Effect of Calcination Conditions on Co3O4 Catalysts in the Total Oxidation of Toluene and Propane
Abstract
:1. Introduction
2. Results and Discussion
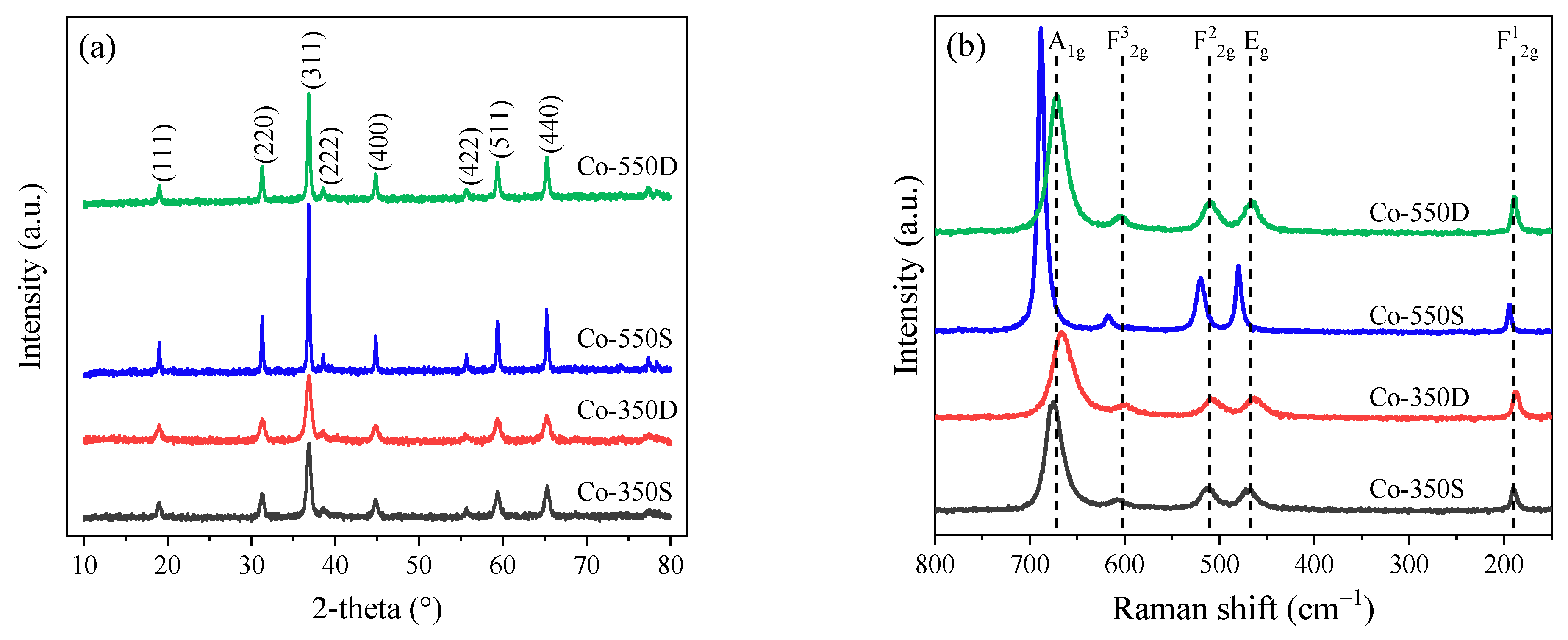
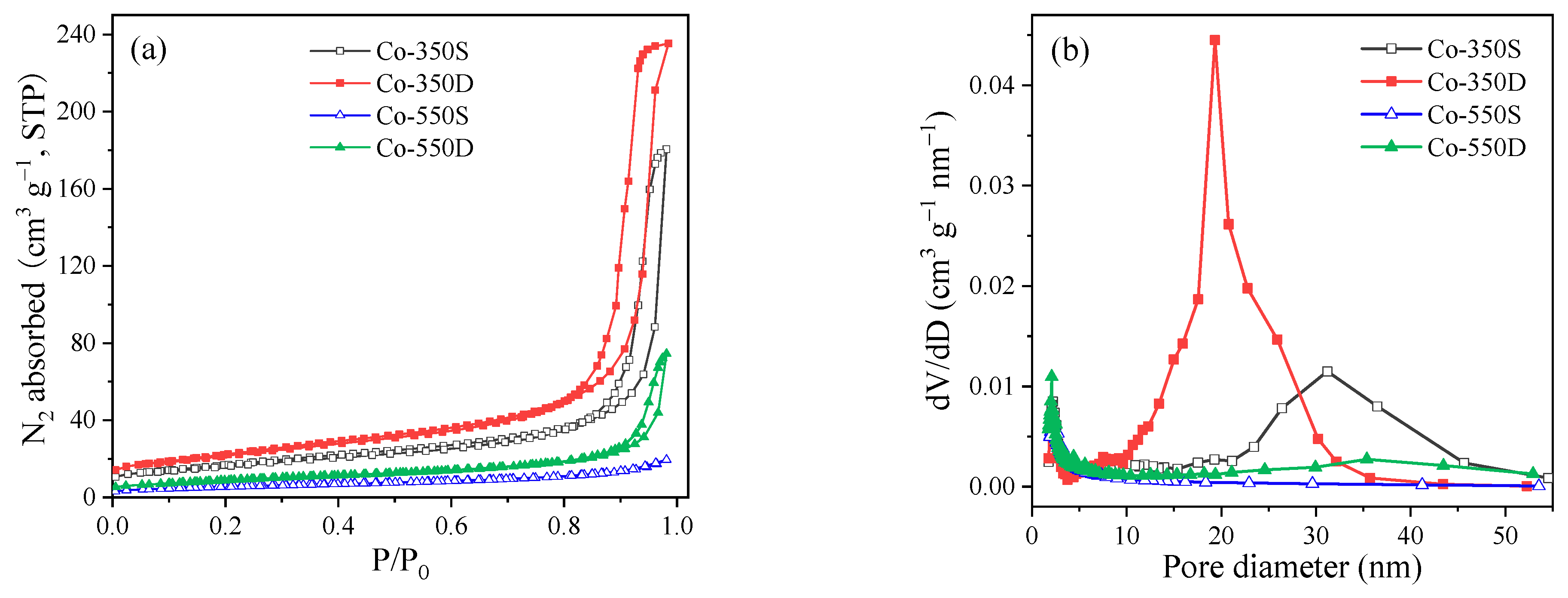
2.1. Structural and Textural Characterization
2.2. Reducibility
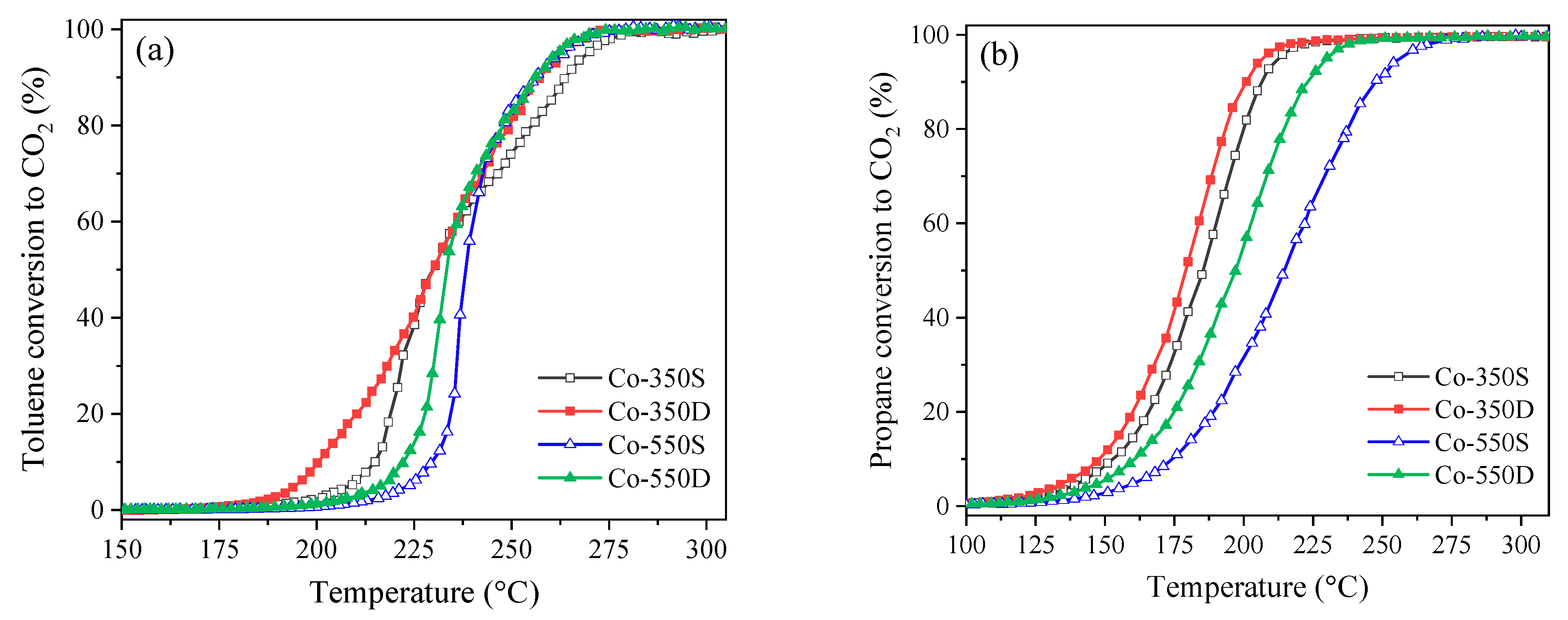
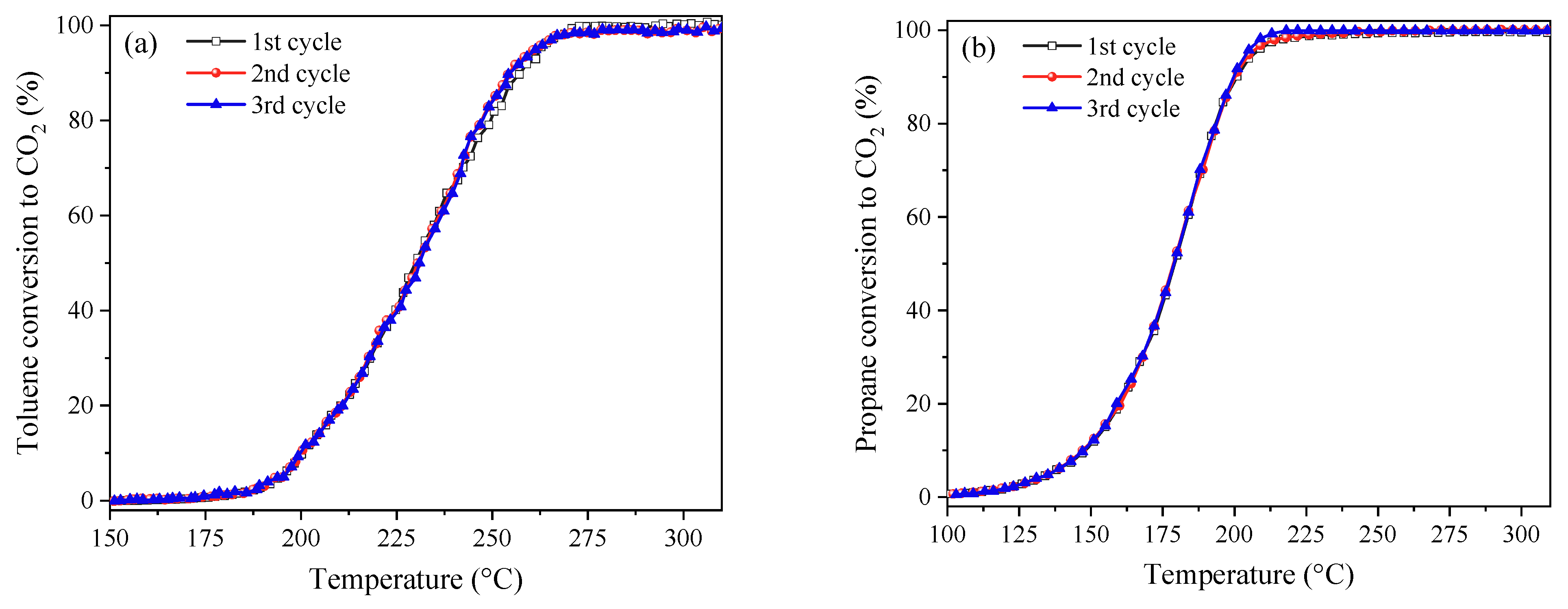
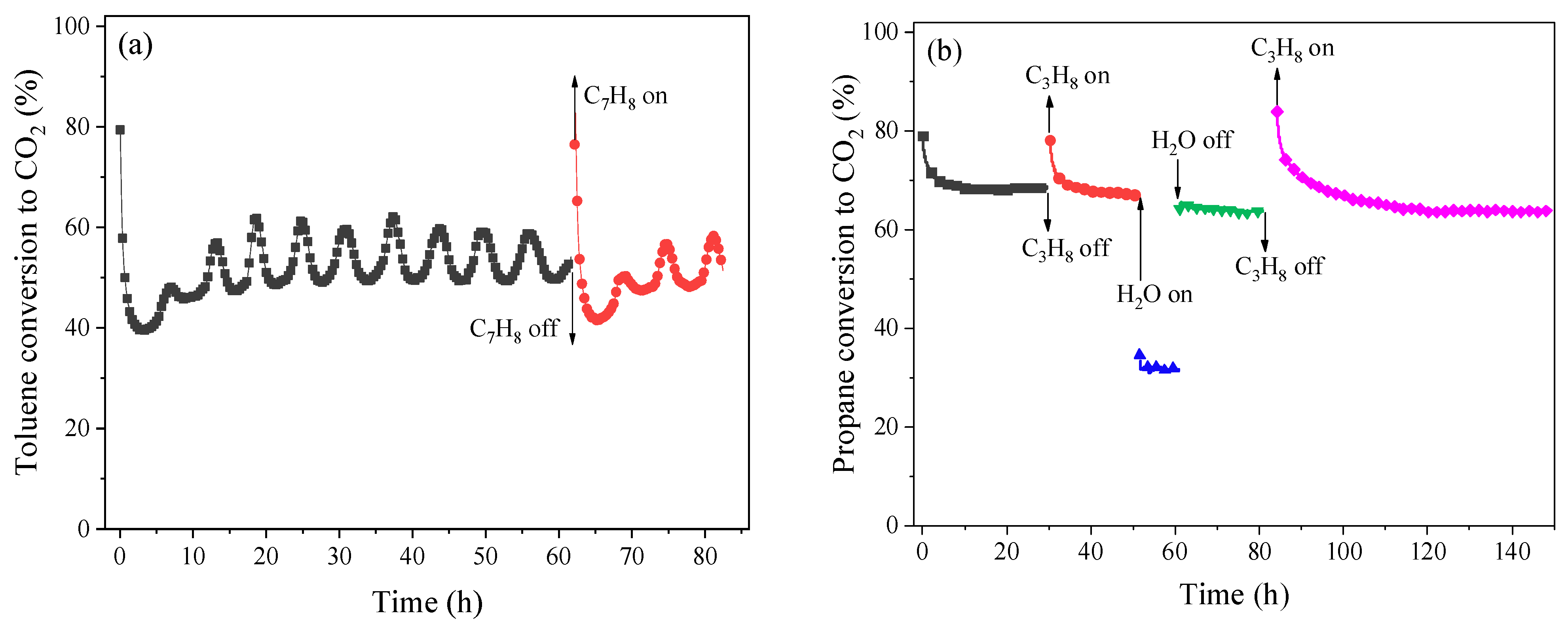
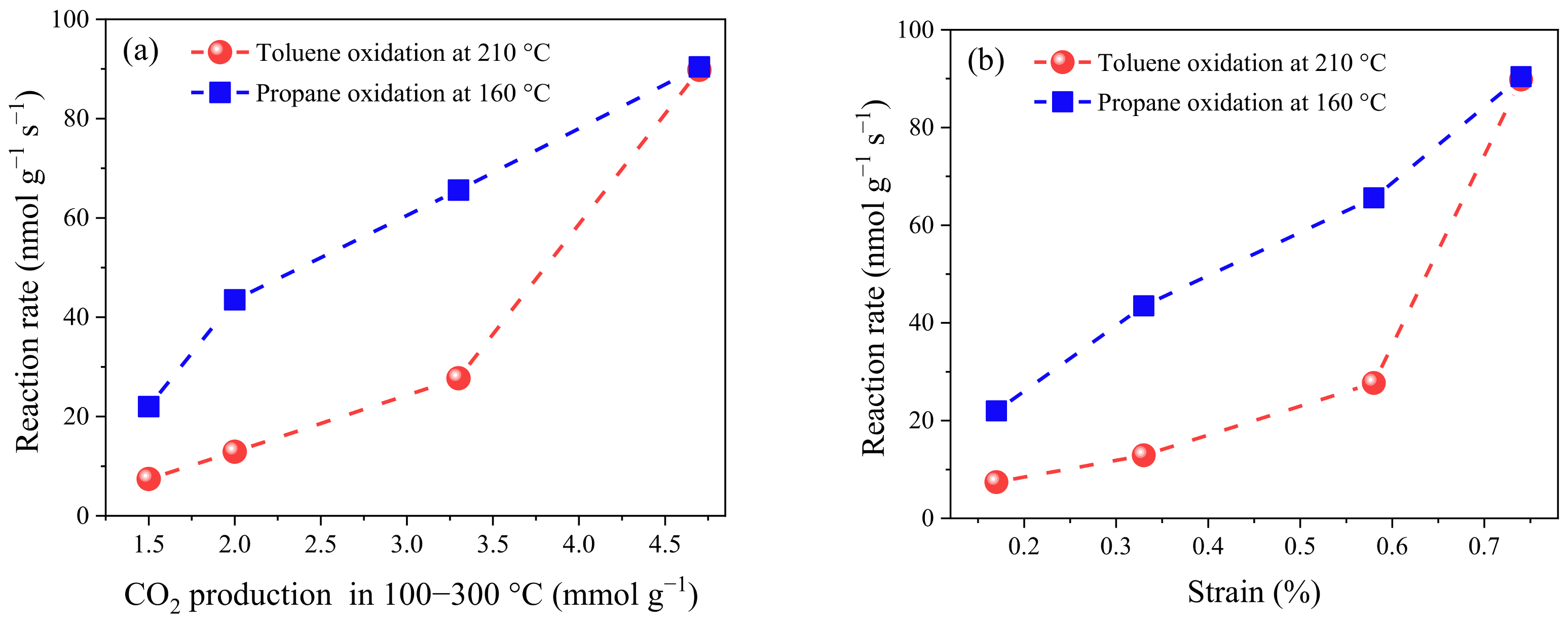
2.3. Catalytic Performance
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B Environ. 2021, 281, 119447. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Shen, Y.; Deng, J.; Impeng, S.; Li, S.; Yan, T.; Zhang, J.; Shi, L.; Zhang, D. Boosting toluene combustion by engineering Co–O strength in cobalt oxide catalysts. Environ. Sci. Technol. 2020, 54, 10342–10350. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, R.; Zhang, H.; Jin, Q.; Song, Z.; Zhang, X. Fabrication of Co3O4 nanospheres and their catalytic performances for toluene oxidation: The distinct effects of morphology and oxygen species. Appl. Catal. A Gen. 2020, 597, 117539. [Google Scholar] [CrossRef]
- Ren, Q.; Mo, S.; Peng, R.; Feng, Z.; Zhang, M.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Controllable synthesis of 3D hierarchical Co3O4 nanocatalysts with various morphologies for the catalytic oxidation of toluene. J. Mater. Chem. A 2018, 6, 498–509. [Google Scholar] [CrossRef]
- Liu, W.; Liu, R.; Zhang, X. Controllable synthesis of 3D hierarchical Co3O4 catalysts and their excellent catalytic performance for toluene combustion. Appl. Surf. Sci. 2020, 507, 145174. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C.; Wang, Z.; Huang, H.; Peng, H.; Li, X. Fabrication of mesoporous Co3O4 oxides by acid treatment and their catalytic performances for toluene oxidation. Appl. Catal. A Gen. 2018, 550, 67–76. [Google Scholar] [CrossRef]
- Ren, Q.; Feng, Z.; Mo, S.; Huang, C.; Li, S.; Zhang, W.; Chen, L.; Fu, M.; Wu, J.; Ye, D. 1D-Co3O4, 2D-Co3O4, 3D-Co3O4 for catalytic oxidation of toluene. Catal. Today 2019, 332, 160–167. [Google Scholar] [CrossRef]
- Han, W.; Tang, Z.; Lin, Q. Morphology-controlled synthesis of the metal–organic framework-derived nanorod interweaved lamellose structure Co3O4 for outstanding catalytic combustion performance. Cryst. Growth Des. 2019, 19, 4546–4556. [Google Scholar] [CrossRef]
- Bao, L.; Zhu, S.; Chen, Y.; Wang, Y.; Meng, W.; Xu, S.; Lin, Z.; Li, X.; Sun, M.; Guo, L. Anionic defects engineering of Co3O4 catalyst for toluene oxidation. Fuel 2022, 314, 122774. [Google Scholar] [CrossRef]
- Li, Y.; Chen, T.; Zhao, S.; Wu, P.; Chong, Y.; Li, A.; Zhao, Y.; Chen, G.; Jin, X.; Qiu, Y.; et al. Engineering cobalt oxide with coexisting cobalt defects and oxygen vacancies for enhanced catalytic oxidation of toluene. ACS Catal. 2022, 12, 4906–4917. [Google Scholar] [CrossRef]
- Garcia, T.; Agouram, S.; Sánchez-Royo, J.F.; Murillo, R.; Mastral, A.M.; Aranda, A.; Vázquez, I.; Dejoz, A.; Solsona, B. Deep oxidation of volatile organic compounds using ordered cobalt oxides prepared by a nanocasting route. Appl. Catal. A Gen. 2010, 386, 16–27. [Google Scholar] [CrossRef]
- Puértolas, B.; Smith, A.; Vázquez, I.; Dejoz, A.; Moragues, A.; Garcia, T.; Solsona, B. The different catalytic behaviour in the propane total oxidation of cobalt and manganese oxides prepared by a wet combustion procedure. Chem. Eng. J. 2013, 229, 547–558. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, F.; Li, J.; You, Z. Dispersion–precipitation synthesis of highly active nanosized Co3O4 for catalytic oxidation of carbon monoxide and propane. Appl. Surf. Sci. 2017, 411, 136–143. [Google Scholar] [CrossRef]
- Yao, J.; Shi, H.; Sun, D.; Lu, H.; Hou, B.; Jia, L.; Xiao, Y.; Li, D. Facet-dependent activity of Co3O4 catalyst for C3H8 combustion. ChemCatChem 2019, 11, 5570–5579. [Google Scholar] [CrossRef]
- Lin, D.; Zheng, Y.; Feng, X.; You, Y.; Wu, E.; Luo, Y.; Qian, Q.; Chen, Q. Highly stable Co3O4 nanoparticles-assembled microrods derived from MOF for efficient total propane oxidation. J. Mater. Sci. 2020, 55, 5190–5202. [Google Scholar] [CrossRef]
- Marin, R.P.; Kondrat, S.A.; Pinnell, R.K.; Davies, T.E.; Golunski, S.; Bartley, J.K.; Hutchings, G.J.; Taylor, S.H. Green preparation of transition metal oxide catalysts using supercritical CO2 anti-solvent precipitation for the total oxidation of propane. Appl. Catal. B Environ. 2013, 140–141, 671–679. [Google Scholar] [CrossRef]
- Tang, W.; Xiao, W.; Wang, S.; Ren, Z.; Ding, J.; Gao, P.-X. Boosting catalytic propane oxidation over PGM-free Co3O4 nanocrystal aggregates through chemical leaching: A comparative study with Pt and Pd based catalysts. Appl. Catal. B Environ. 2018, 226, 585–595. [Google Scholar] [CrossRef]
- He, C.; Ao, C.; Ruan, S.; Xu, K.; Zhang, L. Catalytic combustion of propane over Zr-modified Co3O4 catalysts: An experimental and theoretical study. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128617. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, X.; Li, C.; Chen, X.; Li, W.; Liu, Z.; Liang, C. Defect engineering over Co3O4 catalyst for surface lattice oxygen activation and boosted propane total oxidation. J. Catal. 2022, 413, 150–162. [Google Scholar] [CrossRef]
- Liotta, L.F.; Wu, H.; Pantaleo, G.; Venezia, A.M. Co3O4 nanocrystals and Co3O4–MOx binary oxides for CO, CH4 and VOC oxidation at low temperatures: A review. Catal. Sci. Technol. 2013, 3, 3085. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Z.; Fang, W.; Yao, X.; Zou, G.; Shangguan, W. Low-temperature catalytic oxidation of formaldehyde over Co3O4 catalysts prepared using various precipitants. Chin. J. Catal. 2016, 37, 947–954. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Zhou, H.; Huang, W.; Pu, Z. Complete combustion of methane over Co3O4 catalysts: Influence of pH values. J. Alloys Compd. 2018, 734, 112–120. [Google Scholar] [CrossRef]
- De Rivas, B.; López-Fonseca, R.; Jiménez-González, C.; Gutiérrez-Ortiz, J.I. Synthesis, characterisation and catalytic performance of nanocrystalline Co3O4 for gas-phase chlorinated VOC abatement. J. Catal. 2011, 281, 88–97. [Google Scholar] [CrossRef]
- Dissanayake, S.; Wasalathanthri, N.; Shirazi Amin, A.; He, J.; Poges, S.; Rathnayake, D.; Suib, S.L. Mesoporous Co3O4 catalysts for VOC elimination: Oxidation of 2-propanol. Appl. Catal. A Gen. 2020, 590, 117366. [Google Scholar] [CrossRef]
- Zhang, W.; Díez-Ramírez, J.; Anguita, P.; Descorme, C.; Valverde, J.L.; Giroir-Fendler, A. Nanocrystalline Co3O4 catalysts for toluene and propane oxidation: Effect of the precipitation agent. Appl. Catal. B Environ. 2020, 273, 118894. [Google Scholar] [CrossRef]
- Zhang, W.; Lassen, K.; Descorme, C.; Valverde, J.L.; Giroir-Fendler, A. Effect of the precipitation pH on the characteristics and performance of Co3O4 catalysts in the total oxidation of toluene and propane. Appl. Catal. B Environ. 2021, 282, 119566. [Google Scholar] [CrossRef]
- Chen, H.; Liu, P.; Wei, G.; Huang, Y.; Lin, X.; Liang, X.; Zhu, J. Effect of electron structure on the catalytic activity of LaCoO3 perovskite towards toluene oxidation. Chem. Commun. 2022, 58, 4731–4734. [Google Scholar] [CrossRef]
- Li, J.B.; Jiang, Z.Q.; Qian, K.; Huang, W.X. Effect of calcination temperature on surface oxygen vacancies and catalytic performance towards CO oxidation of Co3O4 nanoparticles supported on SiO2. Chin. J. Chem. Phys. 2012, 25, 103–109. [Google Scholar] [CrossRef]
- Wang, M.; Qi, L.; Li, X. Modulating defective oxygen of Co-based crystals by calcination temperature control for improving the catalytic removal of propane. CrystEngComm 2022, 24, 7902–7905. [Google Scholar] [CrossRef]
- Liu, Z.G.; Chai, S.H.; Binder, A.; Li, Y.Y.; Ji, L.T.; Dai, S. Influence of calcination temperature on the structure and catalytic performance of CuOx-CoOy-CeO2 ternary mixed oxide for CO oxidation. Appl. Catal. A Gen. 2013, 451, 282–288. [Google Scholar] [CrossRef]
- Wu, H.; Pantaleo, G.; Di Carlo, G.; Guo, S.; Marcì, G.; Concepción, P.; Venezia, A.M.; Liotta, L.F. Co3O4 particles grown over nanocrystalline CeO2: Influence of precipitation agents and calcination temperature on the catalytic activity for methane oxidation. Catal. Sci. Technol. 2015, 5, 1888–1901. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Lu, G.; Qiao, D.; Wang, Y.; Guo, Y.; Guo, Y. Catalytic methane combustion over Co3O4/CeO2 composite oxides prepared by modified citrate sol–gel method. Catal. Lett. 2011, 141, 452–458. [Google Scholar] [CrossRef]
- Onrubia, J.A.; Pereda-Ayo, B.; De-La-Torre, U.; González-Velasco, J.R. Key factors in Sr-doped LaBO3 (B = Co or Mn) perovskites for NO oxidation in efficient diesel exhaust purification. Appl. Catal. B Environ. 2017, 213, 198–210. [Google Scholar] [CrossRef]
- Klissurski, D.; Uzunova, E. Synthesis of nickel cobaltite spinel from coprecipitated nickel-cobalt hydroxide carbonate. Chem. Mater. 1991, 3, 1060–1063. [Google Scholar] [CrossRef]
- Xu, R.; Zeng, H.C. Dimensional control of cobalt-hydroxide-carbonate nanorods and their thermal conversion to one-dimensional arrays of Co3O4 nanoparticles. J. Phys. Chem. B 2003, 107, 12643–12649. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, Y.; Li, J.; Gu, L.; Yuan, H.; Xiao, D. Synthesis of 3D-nanonet hollow structured Co3O4 for high capacity supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 6739–6747. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Zhao, Q.; Chen, G. Shape-controlled fabrication of the porous Co3O4 nanoflower clusters for efficient catalytic oxidation of gaseous toluene. J. Hazard. Mater. 2012, 209–210, 385–391. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.-C.C.; Chen, M.; Cao, Y.; He, H.-Y.Y.; Fan, K.-N.N. Dry citrate-precursor synthesized nanocrystalline cobalt oxide as highly active catalyst for total oxidation of propane. J. Catal. 2009, 263, 104–113. [Google Scholar] [CrossRef]
- Lou, Y.; Ma, J.; Cao, X.; Wang, L.; Dai, Q.; Zhao, Z.; Cai, Y.; Zhan, W.; Guo, Y.Y.; Hu, P.; et al. Promoting effects of In2O3 on Co3O4 for CO oxidation: Tuning O2 activation and CO adsorption strength simultaneously. ACS Catal. 2014, 4, 4143–4152. [Google Scholar] [CrossRef]
- González-Prior, J.; López-Fonseca, R.; Gutiérrez-Ortiz, J.I.I.; de Rivas, B. Oxidation of 1,2-dichloroethane over nanocube-shaped Co3O4 catalysts. Appl. Catal. B Environ. 2016, 199, 384–393. [Google Scholar] [CrossRef]
- Tang, W.; Weng, J.; Lu, X.; Wen, L.; Suburamanian, A.; Nam, C.-Y.Y.; Gao, P.-X.X. Alkali-metal poisoning effect of total CO and propane oxidation over Co3O4 nanocatalysts. Appl. Catal. B Environ. 2019, 256, 117859. [Google Scholar] [CrossRef]
- Genty, E.; Brunet, J.; Poupin, C.; Ojala, S.; Siffert, S.; Cousin, R. Influence of CO addition on the toluene total oxidation over Co based mixed oxide catalysts. Appl. Catal. B Environ. 2019, 247, 163–172. [Google Scholar] [CrossRef]
- Tsou, J.; Magnoux, P.; Guisnet, M.; Órfão, J.J.M.; Figueiredo, J.L. Oscillations in the catalytic oxidation of volatile organic compounds. J. Catal. 2004, 225, 147–154. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, X.; Jin, J.; Di, X.; Liang, C.; Liu, Z. Insight into catalytic properties of Co3O4-CeO2 binary oxides for propane total oxidation. Chin. J. Catal. 2020, 41, 679–690. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Xu, W.; Xu, Z.; Chen, J.; Jia, H.; Chen, J. Hydrolysis driving redox reaction to synthesize Mn-Fe binary oxides as highly active catalysts for the removal of toluene. Chem. Eng. J. 2017, 330, 281–293. [Google Scholar] [CrossRef]

| Catalysts | Co wt.% a | Na wt.% a | d (nm) b | a (Å) b | Strain (%) b | Sgeo (m2 g−1) c | SSA (m2 g−1) d | Vpore (cm3 g−1) d |
|---|---|---|---|---|---|---|---|---|
| Co-350S | 72.7 | 0.12 | 16 | 8.088 | 0.58 | 64 | 61 | 0.281 |
| Co-350D | 72.2 | 0.11 | 12 | 8.090 | 0.74 | 81 | 81 | 0.365 |
| Co-550S | 73.9 | 0.17 | 51 | 8.085 | 0.17 | 19 | 20 | 0.032 |
| Co-550D | 73.3 | 0.11 | 29 | 8.086 | 0.33 | 34 | 33 | 0.116 |
| Catalysts | Raman Spectra | CO2 Production in CO-TPR (mmol g−1) | |||
|---|---|---|---|---|---|
| Peak Position of A1g (cm−1) | FWHM of A1g | Peak Ratios of F12g/A1g | 100–300 °C | Total | |
| Co-350S | 675 | 24 | 0.073 | 3.3 | 16.2 |
| Co-350D | 667 | 31 | 0.099 | 4.7 | 16.1 |
| Co-550S | 688 | 11 | 0.036 | 1.5 | 16.7 |
| Co-550D | 670 | 25 | 0.089 | 2.0 | 17.0 |
| Catalyst | WHSV (mL h−1 g−1) | VOC Content (vol.%) | T50/T90 | Ref. | |
|---|---|---|---|---|---|
| Toluene oxidation | Co3O4-HA | 40,000 | 0.1 | 232/240 | [3] |
| Co3O4 microsphere | 60,000 | 0.05 | 243/248 | [4] | |
| Co3O4-cube | 48,000 | 0.1 | 240/248 | [5] | |
| Co3O4-S-160 | 60,000 | 0.05 | 236/250 | [6] | |
| Co3O4-0.01 | 15,000 | 0.1 | 217/226 | [7] | |
| 3D-Co3O4 | 48,000 | 0.1 | 229/238 | [8] | |
| Co3O4–PTA–L2 | 30,000 | 0.3 | 182/188 | [9] | |
| N-Co3O4-200 | 60,000 | 0.1 | 208/218 | [10] | |
| Co3−xO4−y | 72,000 | 0.03 | 171/180 | [11] | |
| Co-350D | 40,000 | 0.1 | 230/257 | This work | |
| Propane oxidation | Co3O4-C100–550 | 12,000 | 0.8 | 200/213 | [12] |
| C-350 | 12,000 | 0.8 | 210/235 | [13] | |
| CoDP | 120,000 | 0.1 | 195/225 | [14] | |
| Co3O4-H | 9990 | 1.0 | 209/239 | [15] | |
| Mic-Co3O4 | 120,000 | 0.8 | 262/374 | [16] | |
| Co-SAS 10% water | 15,000 | 0.5 | 175/200 | [17] | |
| Co3O4-AC | 240,000 | 0.3 | 237/250 | [18] | |
| Co4Zr1 | 60,000 | 0.2 | 210/242 | [19] | |
| Ca-Co3O4-Ac | 120,000 | 0.2 | 236/260 | [20] | |
| Co-350D | 40,000 | 0.1 | 180/201 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Descorme, C.; Valverde, J.L.; Giroir-Fendler, A. Effect of Calcination Conditions on Co3O4 Catalysts in the Total Oxidation of Toluene and Propane. Catalysts 2023, 13, 992. https://doi.org/10.3390/catal13060992
Zhang W, Descorme C, Valverde JL, Giroir-Fendler A. Effect of Calcination Conditions on Co3O4 Catalysts in the Total Oxidation of Toluene and Propane. Catalysts. 2023; 13(6):992. https://doi.org/10.3390/catal13060992
Chicago/Turabian StyleZhang, Weidong, Claude Descorme, Jose Luis Valverde, and Anne Giroir-Fendler. 2023. "Effect of Calcination Conditions on Co3O4 Catalysts in the Total Oxidation of Toluene and Propane" Catalysts 13, no. 6: 992. https://doi.org/10.3390/catal13060992