthetic Biohybrid Systems for Energy and Environmental Applications. ChemPlusChem 2021, 86, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garrido, I. Microalgae immobilization: Current techniques and uses. Bioresour. Technol. 2008, 99, 3949–3964. [Google Scholar] [CrossRef]
- Gisriel, C.J.; Azai, C.; Cardona, T. Recent advances in the structural diversity of reaction centers. Photosynth. Res. 2021, 149, 329–343. [Google Scholar] [CrossRef]
- Rooke, J.C.; Meunier, C.; Leonard, A.; Su, B.L. Energy from photobioreactors: Bioencapsulation of photosynthetically active molecules, organelles, and whole cells within biologically inert matrices. Pure Appl. Chem. 2008, 80, 2345–2376. [Google Scholar] [CrossRef]
- Calzaferri, G.; Huber, S.; Maas, H.; Minkoski, C. Host–Guest Antenna Materials. Angew. Chem. Int. Ed. 2003, 42, 3732–3758. [Google Scholar] [CrossRef]
- Galazzo, J.L.; Bailey, J.E. Growing Saccharomyces cerevisiae in calcium-alginate beads induces cell alterations which accelerate glucose conversion to ethanol. Biotechnol. Bioeng. 1990, 36, 417–426. [Google Scholar] [CrossRef]
- Chen, Y.E.; Yuan, S.; Schröder, W.P. Comparison of methods for extracting thylakoid membranes of Arabidopsis plants. Physiol. Plant. 2016, 156, 3–12. [Google Scholar] [CrossRef]
- Yokthongwattana, K.; Savchenko, T.; Polle, J.E.; Melis, A. Isolation and characterization of a xanthophyll-rich fraction from the thylakoid membrane of Dunaliella salina (green algae). Photochem. Photobiol. Sci. 2005, 45, 1028–1034. [Google Scholar] [CrossRef]
- Komenda, J.; Krynicka, V.; Zakar, T. Isolation of Thylakoid Membranes from the Cyanobacterium Synechocystis sp. PCC 6803 and Analysis of Their Photosynthetic Pigment-protein Complexes by Clear Native-PAGE. Bio Protoc. 2019, 5, e3126. [Google Scholar] [CrossRef]
- Ciesielski, P.N.; Scott, A.M.; Faulkner, C.J.; Berron, B.J.; Cliffel, D.E.; Jennings, G.K. Functionalized nanoporous gold leaf electrode films for the immobilization ofphotosystem I. ACS Nano 2008, 2, 2465–2472. [Google Scholar] [CrossRef]
- Calkins, J.O.; Umasankar, Y.; O’Neill, H.; Ramasamy, R.P. High photo-electrochemical activity of thylakoid-carbon nanotube composites for photosynthetic energy conversion. Energy Environ. Sci. 2013, 6, 1891–1900. [Google Scholar] [CrossRef]
- Ventrella, A.; Catucci, L.; Placido, T.; Longobardi, F.; Agostiano, A. Biomaterials based on photosynthetic membranes as potential sensors for herbicides. Biosens. Bioelectron. 2011, 26, 4747–4752. [Google Scholar] [CrossRef] [PubMed]
- Piletskaya, E.V.; Piletsky, S.A.; Sergeyeva, T.A.; El’skaya, A.V.; Sozinov, A.A.; Marty, J.L.; Rouillon, R. Thylakoid membranes-based test-system for detecting of trace quantities of the photosynthesis-inhibiting herbicides in drinking water. Anal. Chim. Acta 1999, 391, 1–7. [Google Scholar] [CrossRef]
- Lim, S.H.; La, S.W.; Hoang, T.T.H.; Le, Q.T.; Jang, S.; Choo, J.; Vasseghian, Y.; Son, S.J.; Joo, S.W. Carbon capture and biocatalytic oxygen production of photosystem II from thylakoids and microalgae on nanobiomaterials. Bioresour. Technol. 2023, 368, 128279. [Google Scholar]
- Iwuchukwu, I.J.; Vaughn, M.; Myers, N.; O’Neill, H.; Frymier, P.; Bruce, B.D. Self-organized photosynthetic nanoparticle for cell-free hydrogenproduction. Nat. Nanotechnol. 2010, 5, 73–79. [Google Scholar] [CrossRef]
- Ham, M.H.; Choi, J.H.; Boghossian, A.A.; Jeng, E.S.; Graff, R.A.; Heller, D.A.; Chang, A.C.; Mattis, A.; Bayburt, T.H.; Grinkova, Y.V.; et al. Photoelectro-chemical complexes for solar energy conversion that chemically and autonomously regenerate. Nat. Chem. 2010, 2, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Meunier, C.F.; Van Cutsem, P.; Kwon, Y.U.; Su, B.L. Thylakoids entrapped within porous silica gel: Towards living matter able to convert energy. J. Mater. Chem. 2009, 19, 1535–1542. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, J.; Wang, Z.; Wang, H.; Zhang, S.; Wu, Y.; Wang, H.; Li, S.; Jiang, Z. Thylakoid Membrane-Inspired Capsules with Fortified Cofactor Shuttling for Enzyme-Photocoupled Catalysis. J. Am. Chem. Soc. 2022, 144, 4168–4177. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, Y.; Lv, F.; Liu, L.; Gu, Q.; Wang, S. Biomimetic 4D-printed breathing hydrogel actuators by nanothylakoid and thermoresponsive polymer networks. Adv. Healthc. Mater. 2021, 31, 2105544. [Google Scholar] [CrossRef]
- Amao, Y.; Fujimura, M.; Miyazaki, M.; Tadokoro, A.; Nakamura, M.; Shuto, N. A visible light driven electrochemical unctio cell with a function of CO2 conversion to formic acid coupled thylakoid from microalgae and biocatalyst immobilized electrodes. New J. Chem. 2018, 42, 9269–9280. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, L.; Zhang, P.; Lv, F.; Liu, L.; Qi, R.; Wang, Y.; Shen, M.Y.; Yu, H.H.; Bazan, G.; et al. Conducting polymers-thylakoid hybrid materials for water oxidation and photoelectric conversion. Adv. Electr. Mater. 2019, 5, 1800789. [Google Scholar] [CrossRef]
- Mallick, N. Biotechnological potential of immobilized algae for wastewater N, P and metal removal: A review. Biometals 2002, 15, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Nakanishi, T.; Michinobu, T. Immobilization of biomaterials tonano-assembled films (self-assembled monolayers, Langmuir-Blodgett films, and layer-by-layer assemblies) and their related functions. J. Nanosci. Nanotechnol. 2006, 6, 2278–2301. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, N.G.; Stuart, M.A.C.; Fleer, G.J.; Bohmer, M.R. Formation and stability of multilayers of polyelectrolytes. Langmuir 1996, 12, 3675–3681. [Google Scholar] [CrossRef]
- Dementiev, A.A.; Baikov, A.A.; Ptushenko, V.V.; Khomutov, G.B.; Tikhonov, A.N. Biological and polymeric self-assembled hybrid systems: Structure and properties of thylakoid/polyelectrolyte complexes. Biochim. Biophys. Acta Biomembr. 2005, 1712, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Abe, K.; Ishii, A.; Hirano, M.; Rusling, J.F. Photoactivity characteristics of abiodevice using primary photosynthetic reaction centers. Electroanalysis 2005, 17, 2266–2272. [Google Scholar] [CrossRef]
- Zhao, J.Q.; Liu, B.H.; Zou, Y.L.; Xu, C.H.; Kong, J.L. Photoelectric conversion of photosynthetic reaction center in multilayered films fabricated bylayer-by-layer assembly. Electrochim. Acta 2002, 47, 2013–2017. [Google Scholar] [CrossRef]
- Krywko-Cendrowska, A.; di Leone, S.; Bina, M.; Yorulmaz-Avsar, S.; Palivan, C.G.; Meier, W. Recent Advances in Hybrid Biomimetic Polymer-Based Films: From Assembly to Applications. Polymers 2020, 12, 1003. [Google Scholar] [CrossRef]
- Shon, Y.; Kim, H.; Hwang, H.S.; Bae, E.S.; Eom, T.; Park, A.J.; Ahn, W.S.; Wie, J.J.; Shim, B.S. A nanostructured cell-free photosynthetic biocomposite via molecularly controlled layer-by-layer assembly. Sens. Acta B Chem. 2017, 244, 1–10. [Google Scholar] [CrossRef]
- Ng, A.Y.R.; Boruah, B.; Chin, K.F.; Modak, J.M.; Soo, H.S. Photoelectrochemical cells for artificial photosynthesis: Alternatives to water oxidation. ChemNanpoMat 2019, 6, 185–203. [Google Scholar]
- Chai, Y.D.; Pang, Y.L.; Lim, S.; Chong, W.C.; Lai, C.W.; Abdullah, A.Z. Recent Progress on Tailoring the Biomass-Derived Cellulose Hybrid Composite Photocatalysts. Polymers 2022, 14, 5244. [Google Scholar] [CrossRef]
- Wang, X.; Saba, T.; Yiu, H.H.; Howe, R.F.; Anderson, J.A.; Shi, J. Cofactor NAD(P)H Regeneration Inspired by Heterogeneous Pathways. Chem 2017, 2, 621–654. [Google Scholar] [CrossRef] [Green Version]
- Ke, D.; Liu, S.; Dai, K.; Zhou, J.; Zhang, L.; Peng, T. CdS/Regenerated Cellulose Nanocomposite Films for Highly Efficient Photocatalytic H2 Production under Visible Light Irradiation. J. Phys. Chem. C 2009, 113, 16021–16026. [Google Scholar] [CrossRef]
- Tang, A.; Liu, Y.; Wang, Q.; Chen, R.; Liu, W.; Fang, Z.; Wang, L. A new photoelectric ink based on nanocellulose/CdS quantum dots for screen-printing. Carbohydr. Polym. 2016, 148, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Li, J.; Yao, W. CdS@MoS2 Hetero-structured Nanocomposites Are Highly Effective Photo-Catalysts for Organic Dye Degradation. ACS Omega 2020, 5, 27463–27469. [Google Scholar] [CrossRef]
- Yue, Y.; Shen, S.; Cheng, W.; Han, G.; Wu, Q.; Jiang, J. Construction of mechanically robust and recyclable photocatalytic hydrogel based on nanocellulose-supported CdS/MoS2/MMT hybrid for antibiotic degradation. Coll. Surf. A 2022, 636, 128035. [Google Scholar] [CrossRef]
- Długosz, O.; Wąsowicz, N.; Szostak, K.; Banach, M. Photocatalytic properties of coating materials enriched with bentonite/ZnO/CuO nanocomposite. Mater. Chem. Phys. 2021, 260, 124150. [Google Scholar] [CrossRef]
- Wang, S.Q.; Li, J.M.; Liu, W.B. Effect of F, V and Mn co-doping on the catalytic performance of TiO2-pillared bentonite in the photocatalytic denitration. J. Fuel Chem. Technol. 2020, 48, 1131–1139. [Google Scholar] [CrossRef]
- Boukhatem, H.; Djouadi, L.; Abdelaziz, N.; Khalaf, H. Synthesis, characterization and photocatalytic activity of CdS–montmorillonite nanocomposites. Appl. Clay Sci. 2013, 72, 44–48. [Google Scholar] [CrossRef]
- Cui, J.; Wang, G.; Liu, W.; Ke, P.; Tian, Q.; Li, X.; Tian, Y. Synthesis BiVO4 modified by CuO supported onto bentonite for molecular oxygen photocatalytic oxidative desulfurization of fuel under visible light. Fuel 2021, 290, 120066. [Google Scholar] [CrossRef]
- Farooq, A.; Patoary, M.K.; Zhang, M.; Mussana, H.; Li, M.; Naeem, M.A.; Mushtaq, M.; Farooq, A.; Liu, L. Cellulose from sources to nanocellulose and an overview of synthesis and properties of nanocellulose/zinc oxide nanocomposite materials. Int. J. Biol. Macromol. 2020, 154, 1050–1073. [Google Scholar] [CrossRef]
- Reddy, K.R.; Hassan, M.; Gomes, V.G. Hybrid nanostructures based on titanium dioxide for enhanced photocatalysis. Appl. Catal. A Gen. 2015, 489, 1–16. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Basca, R.R.; Benyounes, A.; Axet, R.; Serp, P.; Silva, C.G.; Simva, A.M.T.; Faria, J.L. Synergistic effect between carbon nanomaterials and ZnO for photocatalytic water decontamination. J. Catal. 2015, 331, 172–180. [Google Scholar] [CrossRef]
- Lafatshe, K.; Muiva, C.M.; Kebaabetswe, L.P. Extraction of nanocellulose and in-situ casting of ZnO/cellulose nanocomposite with enhanved photocatalytic and antibacterial activity. Carbohydr. Polym. 2017, 164, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Toro, R.G.; Adel, A.M.; de Caro, T.; Brunetti, B.; Al-Shemy, M.T.; Caschera, D. A Facile One-Pot Approach to the Fabrication of Nanocellulose–Titanium Dioxide Nanocomposites with Promising Photocatalytic and Antimicrobial Activity. Materials 2022, 15, 5789. [Google Scholar] [CrossRef]
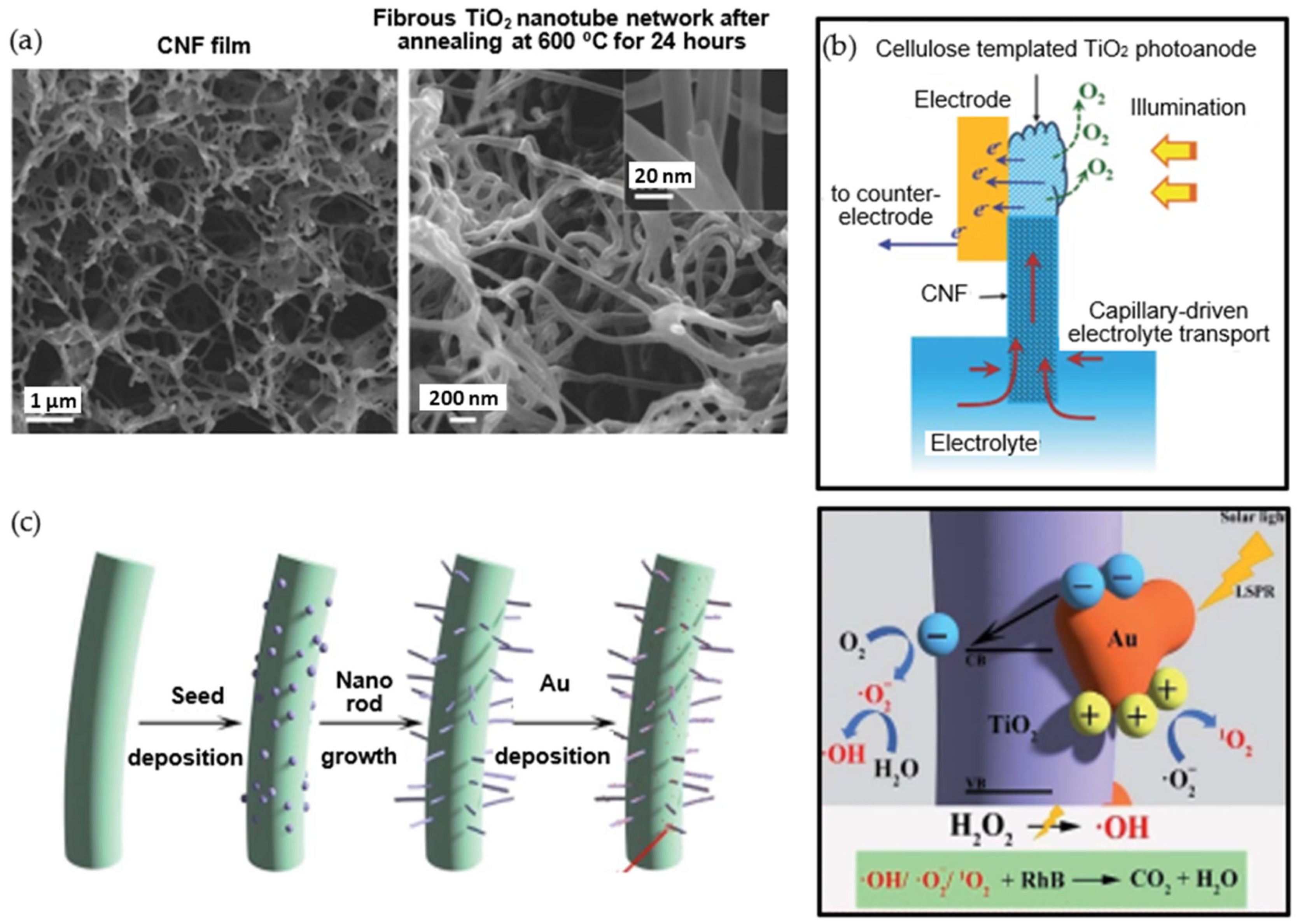
- Li, Z.; Yao, C.; Yu, Y.; Cai, Z.; Wang, X. Highly Efficient Capillary Photoelectrochemical Water Splitting Using Cellulose Nanofiber-Templated TiO2 Photoanodes. Adv. Mater. 2014, 26, 2262–2267. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhan, C.; Kong, F.; Li, W.; Yang, C.; Hsiao, B.S. Facile synthesis of TiO2/CNC nanocomposites for enhanced Cr(VI) photoreduction: Synergistic roles of cellulose nanocrystals. Carbohydr. Polym. 2020, 233, 115838. [Google Scholar] [CrossRef]
- Li, Z.; Yao, C.; Wang, F.; Cai, Z.; Wang, X. Cellulose nanofiber-templated three-dimension TiO2 hierarchical nanowire network for photoelectrochemical photoanode. Nanotechnology 2014, 25, 504005. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.S.; Chen, J.; Slabon, A.; Mathew, A.P. Converting cellulose nanocrystals into photocatalysts by functionalization with titanium dioxide nanorods and gold nanocrystals. RSC Adv. 2020, 10, 37374–37381. [Google Scholar] [CrossRef]
- Guo, Z.; Fu, S. Preparation of gold nanoparticles on nanocellulose and their catalytic activity for reduction of NO2−. BioResources 2019, 14, 3057–3068. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, M.; Liu, H.; Shang, S.; Wang, S.; Liimatainen, H. Facile synthesis of palladium and gold nanoparticles by using dialdehyde nanocellulose as template and reducing agent. Carbohydr. Polym. 2018, 186, 132–139. [Google Scholar] [CrossRef]
- Tavker, N.; Sharma, M. Enhanced photocatalytic activity of nanocellulose supported zinc oxide composite for RhB dye as well as ciprofloxacin drug under sunlight/visible light. AIP Conf. Proc. 2018, 1961, 030013. [Google Scholar]
- Zhou, Y.; Ding, E.; Li, W. Synthesis of TiO2 nanocubes induced by cellulose nanocrystal (CNC) at low temperature. Mater. Lett. 2007, 61, 5050–5052. [Google Scholar] [CrossRef]
- Carrasco, S.; Epinosa, E.; Gonzalez, Z.; Cruz-Yusta, M.; Sanchez, L.; Rodrigues, A. Simple Route to Prepare Composite Nanocellulose Aerogels: A Case of Photocatalytic De-Nox Materials Application. ACS Sustain. Chem. Eng. 2023, 11, 2354–2363. [Google Scholar] [CrossRef]
- Evdokimova, O.L.; Belousova, M.E.; Evdokimova, A.V.; Kusova, T.V.; Baranchikov, A.E.; Antonets, K.S.; Nizhnikov, A.A.; Agafonov, A.V. Fast and simple approach for production of antibacterial nanocellulose/cuprous oxide hybrid films. Cellulose 2021, 28, 2931–2945. [Google Scholar] [CrossRef]
- Zheng, A.; Sabidi, S.; Ohno, T.; Maeda, T.; Andou, Y. Cu2O/TiO2 decorated on cellulose nanofiber/reduced graphene hydrogel for enhanced photocatalytic activity and its antibacterial applications. Chemosphere 2022, 286, 131731. [Google Scholar] [CrossRef]
- Luo, Y.; Xing, L.; Hu, C.; Zhang, W.; Lin, X.; Gu, J. Facile synthesis of nanocellulose-based Cu2O/Ag heterostructure as a surface-enhanced Raman scattering substrate for trace dye detection. Int. J. Biol. Macromol. 2022, 205, 366–375. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, L.; Ma, J.; Alam, N.; Zhou, X.; Ni, Y. Nano-Cellulose/MOF Derived Carbon Doped CuO/Fe3O4 Nanocomposite as High Efficient Catalyst for Organic Pollutant Remedy. Nanomaterials 2019, 9, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farahani, M.S.V.; Nikzad, M.; Ghorbani, M. Fabrication of Fe-doped ZnO/nanocellulose nanocomposite as an efficient photocatalyst for degradation of methylene blue under visible light. Cellulose 2022, 29, 7277–7299. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Lu, H.; Ma, J.; Zhou, X.; Wang, Z.; Yi, C. Macro-/nanoporous Al-doped ZnO/cellulose composites based on tunable cellulose fiber sizes for enhancing photocatalytic properties. Carbohydr. Polym. 2020, 250, 116873. [Google Scholar] [CrossRef] [PubMed]
- Pathania, D.; Kumari, M.; Gupta, V.K. Fabrication of ZnS–cellulose nanocomposite for drug delivery, antibacterial and photocatalytic activity. Mater. Des. 2015, 87, 1056–1064. [Google Scholar] [CrossRef]
- Thi, H.N.; Thi, N.L.; Van, T.V.; Xuam, N.P. Anchoring Ag3PO4 nanoparticles on MIL-101(Fe)@nanocellulose composite for tetracycline degradation. Viet. J. Catl. Adsorp. 2021, 10, 125–136. [Google Scholar]
- Shan, Y.; Guo, Y.; Wang, Y.; Du, X.; Yu, J.; Juo, H.; Wu, H.; Boury, B.; Xiao, H.; Huang, L.; et al. Nanocellulose-derived carbon/g-C3N4 heterojunction with a hybrid electron transfer pathway for highly photocatalytic hydrogen peroxide production. J. Colloid Interf. Sci. 2021, 599, 507–518. [Google Scholar] [CrossRef]
- Li, X.B.; Tung, C.H.; Wu, L.Z. Semiconducting quantum dots for artificial photosynthesis. Nat. Rev. Chem. 2018, 2, 160–173. [Google Scholar] [CrossRef]
- Xiong, R.; Yu, S.; Smith, M.J.; Zhou, J.; Krecker, M.; Zhang, L.; Nepal, D.; Bunning, T.J.; Tsukruk, V.V. Self-Assembly of Emissive Nanocellulose/Quantum Dot Nanostructures for Chiral Fluorescent Materials. ACS Nano 2019, 18, 9074–9081. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Y.; Ti, P.; Su, G.; Yuan, Q. Graphene oxide quantum dots attached on wood-derived nanocellulose to fabricate a highly sensitive humidity sensor. Carbohydr. Polym. 2022, 288, 119312. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Gao, S.; Niu, Y.; Liu, H.; Mei, C.; Cai, J.; Xu, C. Preparation and Properties of Cyanobacteria-Based Carbon Quantum Dots/Polyvinyl Alcohol/Nanocellulose Composite. Polymers 2020, 12, 1143. [Google Scholar] [CrossRef]
- Afsharipour, R.; Shabani, A.M.H.; Dadfarnia, S. A selective off–on fluorescent aptasensor for alpha-fetoprotein determination based on N-carbon quantum dots and oxidized nanocellulose. J. Photochem. Photobiol. A 2022, 428, 113872. [Google Scholar] [CrossRef]
- Guo, X.; Xu, D.; Yuan, H.; Luo, Q.; Tang, S.; Liu, L.; Wu, Y. A novel fluorescent nanocellulosic hydrogel based on carbon dots for efficient adsorption and sensitive sensing in heavy metals. J. Mater. Chem. A 2019, 7, 27081–27088. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, W. Metal–organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef]
- Gibbons, B.; Cai, M.; Morrios, A.J. A Potential Roadmap to Integrated Metal Organic Framework Artificial Photosynthetic Arrays. J. Am. Chem. Soc. 2022, 144, 17723–17736. [Google Scholar] [CrossRef]
- Lan, G.; Fan, Y.; Shi, W.; You, E.; Veroneau, S.S.; Lin, W. Biomimetic active sites on monolayered metal–organic frameworks for artificial photosynthesis. Nat. Catal. 2022, 5, 1006–1018. [Google Scholar] [CrossRef]
- Seo, J.Y.; Song, Y.; Lee, J.H.; Kim, H.; Cho, S.; Baek, K.Y. Robust Nanocellulose/Metal–Organic Framework Aerogel Composites: Superior Performance for Static and Continuous Disposal of Chemical Warfare Agent Simulants. ACS Appl. Mater. Interfaces 2021, 13, 33516–33523. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, X.; Zhang, G.L.; Hao, H.; Zhu, B.W.; Hou, H.M.; Bi, J. Hierarchical Porous Nanocellulose Aerogels Loaded with Metal–Organic Framework Particles for the Adsorption Application of Heterocyclic Aromatic Amines. ACS Appl. Mater. Interfacace 2022, 14, 29131–29143. [Google Scholar] [CrossRef]
- Mai, T.; Li, D.D.; Ma, M.G. Collaboration of two-star nanomaterials: The applications of nanocellulose-based metal organic frameworks composites. Carbohydr. Polym. 2023, 302, 120359. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, E.; Xie, Y.; Liu, D.; Hu, Y.; Shi, Z.; Xiong, C.; Yang, Q. Hydrangea-like nanocellulose microspheres with high dye adsorption and drug encapsulation prepared by emulsion method. Carbohydr. Polym. 2022, 296, 119947. [Google Scholar] [CrossRef]
- Ruiz-Palmero, C.; Soriano, M.L.; Valcarcel, M. Gels based on nanocellulose with photosensitive ruthenium bipyridine moieties as sensors for silver nanoparticles in real samples. Sens. Actuators 2016, 299, 31–37. [Google Scholar] [CrossRef]
- Fujisawa, S.; Togawa, E.; Kuroda, K. Nanocellulose-stabilized Pickering emulsions and their applications. Sci. Technol. Adv. Mater. 2017, 18, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Tanpichai, S.; Biswas, S.K.; Withayakran, S.; Yano, H. Optically Transparent Nanocellulose-Reinforced Composites via Pickering Emulsification. Compos. A 2020, 132, 105811. [Google Scholar] [CrossRef]
- Svagan, A.J.; Busko, D.; Avlasevich, Y.; Glasser, G.; Baluschev, S.; Landfester, K. Photon energy upconversion nanopaper: A bioinspired oxygen protection strategy. ACS Nano 2014, 8, 8198–8207. [Google Scholar] [CrossRef] [PubMed]
- Bencurova, E.; Shityakov, S.; Schaack, D.; Kaltdor, M.; Sarukhanyan, E.; Hilgarth, A.; Rath, C.; Montenegro, S.; Roth, G.; Lopes, D.; et al. Nanocellulose Composites as Smart Devices with Chassis, Light-Directed DNA Storage, Engineered Electronic Properties, and Chip Integration. Front. Bioeng. Biotechnol. 2022, 10, 869111. [Google Scholar] [CrossRef] [PubMed]
- Singh-Rachford, T.N.; Castellano, F.N. Photon upconversion based on sensitized triplet–triplet annihilation. Coord. Chem. Rev. 2010, 254, 2560–2573. [Google Scholar] [CrossRef]
- Mabrouk, A.B.; Salon, M.C.B.; Magnin, A.; Belgacem, M.N.; Boufi, S. Cellulose-based nanocomposites prepared via mini-emulsion polymerization: Understanding the chemistry of the nanocellulose/matrix interface. Colloids Surf. A 2014, 448, 1–8. [Google Scholar] [CrossRef]
- Paulraj, T.; Wennmalm, S.; Wieland, D.C.F.; Riazanova, A.V.; Dėdinaitė, A.; Pomorski, T.G.; Cárdenas, M.; Svagan, A.J. Primary cell wall inspired micro containers as a step towards a synthetic plant cell. Nat. Commun. 2020, 11, 958. [Google Scholar] [CrossRef] [Green Version]
- Casanova, F.; Pereira, C.F.; Ribeiro, A.B.; Freixa, R.; Costa, E.; Pintado, M.E.; Fernandes, J.C.; Ramos, O.L. Novel Micro-and Nanocellulose-Based Delivery Systems for Liposoluble Compounds. Nanomaterials 2021, 11, 2593. [Google Scholar] [CrossRef]
- Paulraj, T.; Wennmalm, S.; Wieland, D.C.F.; Dėdinaitė, A.; Pomorski, T.G.; Cardenas, M.; Svagan, A.J. Assembly of Primary Cell-Wall inspired Micro-containers, Plantosomes, as a step towards a Synthetic Plant-Cell. Ph.D. Thesis, KTH, Stockholm, Sweden, 2019. [Google Scholar]
- Lombardo, S.; Villares, A. Engineered Multilayer Microcapsules Based on Polysaccharides Nanomaterials. Molecules 2020, 25, 4420. [Google Scholar] [CrossRef]
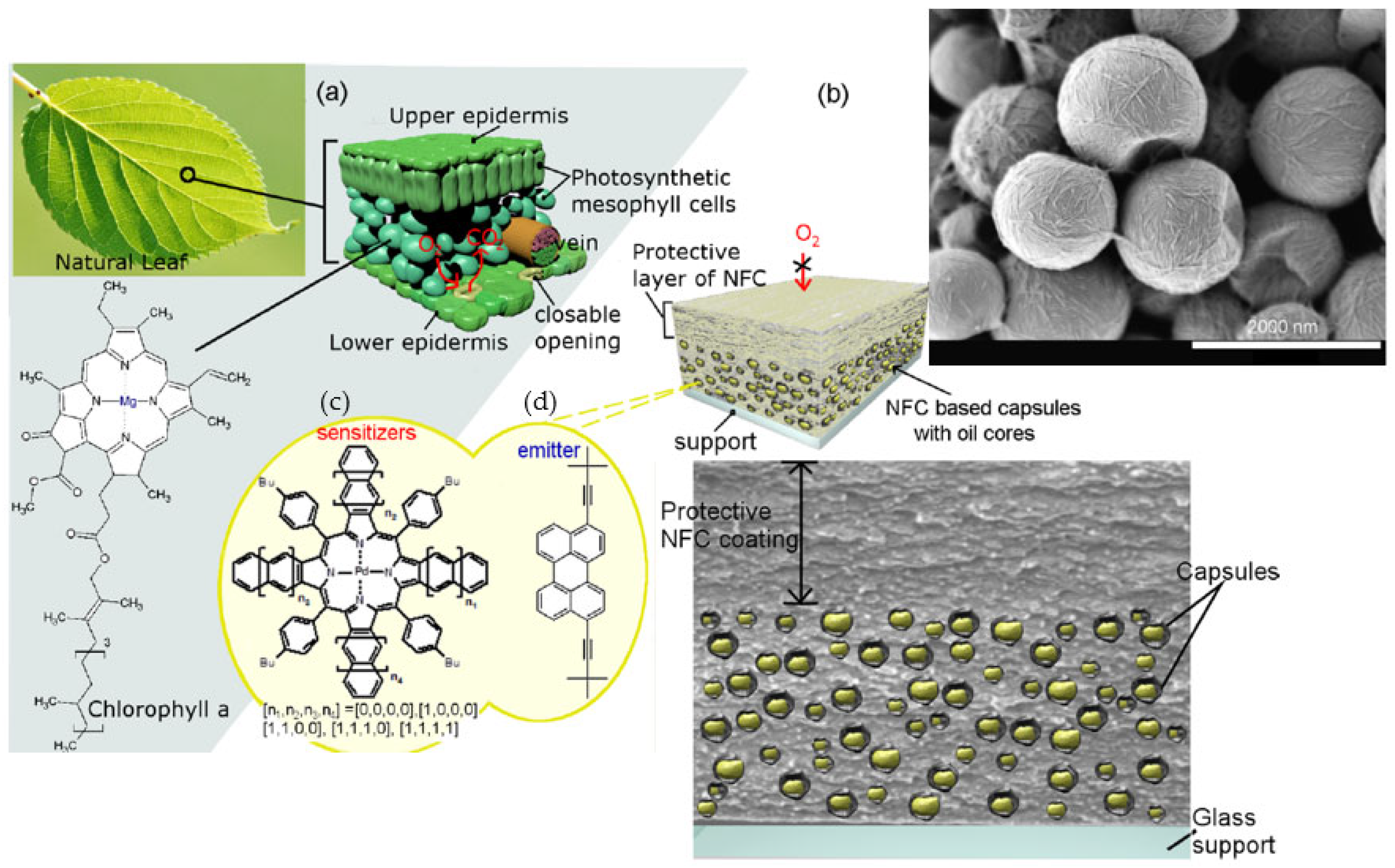
- Svagan, A.J.; Koch, C.B.; Hedenqvist, M.S.; Nilsson, F.; Glasser, G.; Baluschev, S.; Andersen, M.L. Liquid-core nanocellulose-shell capsules with tunable oxygen permeability. Carbohydr. Polym. 2016, 136, 292–299. [Google Scholar] [CrossRef]
- Buschhaus, C.; Jetter, R. Composition differences between epicuticular and intracuticular wax substructures: How do plants seal their epidermal surfaces? J. Exp. Bot. 2011, 62, 841–853. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. High performance green barriers based on nanocellulose. Sustain. Chem. Proc. 2014, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Paulraj, T.; Riazanova, A.V.; Yao, K.; Andersson, R.L.; Müllertz, A.; Svagan, A.J. Bioinspired Layer-by-Layer Microcapsules Based on Cellulose Nanofibers with Switchable Permeability. Biomacromolecules 2017, 18, 1401–1410. [Google Scholar] [CrossRef]
- Paulraj, T.; Riazanova, A.V.; Svagan, A.J. Bioinspired capsules based on nanocellulose, xyloglucan and pectin—The influence of capsule wall composition on permeability properties. Acta Biomater. 2018, 69, 196–205. [Google Scholar] [CrossRef]
- Kak, A.; Parhi, A.; Rasco, B.A.; Tang, J.; Sablani, S.S. Improving the Oxygen Barrier of Microcapsules using Cellulose Nanofibers. Int. J. Food Sci. Technol. 2021, 56, 4258–4267. [Google Scholar] [CrossRef]
- Oppelt, K.T.; Wöß, E.; Stiftinger, M.; Schöfberger, W.; Buchberger, W.; Knör, G. Photocatalytic reduction of artificial and natural nucleotide co-factors with a chlorophyll-like tin-dihydroporphyrin sensitizer. Inorg. Chem. 2013, 52, 11910–11922. [Google Scholar] [CrossRef]
- Alvarado, D.R.; Argyropoulos, D.S.; Scholle, F.; Peddinti, B.S.; Ghiladi, R.A. A facile strategy for photoactive nanocellulose-based antimicrobial materials. Green Chem. 2019, 21, 3424–3435. [Google Scholar] [CrossRef]
- Nam, D.H.; Park, C.B. Visible light driven NADH regeneration sensitized by proflavine for biocatalysis. ChemBioChem 2012, 13, 1278–1282. [Google Scholar] [CrossRef]
- Spagnul, C.; Turner, L.C.; Boyle, R.W. Immobilized photosensitizers for antimicrobial applications. J. Photochem. Photobiol. B 2015, 150, 11–30. [Google Scholar] [CrossRef]
- Monteiro, C.J.; Neves, M.G.P.; Nativi, C.; Almeida, A.; Faustino, M.A. Porphyrin Photosensitizers Grafted in Cellulose Supports: A Review. Int. J. Mol. Sci. 2023, 24, 3475. [Google Scholar] [CrossRef]
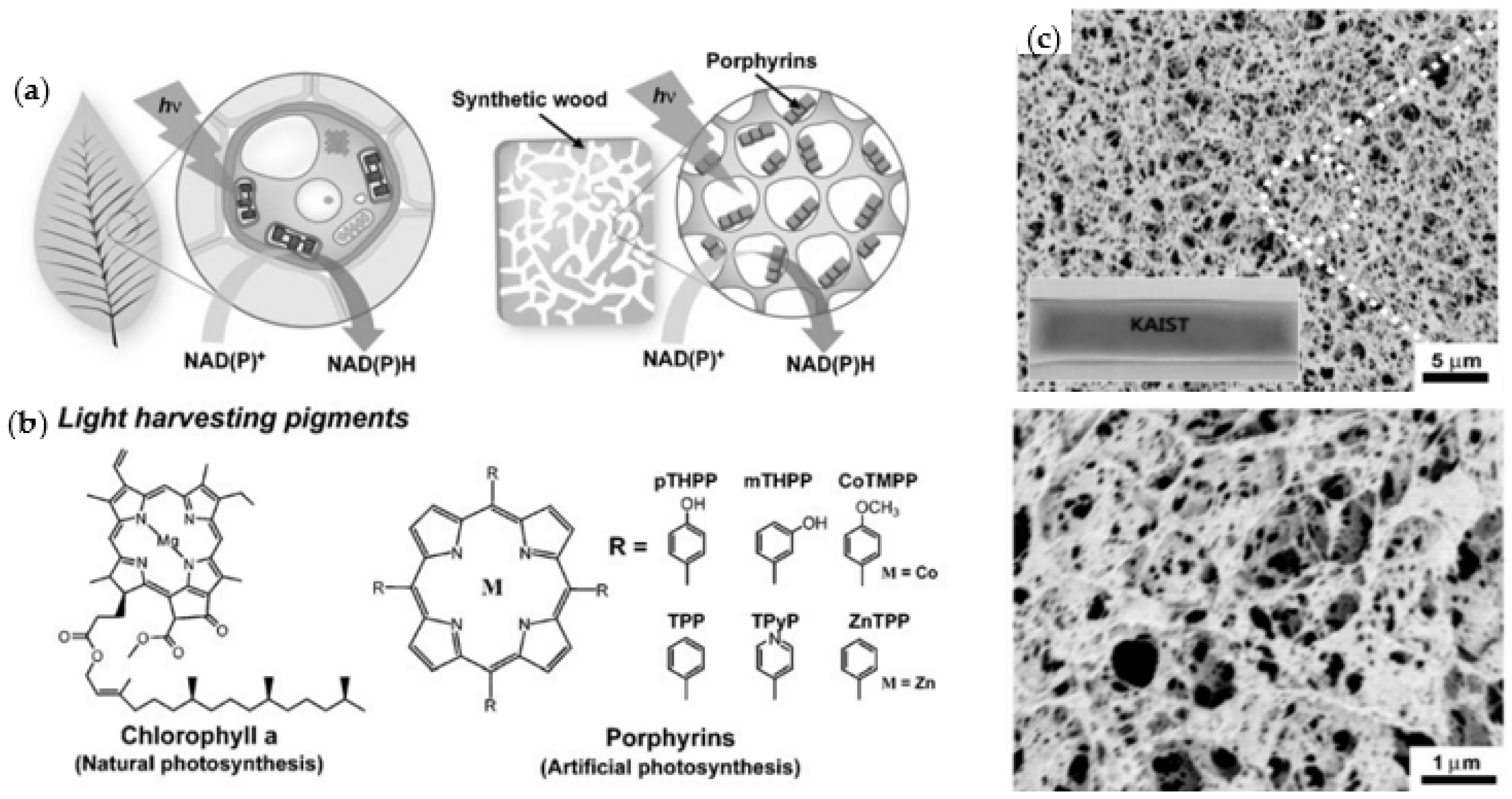
- Lee, M.; Kim, J.H.; Lee, S.H.; Lee, S.H.; Park, C.B. Biomimetic artificial photosynthesis by light-harvesting synthetic wood. ChemSusChem 2011, 4, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Kumar, V.; Baker, S.N.; Baker, G.A.; Pandey, S. J-aggregation of ionic liquid solutions of meso-tetrakis (4-sulfonatophenyl)porphyrin. Phys. Chem. Chem. Phys. 2010, 12, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, M.; Lee, J.S.; Park, C.B. Self-assembled light-harvesting peptide nanotubes for mimicking natural photosynthesis. Angew. Chem. Int. Ed. 2012, 51, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Hou, Q.; He, Z.; Liu, Z.; Ni, Y. Cellulose nanocrystals (CNC) as carriers for a spirooxazine dye and its effect on photochromic efficiency. Carbohydr. Polym. 2014, 111, 419–424. [Google Scholar] [CrossRef]
- Sun, B.; He, Z.; Hou, Q.; Liu, Z.; Cha, R.; Ni, Y. Interaction of a spirooxazine dye with latex and its photochromic efficiency on cellulosic paper. Carbohydr. Polym. 2013, 95, 598–605. [Google Scholar] [CrossRef]
- Filipponen, I.; Sadeghifar, H.; Argyrpoulos, D.S. Photoresponsive Cellulose Nanocrystals. Nanomater. Nanotechnol. 2011, 1, 34–43. [Google Scholar] [CrossRef]
- Wang, E.Z.; Wang, Y.; Xiao, D. Polymer Nanocomposites for Photocatalytic Degradation and Photoinduced Utilizations of Azo-Dye. Polymers 2021, 13, 1215. [Google Scholar] [CrossRef]
- Rashid, M.A.M.; Hayati, D.; Kwak, K.; Hong, J. Theoretical Investigation of Azobenzene-Based Photochromic Dyes for Dye-Sensitized Solar Cell. Nanomaterials 2020, 10, 914. [Google Scholar] [CrossRef]
- Chauhan, P.; Hadad, C.; Lopez, A.H.; Silvestrini, S.; La Parola, V.; Frison, E.; Maggini, M.; Prato, M.; Carofiglio, T. A nanocellulose–dye conjugate for multi-format optical pH-sensing. Chem. Commun. 2014, 50, 9493–9496. [Google Scholar] [CrossRef]
- Chiappone, A.; Bella, F.; Nair, J.R.; Meligrana, G.; Bongiovanni, R.; Gerbaldi, C. Structure-performance correlation of nanocellulose-based polymer electrolytes for efficient quasi-solid DSSCs. ChemElectroChem 2014, 1, 1350–1358. [Google Scholar] [CrossRef]
- Bapat, V.A.; Kishor, P.B.; Jalaja, N.; Jaion, S.M.; Penna, S. Plant Cell Cultures: Biofactories for the Production of Bioactive Compounds. Agronomy 2023, 13, 858. [Google Scholar] [CrossRef]
- Zhang, C.S.; Zhu, X.; Liao, Q.; Wang, Y.; Li, J.; Ding, Y.; Wang, H. Performance of a groove-type photobioreactor for hydrogen production by immobilized photosynthetic bacteria. Int. J. Hydrogen Energy 2010, 35, 5284–5292. [Google Scholar] [CrossRef]
- Meunier, C.F.; Yang, X.Y.; Rooke, J.C.; Su, B.L. Biofuel cells Based on the Immobilization of Photosynthetically Active Bioentities. ChemCatChem 2011, 3, 476–488. [Google Scholar] [CrossRef]
- Caldwell, G.S.; In-na, P.; Hart, R.; Sharp, E.; Stefanova, A.; Pickersgill, M.; Walker, M.; Unthank, M.; Perry, J.; Lee, J.G.M. Immobilising Microalgae and Cyanobacteria as Biocomposites: New Opportunities to Intensify Algae Biotechnology and Bioprocessing. Energies 2021, 14, 2566. [Google Scholar] [CrossRef]
- Meunier, C.F.; Dandoy, P.; Su, B.L. Encapsulation of cells within silica matrixes: Towards a new advance in the conception of living hybrid materials. J. Coll. Interf. Sci. 2010, 342, 211–224. [Google Scholar] [CrossRef]
- Song, W.; Rashid, N.; Choi, W.; Lee, K. Biohydrogen production by immobilized Chlorella sp. using cycles of oxygenic photosynthesis and anaerobiosis. Biores. Technol. 2011, 102, 8676–8681. [Google Scholar] [CrossRef]
- Leino, H.; Kosourov, S.N.; Saari, L.; Sivonen, K.; Tsygankov, A.A.; Aro, E.M.; Allahverdiyeva, Y. Extended H2 photoproduction by N2-fixing cyanobacteria immobilized in thin alginate films. Int. J. Hydrogen Energy 2012, 37, 151–161. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Bashan, Y. Immobilized microalgae for removing pollutants: Review of practical aspects. Biores. Technol. 2010, 101, 1611–1627. [Google Scholar] [CrossRef]
- Garbisu, C.; Gil, J.M.; Bazin, M.J.; Hall, D.O.; Serra, J.L. Removal of nitrate from water by foam-immobilized Phormidium laminosum in batch and continuous-flow bioreactors. J. Appl. Phycol. 1991, 3, 221–234. [Google Scholar] [CrossRef]
- Park, M.; Lee, D.; Hyun, J. Nanocellulose-alginate hydrogel for cell encapsulation. Carbohydr. Polym. 2015, 116, 223–228. [Google Scholar] [CrossRef]
- Ng, F.L.; Phang, S.M.; Periasamy, V.; Yunus, K.; Fisher, A.C. Enhancement of Power Output by using Alginate Immobilized Algae in Biophotovoltaic Devices. Sci. Rep. 2017, 7, 16237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosourov, S.N.; Seibert, M. Hydrogen photoproduction by nutrient-deprived Chlamydomonas reinhardtii cells immobilized within thin alginate films under aerobic and anaerobic conditions. Biotechnol. Bioeng. 2009, 102, 50–58. [Google Scholar] [CrossRef]
- Touloupakis, E.; Rontogiannis, G.; Benavides, A.M.S.; Cicchi, B.; Ghanotakis, D.F.; Torzillo, G. Hydrogen production by immobilized Synechocystis sp. PCC 6803. Int. J. Hydrogen Energy 2016, 41, 15181–15186. [Google Scholar] [CrossRef]
- Vajravel, S.; Sirin, S.; Kosourov, S.; Allahverdiyeva, Y. Towards sustainable ethylene production with cyanobacterial artificial biofilms. Green Chem. 2020, 22, 6404–6414. [Google Scholar] [CrossRef]
- Gosse, J.L.; Engel, B.J.; Hui, J.C.H.; Harwood, C.S.; Flickinger, M.C. Progress toward a biomimetic leaf: 4000 h of hydrogen production by coating-stabilized nongrowing photosynthetic Rhodopseudomonas palustris. Biotechnol. Prog. 2010, 26, 907–918. [Google Scholar] [PubMed]
- Piskorska, M.; Soule, T.; Gosse, J.L.; Milliken, C.; Flickinger, M.C.; Smith, G.W.; Yeager, C.M. Preservation of H-2 production activity in nanoporous latex coatings of Rhodopseudomonas palustris CGA009 during dry storage at ambient temperatures. Microb. Biotechnol. 2013, 6, 515–525. [Google Scholar] [CrossRef]
- Gosse, J.L.; Engel, B.J.; Rey, F.E.; Harwood, C.S.; Scriven, L.E.; Flickinger, M.C. Hydrogen production by photoreactive nanoporous latex coatings of nongrowing Rhodopseudomonas palustris CGA009. Biotechnol. Prog. 2007, 23, 124–130. [Google Scholar] [CrossRef]
- Eroglu, E.; Smith, S.M.; Raston, C.L. Application of Various Immobilization Techniques for Algal Bioprocesses. In Biomass and Biofuels from Microalgae; Moheimani, N.R., McHenry, M.P., de Boer, K., Bahri, P.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 19–44. [Google Scholar]
- Han, M.; Zhang, C.; Ho, S.H. Immobilized microalgal system: An achievable idea for upgrading current microalgal wastewater treatment. Environ. Sci. Ecotechnol. 2023, 14, 100227. [Google Scholar] [CrossRef]
- Chen, Z.; Osman, A.I.; Rooney, D.W.; Oh, W.D.; Yap, P.S. Remediation of Heavy Metals in Polluted Water by Immobilized Algae: Current Applications and Future Perspectives. Sustainability 2023, 15, 5128. [Google Scholar] [CrossRef]
- Abe, K.; Matsumara, I.; Imamaki, A.; Hirano, M. Removal of inorganic nitrogen sources from water by the algal biofilm of the aerial microalga Trentepohlia aurea. World J. Mircobiol. Biotechnol. 2003, 19, 325–328. [Google Scholar] [CrossRef]
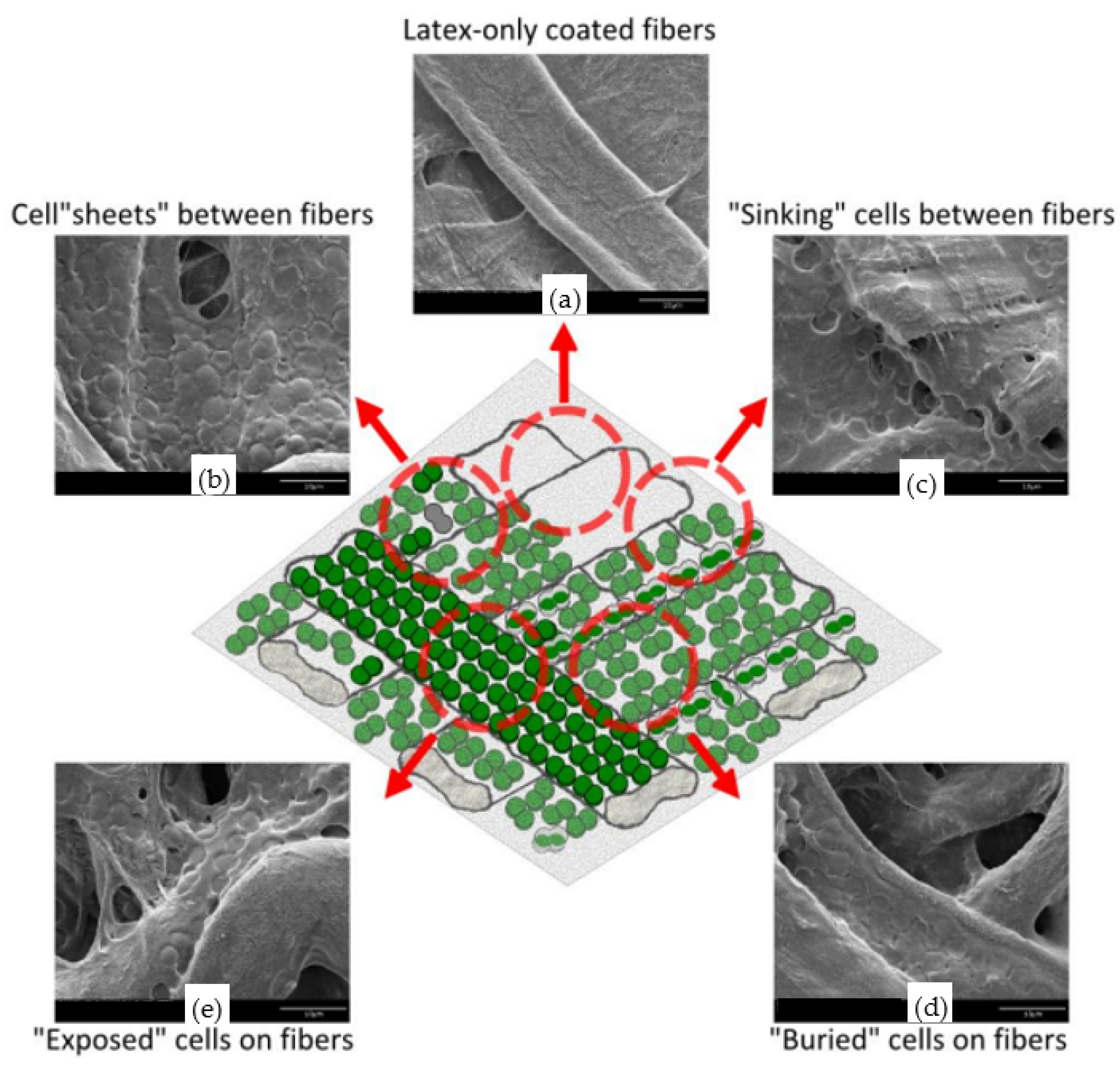
- Bernal, O.I.; Mooney, C.B.; Flickinger, M.C. Specific photosynthetic rate enhancement by cyanobacteria coated onto paper enables engineering of highly reactive cellular biocomposite “leaves”. Biotechnol. Bioeng. 2014, 111, 1993–2008. [Google Scholar] [CrossRef]
- Zuniga, B.; Ivan, O. Mimicking the Plant Leaf: Cellular Composite Materials for Capturing Solar Energy and Gas-Phase Biocatalysis. Ph.D. Thesis, NC State University, Raleigh, NC, USA, 2014. [Google Scholar]
- Mota, M.; Yelshin, A.; Fidaleo, M.; Flickinger, M.C. Modelling diffusivity in porous polymeric membranes with an intermediate layer containing microbial cells. Biochem. Eng. J. 2007, 37, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Gosse, J.L.; Chinn, M.S.; Gruden, A.M.; Bernal, O.I.; Jenkins, J.S.; Yeager, C.; Kosourov, S.; Seibert, M.; Flickinger, M.C. A versatile method for preparation of hydrated microbial–latex biocatalytic coatings for gas absorption and gas evolution. J. Ind. Microbiol. Biotechnol. 2012, 39, 1269–1278. [Google Scholar] [CrossRef]
- Jenkins, J.S.; Flickinger, M.C.; Velev, O.D. Engineering Cellular Photocomposite Materials Using Convective Assembly. Materials 2013, 6, 1803–1825. [Google Scholar] [CrossRef] [Green Version]
- Sagir, E.; Alipour, S. Photofermentative hydrogen production by immobilized photosynthetic bacteria: Current perspectives and challenges. Renew. Sustain. Energy Rev. 2021, 141, 110796. [Google Scholar] [CrossRef]
- Markov, S.A.; Bazin, M.J.; Hall, M.O. Hydrogen photoproduction and carbon dioxide uptake by immobilized Anabaena variabilis in a hollow-fiber photobioreactor. Enzyme Microb. Technol. 1995, 17, 306–310. [Google Scholar] [CrossRef]
- Evans, B.R. Method for Culturing Photosynthetic Microorganisms on Microbial Cellulose. U.S. Patent 20120077250A1, 29 March 2012. [Google Scholar]
- Sueoka, N. Composition of Minimal Salts Medium for Algae Modified by Increased Phosphate and Magnesium Concentrations. Proc. Natl. Acad. Sci. USA 1960, 46, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.M.; Chen, J.H.; Nagarajan, D.; Lee, C.K.; Varjani, S.; Lee, S.J.; Chang, J.S. Immobilization of Chlorella sorokiniana AK-1 in bacterial cellulose by co-culture and its application in wastewater treatment. J. Taiwan Inst. Chem. Eng. 2022, 137, 104286. [Google Scholar] [CrossRef]
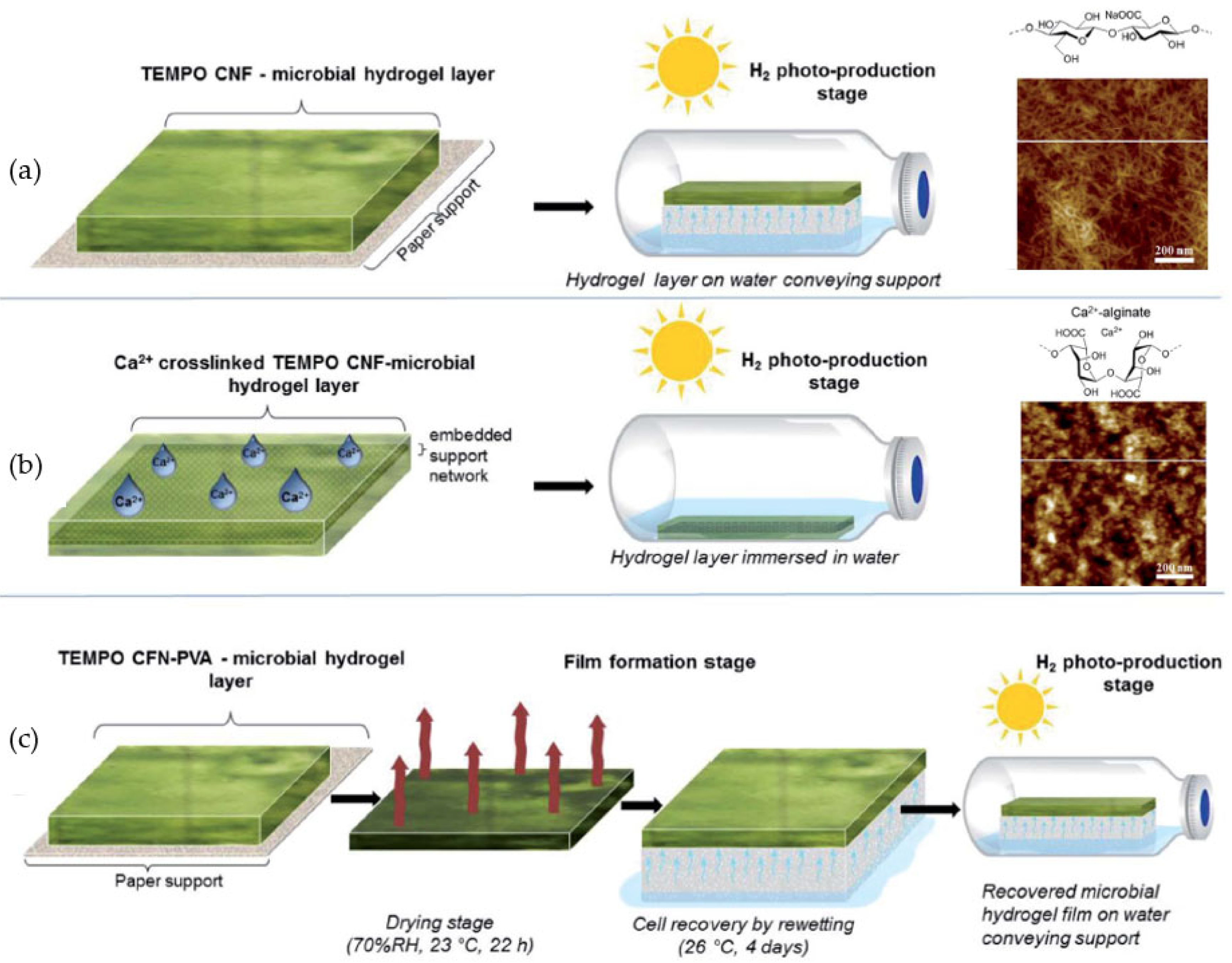
- Jämsä, M.; Kosourov, S.; Rissanen, V.; Hakalahti, M.; Pere, J.; Ketoja, J.A.; Tammelin, T.; Allahverhiyeva, Y. Versatile templates from cellulose nanofibrils for photosynthetic microbial biofuel production. J. Mater. Chem. A 2018, 6, 5825–5835. [Google Scholar] [CrossRef] [Green Version]
- Rissanen, V.; Vajravel, S.; Kosourov, S.N.; Arola, S.; Kontturi, E.; Allahverdiyeva, Y.; Tammelin, T. Nanocellulose-based mechanically stable immobilization matrix for enhanced ethylene production: A framework for photosynthetic solid-state cell factories. Green Chem. 2021, 23, 3715–3724. [Google Scholar] [CrossRef]
- Lungu, A.; Cernencu, A.I.; Dinescu, S.; Balahura, R.; Mereuta, P.; Costache, M.; Syverud, K.; Stancu, I.C.; Iovu, H. Nanocellulose-enriched hydrocolloid-based hydrogels designed using a Ca2+ free strategy based on citric acid. Mater. Des. 2021, 197, 109200. [Google Scholar] [CrossRef]
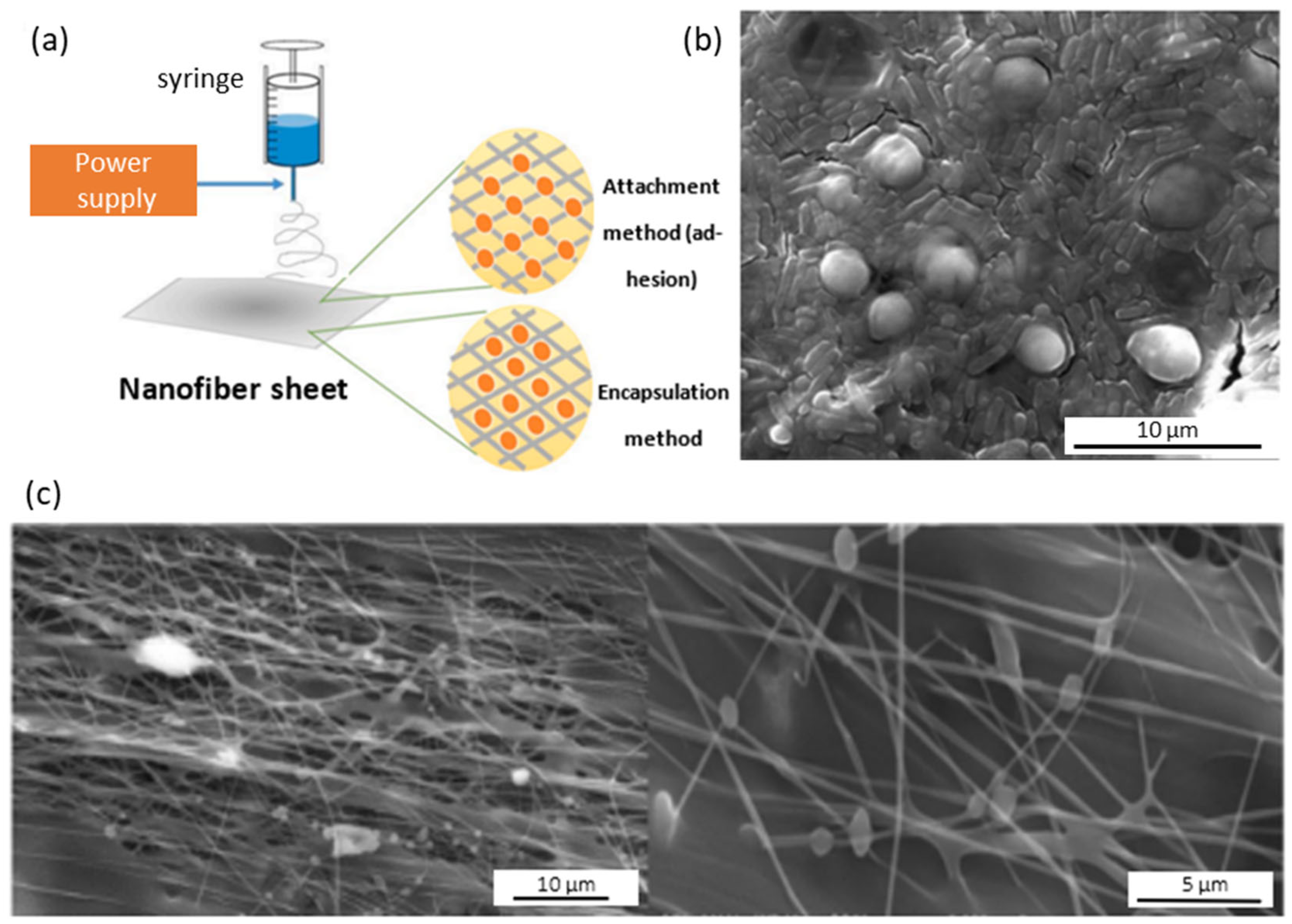
- Sarioglu, O.F.; Yasa, O.; Celebioglu, A.; Uyar, Y.; Tekinay, T. Efficient ammonium removal from aquatic environments by Acinetobacter calcoaceticus STB1 immobilized on an electrospun cellulose acetate nanofibrous web. Green Chem. 2013, 15, 2566–2572. [Google Scholar] [CrossRef] [Green Version]
- San, N.O.; Celebioglu, A.; Tümtas, Y.; Uyar, T.; Tekinay, T. Reusable bacteria immobilized electrospun nanofibrous webs for decolorization of methylene blue dye in wastewater treatment. RSC Adv. 2014, 4, 32249–32255. [Google Scholar] [CrossRef] [Green Version]
- Stojanoc, S.; Berlec, A. Electrospun Nanofibers as Carriers of Microorganisms, Stem Cells, Proteins, and Nucleic Acids in Therapeutic and Other Applications. Front. Bioeng. Biotechnol. 2020, 8, 130. [Google Scholar] [CrossRef]
- Zamel, D.; Khan, A.U. Bacterial immobilization on cellulose acetate based nanofibers for methylene blue removal from wastewater: Mini-review. Inorg. Chem. Commun. 2021, 131, 108766. [Google Scholar] [CrossRef]
- Jayani, T.; Sanjeev, B.; Marimuthu, S.; Uthandi, S. Bacterial Cellulose Nano Fiber (BCNF) as carrier support for the immobilization of probiotic Lactobacillus acidophilus 016. Carbohydr. Polym. 2020, 250, 116965. [Google Scholar] [CrossRef]
- Sarioglu, O.F.; Keskin, N.O.S.; Celebioglu, A.; Tekinay, T.; Uyar, T. Bacteria encapsulated electrospun nanofibrous webs for remediation of methylene blue dye in water. Colloid Surf. B 2017, 152, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Zamel, D.; Hassanin, A.H.; Ellethy, R.; Singer, G.; Abdelmoneim, A. Novel Bacteria Immobilized Cellulose Acetate/Poly (ethylene oxide) Nanofibrous Membrane for Wastewater Treatment. Sci. Rep. 2019, 12, 18994. [Google Scholar] [CrossRef] [Green Version]
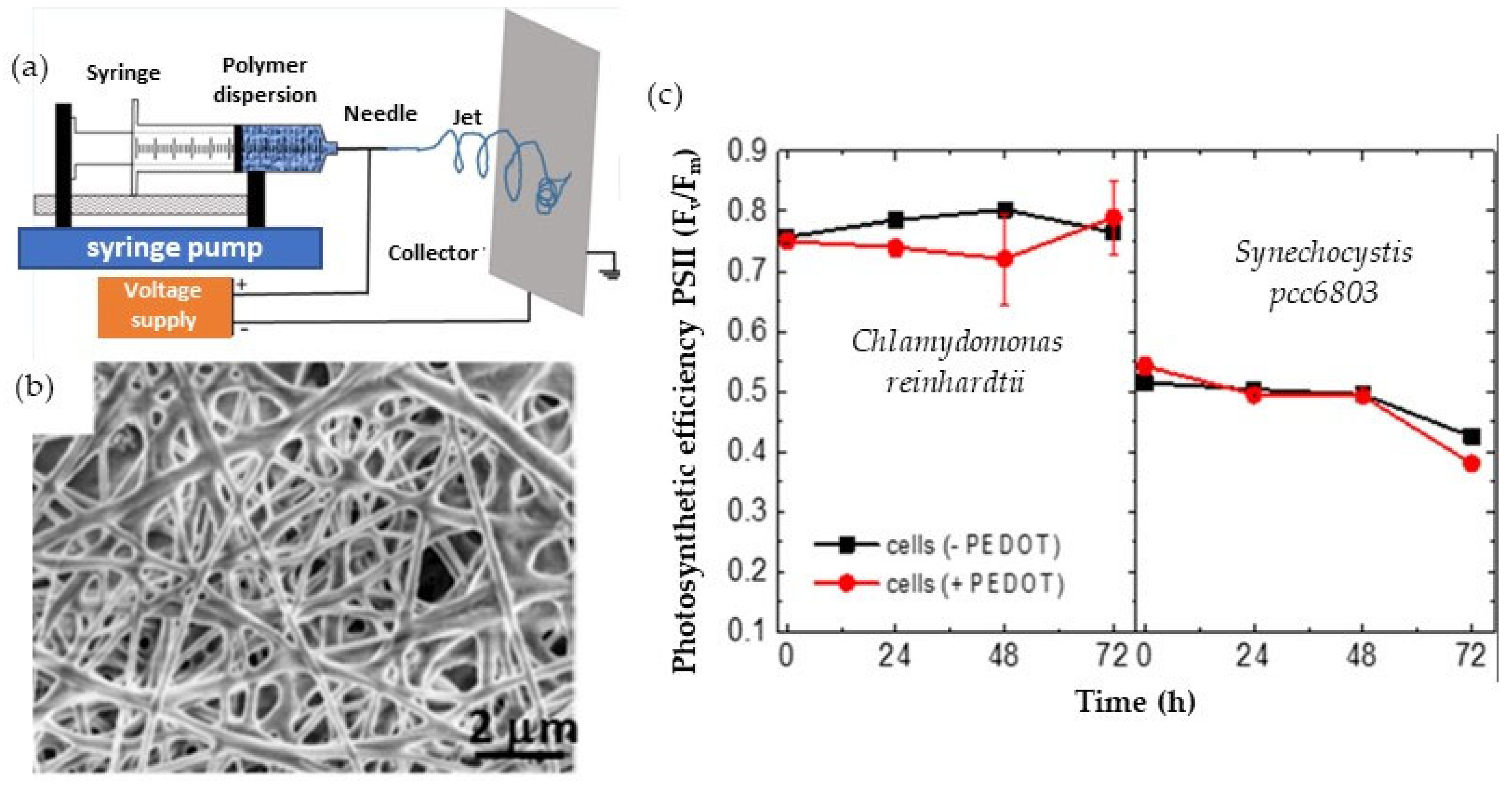
- Bedford, N.M.; Winget, G.D.; Punnamaraju, S.; Steckl, A.J. Immobilization of stable thylakoid vesicles in conductive nanofibers by electrospinning. Biomacromolecules 2011, 12, 778–784. [Google Scholar] [CrossRef]
- Shi, Z.; Phillips, G.O.; Yang, G. Nanocellulose electroconductive composites. Nanoscale 2013, 5, 3194–3201. [Google Scholar] [CrossRef]
- Han, J.; Wang, S.; Zhu, S.; Huang, C.; Yue, Y.; Mei, C.; Xu, X.; Xia, C. Electrospun Core–Shell Nanofibrous Membranes with Nanocellulose-Stabilized Carbon Nanotubes for Use as High-Performance Flexible Supercapacitor Electrodes with Enhanced Water Resistance, Thermal Stability, and Mechanical Toughness. ACS Appl. Mater. Interfaces 2019, 11, 44624–44635. [Google Scholar] [CrossRef]
- Muller, D.; Silva, J.P.; Rambo, C.R.; Barra, G.M.O.; Dourado, F.; Gama, F.M. Neuronal cells’ behavior on polypyrrole coated bacterial nanocellulose three-dimensional (3D) scaffolds. J. Biomater. Sci. Polym. Ed. 2013, 24, 1368–1377. [Google Scholar] [CrossRef]
- Moyo, S.; Gumbi, N.N.; De Kock, L.A.; Nxumalo, E.N. A mini-review of nanocellulose-based nanofiber membranes incorporating carbon nanomaterials for dye wastewater treatment. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100714. [Google Scholar] [CrossRef]
- Latonen, R.M.; Cabrera, J.A.W.; Lund, S.; Kosourov, S.; Vajravel, S.; Boeva, Z.; Wang, X.; Xu, C.; Allahverdiyeva, Y. Electrospinning of Electroconductive Water-Resistant Nanofibers of PEDOT–PSS, Cellulose Nanofibrils and PEO: Fabrication, Characterization, and Cytocompatibility. ACS Appl. Bio Mater. 2021, 4, 483–493. [Google Scholar] [CrossRef]
- Ge, L.; Yin, J.; Yan, D.; Hong, W.; Jiao, T. Construction of nanocrystalline cellulose-based composite fiber films with excellent porosity performances via an electrospinning strategy. ACS Omega 2021, 6, 4958–4967. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green Light Drives Leaf Photosynthesis More Efficiently than Red Light in Strong White Light: Revisiting the Enigmatic Question of Why Leaves are Green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Wu, S.; Zhang, H.; Zhang, X.; Zhuang, J.; Hu, C.; Liu, Y.; Lei, B.; Ma, L.; Wang, X. Enhanced Biological Photosynthetic Efficiency Using Light-Harvesting Engineering with Dual-Emissive Carbon Dots. Adv. Funct. Mater. 2018, 28, 1804004. [Google Scholar] [CrossRef]
- Jin, H.; Li, M.; Duan, S.; Fu, M.; Dong, X.; Liu, B.; Feng, D.; Wang, J.; Wang, H.B. Optimization of Light-Harvesting Pigment Improves Photosynthetic Efficiency. Plant Physiol. 2016, 172, 1720–1731. [Google Scholar] [CrossRef] [Green Version]
- Mehes, G.; Vagin, M.; Mulla, M.Y.; Granberg, H.; Che, C.; Beni, V.; Crispin, X.; Berggren, M.; Stavrinidou, E.; Simon, D.T. Solar Heat-Enhanced Energy Conversion in Devices Based on Photosynthetic Membranes and PEDOT:PSS-Nanocellulose Electrodes. Adv. Sustain. Syst. 2019, 4, 1900100. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Nam, D.H.; Park, C.B. Nanobiocatalytic assemblies for artificial photosynthesis. Curr. Opin. Biotechnol. 2014, 28, 1–9. [Google Scholar] [CrossRef]
- Tian, L.; Xin, Q.; Zhai, C.; Xie, G.; Akram, M.Z.; Wang, W.; Ma, R.; Jia, X.; Guo, B.; Gong, J.R. Nanoarray Structures for Artificial Photosynthesis. Small 2021, 17, 2006530. [Google Scholar] [CrossRef] [PubMed]
- Lehmeier, C.; Pajor, R.; Lundgren, M.R.; Mathers, A.; Sloan, J.; Bauch, M.; Mitchell, A.; Bellasio, C.; Green, A.; Bouyer, D.; et al. Cell density and airspace patterning in the leaf can be manipulated to increase leaf photosynthetic capacity. Plant J. 2017, 92, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Kuruvinashetti, K.; Packirisamy, M. Arraying of microphotosynthetic power cells for enhanced power output. Microsyst. Nanoeng. 2022, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shin, D.; Choi, J.; Ki, C.S.; Hyun, J. Mimicry of the plant leaf with a living hydrogel sheet of cellulose nanofibers. Carbohydr. Polym. 2022, 290, 119485. [Google Scholar] [CrossRef]
- Fourmann, O.; Hausmann, M.K.; Neels, A.; Schubert, M.; Nyström, G.; Zimmermann, T.; Siquieira, G. 3D printing of shape-morphing and antibacterial anisotropic nanocellulose hydrogels. Carbohydr. Polym. 2021, 259, 117716. [Google Scholar] [CrossRef]
- Samyn, P.; Pappa, M.; Lama, S.; Vandamme, D. Algae for Nanocellulose Production. In Bioprospecting Algae for Nanosized Materials; Thangadurai, D., Sangeetha, J., Prasad, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 293–343. [Google Scholar]
- Zhu, X.G.; Hasanuzzaman, M.; Jajoo, A.; Lawson, T.; Lin, R.; Liu, C.M.; Liu, L.N.; Liu, Z.; Lu, C.; Moustakas, M.; et al. Improving photosynthesis through multidisciplinary efforts: The next frontier of photosynthesis research. Front. Plant. Sci. 2022, 13, 967203. [Google Scholar] [CrossRef]
- Dogutan, D.K.; Nocera, D.G. Artificial Photosynthesis at Efficiencies Greatly Exceeding That of Natural Photosynthesis. Acc. Chem. Res. 2019, 52, 3143–3148. [Google Scholar] [CrossRef]


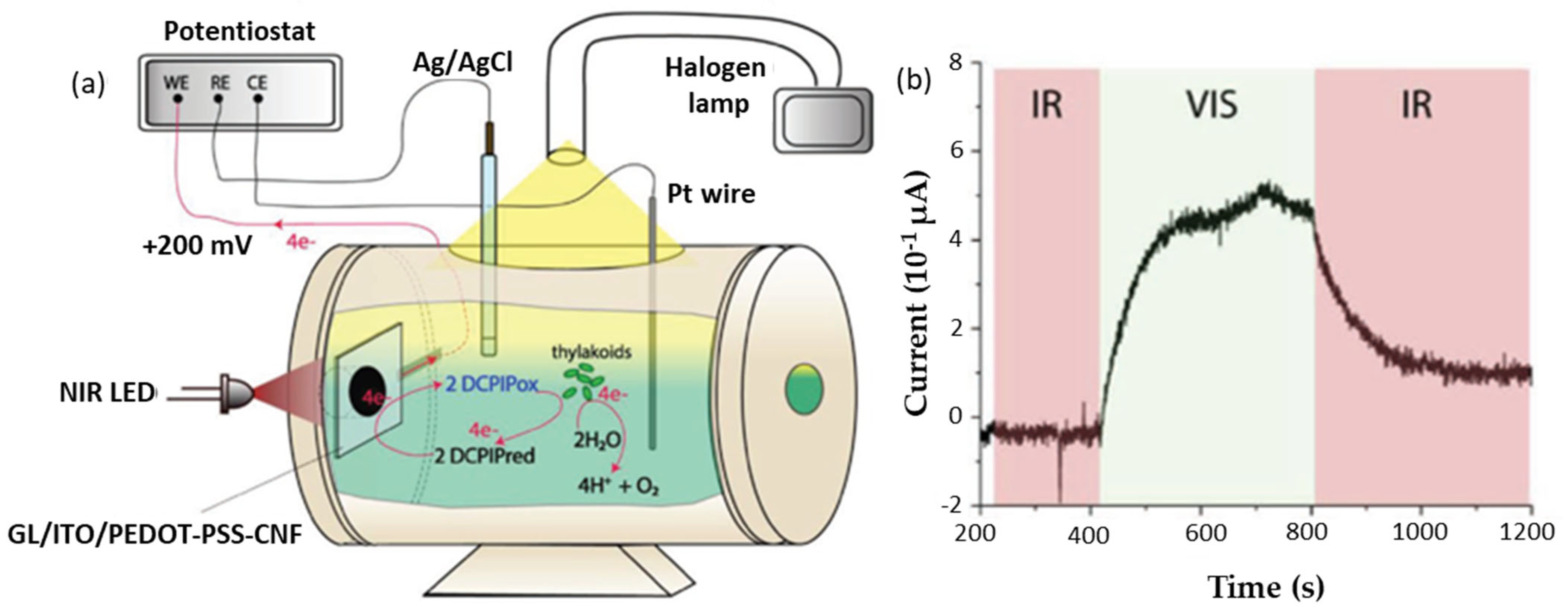
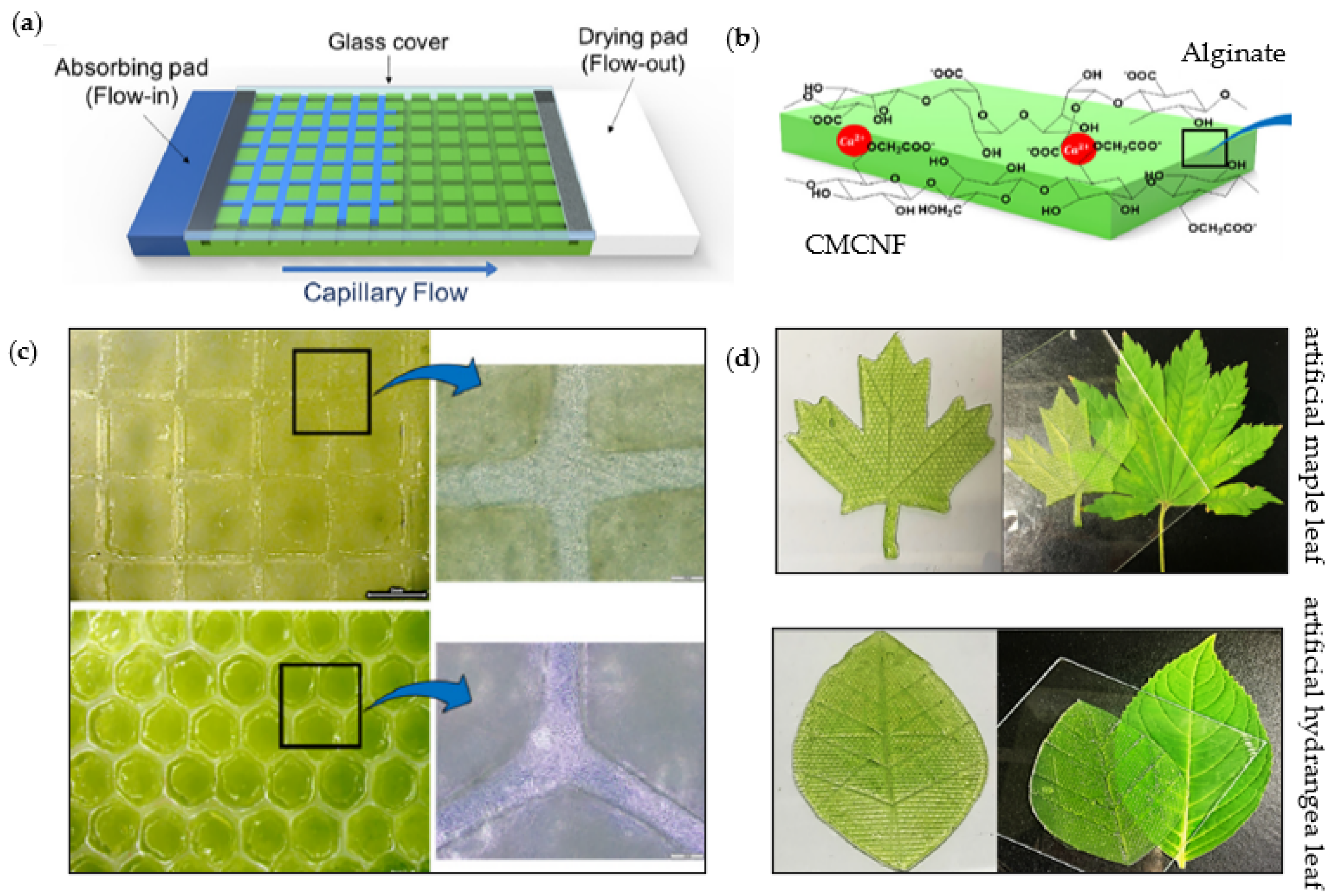
| Photoreactive Nanoparticles | Nano-Cellulose | Processing for Incorporation within Photoreactive Nanocellulose Composites | Reference |
|---|---|---|---|
| Metals | |||
| Au | CNC | Reduction from HAuCl4 | [188] |
| Pd | CNF | Direct reduction of Pd2+ ions | [189] |
| Metal oxides | |||
| ZnO | CNC | In-situ casting from Zn(NO3)2 | [180] |
| ZnO | CNC | Co-precipitation from Zn(CH2CO2) | [190] |
| TiO2 | CNC | Hydrolysis of TiCl4 | [191] |
| TiO2 | CNC | Reduction of TiOOSO4·H2SO4·H2O | [185] |
| TiO2 | CNF | Heterocoagulation of CNC and TiO2 NP | [192] |
| Cu2O | CNC | Microwave-assisted polyol synthesis | [193] |
| Cu2O/TiO2 | CNF | Hydrothermal reaction from CuSO4 and NaOH | [194] |
| Cu2O/Ag | CNF | Reduction from CuSO4 and AgNO3 | [195] |
| CuO/Fe3O4 | MFC | In-situ co-precipitation of Cu(CH3COO)2 and Fe(NO3)3·9H2O | [196] |
| Fe-doped ZnO | CNC | In-situ precipitation from Zn(CH3CO2)2 and FeCl3 | [197] |
| Al-doped ZnO | MFC/CNF | In-situ reduction from ZnCl2 and AlCl3 | [198] |
| Metal sulphides | |||
| CdS | CNF | Quantum dots formation from CdCl2 and Na2S | [170] |
| ZnS | CNC | In-situ co-precipitation from ZnNO3 precursor | [199] |
| MoS2/CdS | CNF | Coagulation casting of Na2MoO4·2H2O and Cd(CH3CO2)2·2H2O | [172] |
| Others | |||
| Ag3PO4 | CNC | In-situ synthesis from Na2HPO4·12H2O and AgNO3 | [200] |
| Graphitic carbon nitride | CNF | Thermal polymerization of bi-component precursor | [201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samyn, P.; Rastogi, V.K.; Baba, N.S.S.; Van Erps, J. Role of Nanocellulose in Light Harvesting and Artificial Photosynthesis. Catalysts 2023, 13, 986. https://doi.org/10.3390/catal13060986
Samyn P, Rastogi VK, Baba NSS, Van Erps J. Role of Nanocellulose in Light Harvesting and Artificial Photosynthesis. Catalysts. 2023; 13(6):986. https://doi.org/10.3390/catal13060986
Chicago/Turabian StyleSamyn, Pieter, Vibhore Kumar Rastogi, Neelisetty Sesha Sai Baba, and Jürgen Van Erps. 2023. "Role of Nanocellulose in Light Harvesting and Artificial Photosynthesis" Catalysts 13, no. 6: 986. https://doi.org/10.3390/catal13060986






