Exsolved Nanoparticles Decorated Double Perovskites as High-Performance Anodes for Direct-Ammonia Solid Oxide Fuel Cells
Abstract
:1. Introduction
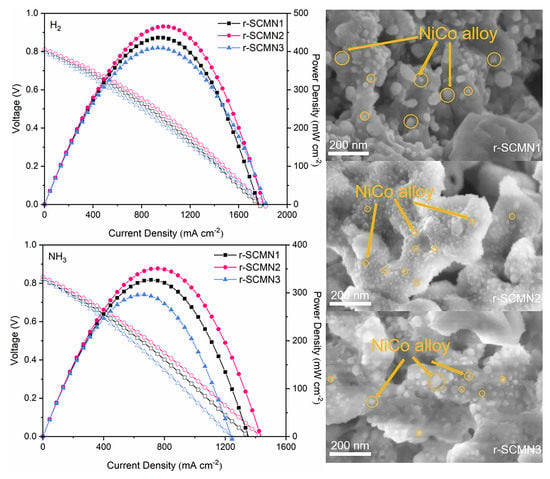
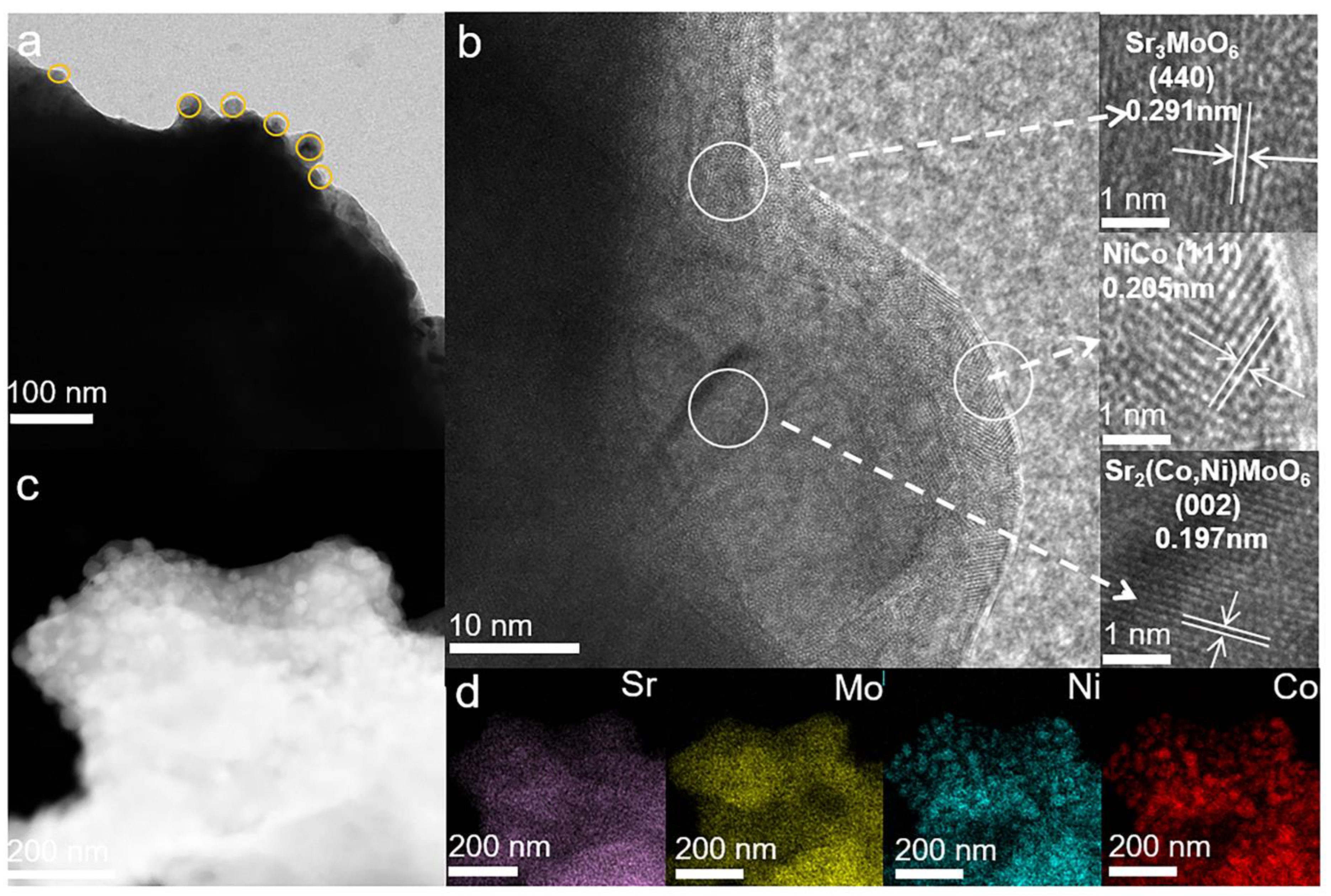
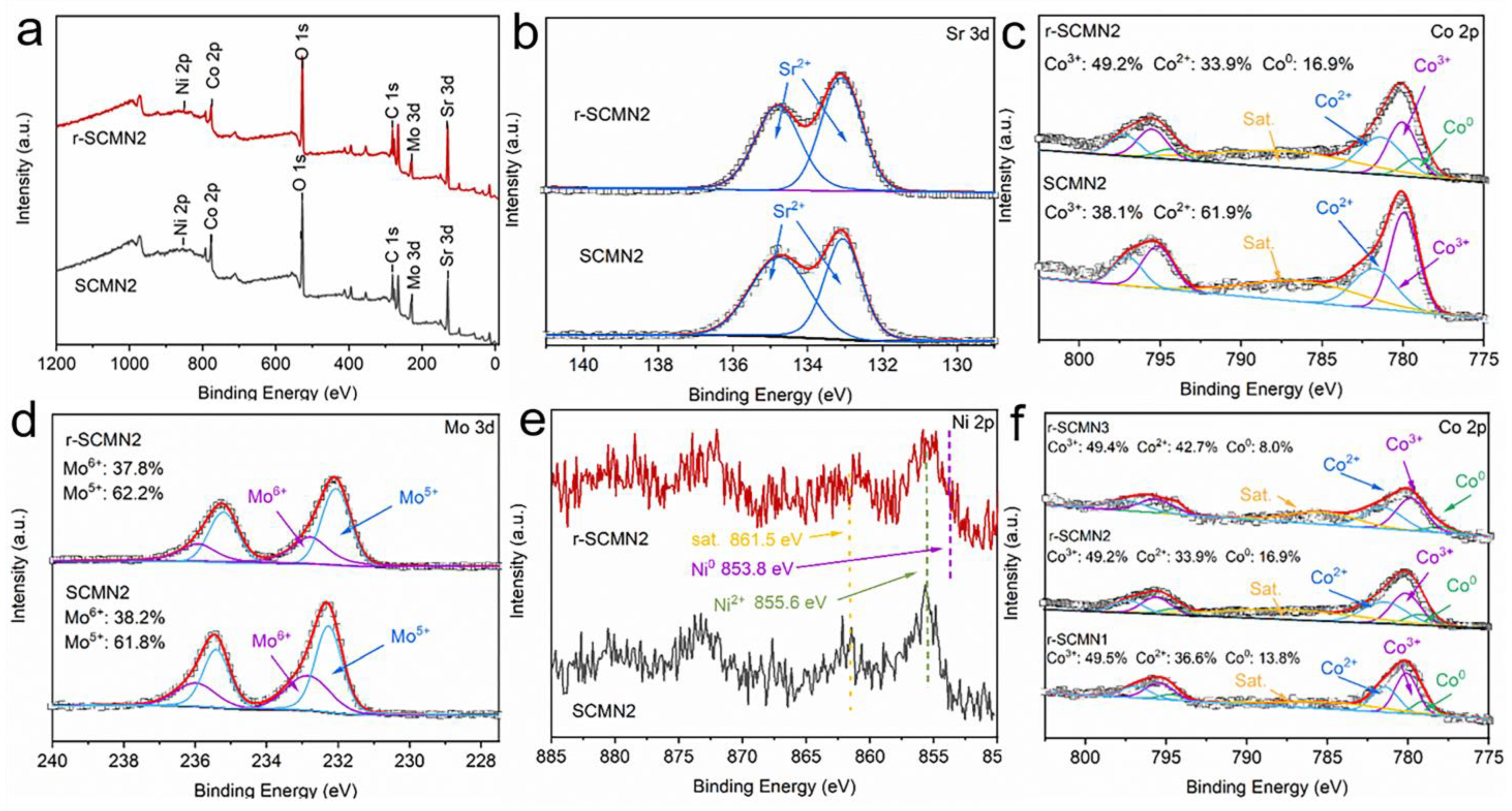
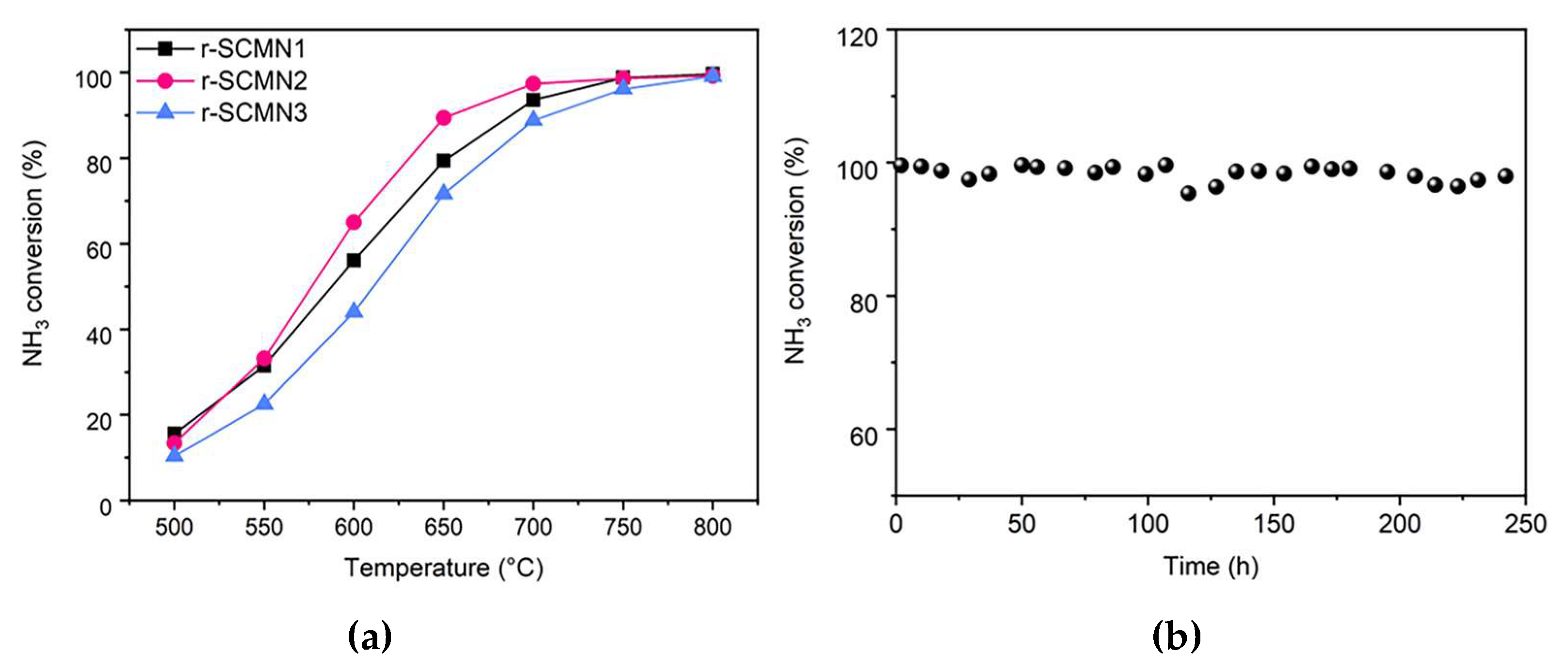
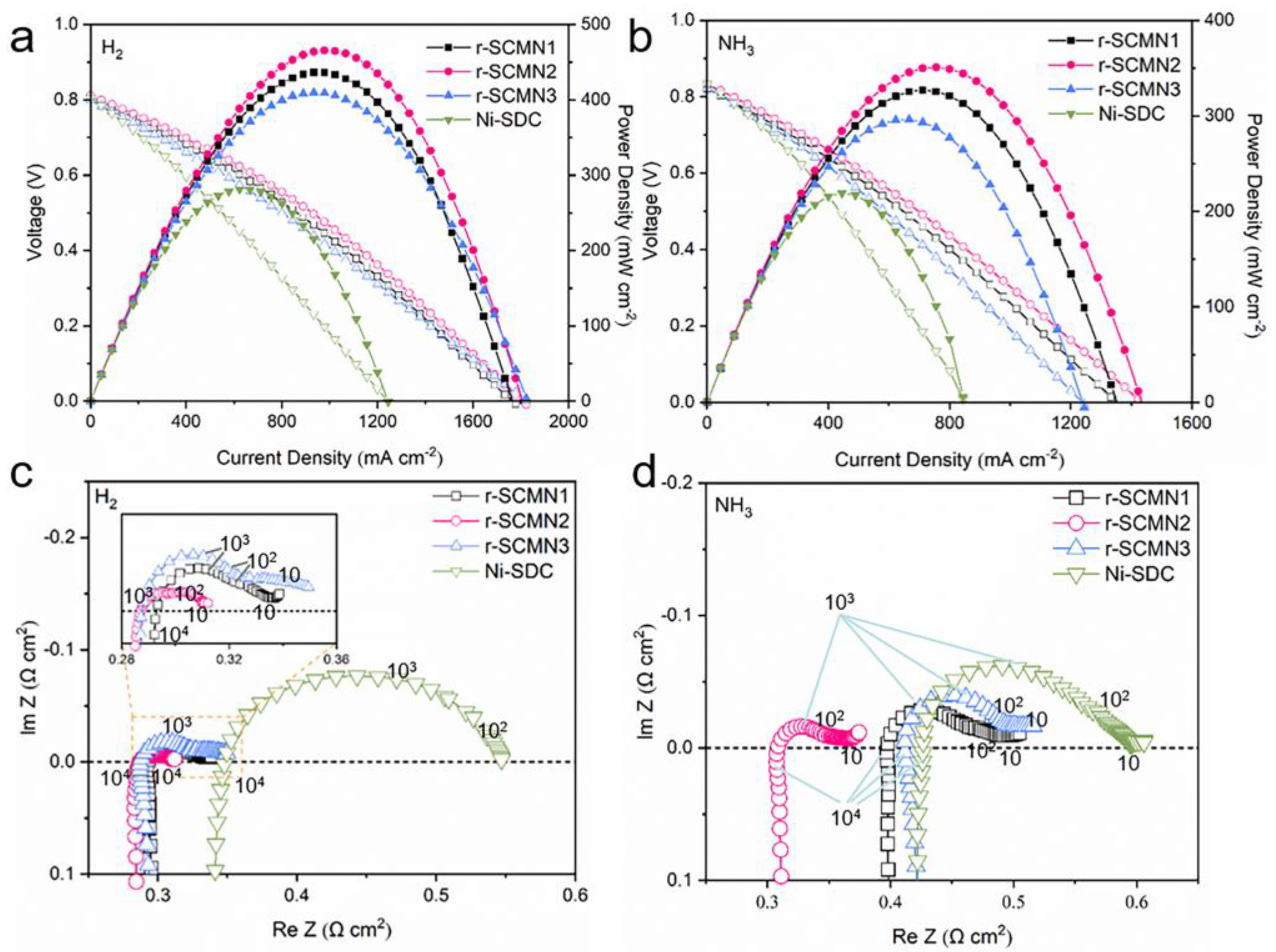
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis and Fabrication
3.2. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seo, H.G.; Kim, D.H.; Seo, J.; Jeong, S.J.; Kim, J.; Tuller, H.L.; Son, J.W.; Jung, W. High-Performance and Durable Fuel Cells Using Co/Sr-Free Fluorite-Based Mixed Conducting (Pr,Ce)O2−δ Cathode. Adv. Energy Mater. 2022, 12, 2202101. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, E.; Chen, Y.; Liu, Y.; Zhang, L.; Zhang, W.; Luo, Z.; Kane, N.; Zhao, B.; Soule, L.; et al. An Active and Robust Air Electrode for Reversible Protonic Ceramic Electrochemical Cells. ACS Energy Lett. 2021, 6, 1511–1520. [Google Scholar] [CrossRef]
- Teketel, B.S.; Beshiwork, B.A.; Tian, D.; Zhu, S.; Desta, H.G.; Kashif, K.; Chen, Y.; Lin, B. Promoted Performance of Layered Perovskite PrBaFe2O5+δ Cathode for Protonic Ceramic Fuel Cells by Zn Doping. Catalysts 2022, 12, 488. [Google Scholar] [CrossRef]
- Sun, M.; He, Q.; Kuang, X.; Zhang, Q.; Ye, S.; Huang, B. Probing Oxide-Ion Conduction in Low-Temperature SOFCs. Nano Energy 2018, 50, 88–96. [Google Scholar] [CrossRef]
- Sarner, S.; Schreiber, A.; Menzler, N.H.; Guillon, O. Recycling Strategies for Solid Oxide Cells. Adv. Energy Mater. 2022, 12, 2201805. [Google Scholar] [CrossRef]
- Sravani, B.; Reddy, Y.V.M.; Park, J.P.; Venu, M.; Sarma, L.S. Design of Bimetallic PtFe-Based Reduced Graphene Oxide as Efficient Catalyst for Oxidation Reduction Reaction. Catalysts 2022, 12, 1528. [Google Scholar] [CrossRef]
- Subotić, V.; Hochenauer, C. Analysis of Solid Oxide Fuel and Electrolysis Cells Operated in A Real-System Environment: State-of-the-Health Diagnostic, Failure Modes, Degradation Mitigation and Performance Regeneration. Prog. Energy Combust. Sci. 2022, 93, 101011. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, W.; Kane, N.; Luo, Z.; Pei, K.; Sasaki, K.; Choi, Y.; Chen, Y.; Ding, D.; Liu, M. An Efficient Bifunctional Air Electrode for Reversible Protonic Ceramic Electrochemical Cells. Adv. Funct. Mater. 2021, 31, 2105386. [Google Scholar] [CrossRef]
- Yi, Y.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Perovskite-Based Nanocomposites as High-Performance Air Electrodes for Protonic Ceramic Cells. Curr. Opin. Green Sustain. Chem. 2022, 38, 100711. [Google Scholar] [CrossRef]
- Dhongde, V.; Singh, A.; Kala, J.; Anjum, U.; Haider, M.A. Suddhasatwa Basu, Radio-Frequency Magnetron Sputtered Thin-Film La0.5Sr0.5Co0.95Nb0.05O3−δ Perovskite Electrodes for Intermediate Temperature Symmetric Solid Oxide Fuel Cell (IT-SSOFC). Mater. Rep. Energy 2022, 2, 100095. [Google Scholar]
- Du, N.; Roy, C.; Peach, R.; Turnbull, M.; Thiele, S.; Bock, C. Anion-exchange Membrane Water Electrolyzers. Chem. Rev. 2022, 122, 11830–11895. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Liu, J.; Luo, W.; Fan, Y.; Zhang, J.; Luo, J.L.; Fu, X.Z. Unraveling the Enhanced Kinetics of Sr2Fe1+xMo1−xO6−δ Electrocatalysts for High-Performance Solid Oxide Cells. Adv. Energy Mater. 2021, 11, 2102845. [Google Scholar] [CrossRef]
- Pan, J.; Ye, Y.; Zhou, M.; Sun, X.; Ling, Y.; Yashiro, K.; Chen, Y. Improving the Activity and Stability of Ni-Based Electrodes for Solid Oxide Cells through Surface Engineering: Recent Progress and Future Perspectives. Mater. Rep. Energy 2021, 1, 100025. [Google Scholar] [CrossRef]
- Afif, A.; Radenahmad, N.; Cheok, Q.; Shams, S.; Kim, J.H.; Azad, A.K. Ammonia-Fed Fuel Cells: A Comprehensive Review. Renew. Sustain. Energy Rev. 2016, 60, 822–835. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Wang, W.; Zhou, C.; Zhong, Y.; Yang, G.; Zhou, W.; Liu, M.; Shao, Z. Self-assembled Triple-Conducting Nanocomposite as a Superior Protonic Ceramic Fuel Cell Cathode. Joule 2019, 3, 2842–2853. [Google Scholar] [CrossRef]
- Wang, Q.; Ricote, S.; Wang, Y.; Hendriksen, P.V.; Wang, J.; Chen, M. Ba0.5Gd0.8La0.7Co2O6−δ Infiltrated BaZr0.8Y0.2O3−δ Composite Oxygen Electrodes for Protonic Ceramic Cells. J. Electrochem. Soc. 2022, 169, 014513. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Xu, M.; Wang, W.; Zhang, Y.; Yang, G.; Ran, R.; Zhou, W.; Shao, Z. A Cobalt-Free Multi-Phase Nanocomposite as Near-ideal Cathode of Intermediate-Temperature Solid Oxide Fuel Cells Developed by Smart Self-Assembly. Adv. Mater. 2020, 32, 1906979. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, D.; Zhu, Y.; Liang, M.; Yang, M.; Yang, G.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Robust Bifunctional Phosphorus-Doped Perovskite Oxygen Electrode for Reversible Proton Ceramic Electrochemical Cells. Chem. Eng. J. 2022, 450, 137787. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in Material Selection for Solid Oxide Fuel Cell Technology: A Review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Li, M.; Dong, J.; Chen, Z.; Huang, K.; Xiong, K.; Li, R.; Rao, M.; Chen, C.; Ling, Y.; Lin, B. Excessive Na-Doped La0.75Sr0.25Cr0.5Fe0.4Cu0.1O3−δ Perovskite as an Additional Internal Reforming Catalyst for Direct Carbon Dioxide-Ethanol Solid Oxide Fuel Cells. Catalysts 2022, 12, 1600. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, X.; Zhou, Y.; Luo, Z.; Nam, G.; Ding, Y.; Li, T.; Liu, Z.; Ahn, Y.; Kane, N.; et al. A Solid Oxide Fuel Cell Runs on Hydrocarbon Fuels with Exceptional Durability and Power Output. Adv. Energy Mater. 2022, 12, 2202928. [Google Scholar] [CrossRef]
- Song, Y.; Chen, J.; Yang, M.; Xu, M.; Liu, D.; Liang, M.; Wang, Y.; Ran, R.; Wang, W.; Ciucci, F.; et al. Realizing Simultaneous Detrimental Reactions Suppression and Multiple Benefits Generation from Nickel Doping Toward Improved Protonic Ceramic Fuel Cell Performance. Small 2022, 18, 2200450. [Google Scholar] [CrossRef] [PubMed]
- Zvonareva, I.; Fu, X.; Medvedev, D.; Shao, Z. Electrochemistry and Energy Conversion Features of Protonic Ceramic Cells with Mixed Ionic-Electronic Electrolytes. Energy Environ. Sci. 2022, 15, 439–465. [Google Scholar] [CrossRef]
- Cao, J.; Ji, Y.; Shao, Z. Perovskites for Protonic Ceramic Fuel Cells: A review. Energy Environ. Sci. 2022, 15, 2200–2232. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, S.; Yin, Y.; Bi, L. Taking Advantage of Li-Evaporation in LiCoO2 as Cathode for Proton-Conducting Solid Oxide Fuel Cells. J. Adv. Ceram. 2022, 11, 1849–1859. [Google Scholar] [CrossRef]
- Jo, M.; Bae, H.; Park, K.; Hamayun, M.A.; Park, G.; Kim, J.H.; Lee, K.T.; Lee, K.; Song, S.; Park, J. Layered Barium Cobaltite Structure Materials Containing Perovskite and CdI2-Based Layers for Reversible Solid Oxide Cells with Exceptionally High Performance. Chem. Eng. J. 2023, 451, 138954. [Google Scholar] [CrossRef]
- Park, K.; Bae, H.; Kim, H.K.; Choi, I.G.; Jo, M.; Park, G.M.; Asif, M.; Bhardwaj, A.; Lee, K.S.; Kim, Y.C.; et al. Understanding the Highly Electrocatalytic Active Mixed Triple Conducting NaxCa3−xCo4O9−δ Oxygen Electrode Materials. Adv. Energy Mater. 2023, 13, 2202999. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, Z.; Song, Y.; Yang, G.; Qian, W.; Yang, M.; Zhu, Y.; Ran, R.; Wang, W.; Zhou, W.; et al. High-Entropy Perovskite Oxide: A New Opportunity for Developing Highly Active and Durable Air Electrode for Reversible Protonic Ceramic Electrochemical Cells. Nano-Micro Lett. 2022, 14, 217. [Google Scholar] [CrossRef]
- Chen, L.; Xu, J.; Wang, X.; Xie, K. Sr2Fe1.5+xMo0.5O6−δ Cathode with Exsolved Fe Nanoparticles for Enhanced CO2 Electrolysis. Int. J. Hydrogen Energy 2020, 45, 11901–11907. [Google Scholar] [CrossRef]
- Liu, C.; Li, S.; Gao, J.; Bian, L.; Hou, Y.; Wang, L.; Peng, J.; Bao, J.; Song, X.; An, S. Enhancing CO2 Catalytic Adsorption on an Fe Nanoparticle-Decorated LaSrFeO4+δ Cathode for CO2 Electrolysis. ACS Appl. Mater. Interfaces 2021, 13, 8229–8238. [Google Scholar] [CrossRef]
- Akimoto, W.; Fujimoto, T.; Saito, M.; Inaba, M.; Yoshida, H.; Inagaki, T. Ni-Fe/Sm-doped CeO2 Anode for Ammonia-Fueled Solid Oxide Fuel Cells. Solid State Ion. 2014, 256, 1–4. [Google Scholar] [CrossRef]
- Weissenberger, T.; Zapf, R.; Pennemann, H.; Kolb, G. Effect of the Active Metal on the NOx Formation during Catalytic Combustion of Ammonia SOFC Off-Gas. Catalysts 2022, 12, 1186. [Google Scholar] [CrossRef]
- Li, H.; Song, Y.; Xu, M.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Exsolved Alloy Nanoparticles Decorated Ruddlesden-Popper Perovskite as Sulfur-Tolerant Anodes for Solid Oxide Fuel Cells. Energy Fuels 2020, 34, 11449–11457. [Google Scholar] [CrossRef]
- Miyazaki, K.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. Development of Ni-Ba(Zr,Y)O3 Cermet Anodes for Direct Ammonia-Fueled Solid Oxide Fuel Cells. J. Power Sources 2017, 365, 148–154. [Google Scholar] [CrossRef]
- Wan, Z.; Tao, Y.; Shao, J.; Zhang, Y.; You, H. Ammonia as an Effective Hydrogen Carrier and a Clean Fuel for Solid Oxide Fuel Cells. Energy Convers. Manag. 2021, 228, 113729. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.; Pei, K.; Pan, Y.; Xu, K.; Ding, Y.; Zhao, B.; Sasaki, K.; Choi, Y.; Chen, Y.; et al. An Efficient and Durable Anode for Ammonia Protonic Ceramic Fuel Cells. Energy Environ. Sci. 2022, 15, 287. [Google Scholar] [CrossRef]
- Li, Y.; Pillai, H.S.; Wang, T.; Hwang, S.; Zhao, Y.; Qiao, Z.; Mu, Q.; Karakalos, S.; Chen, M.; Yang, J.; et al. High-Performance Ammonia Oxidation Catalysts for Anion-Exchange Membrane Direct Ammonia Fuel Cells. Energy Environ. Sci. 2021, 14, 1449–1460. [Google Scholar] [CrossRef]
- Du, Z.; Zhao, H.; Li, S.; Zhang, Y.; Chang, X.; Xia, Q.; Chen, N.; Gu, L.; Świerczek, K.; Li, Y.; et al. Exceptionally High Performance Anode Material Based on Lattice Structure Decorated Double Perovskite Sr2FeMo2/3Mg1/3O6−δ for Solid Oxide Fuel Cells. Adv. Energy Mater. 2018, 8, 1800062. [Google Scholar] [CrossRef]
- Jacobs, R.; Mayeshiba, T.; Booske, J.; Morgan, D. Material Discovery and Design Principles for Stable, High Activity Perovskite Cathodes for Solid Oxide Fuel Cells. Adv. Energy Mater. 2018, 8, 1702708. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Du, Z.; Zhang, Y.; Dong, X.; Zhao, H. Medium-Entropy Perovskites Sr(FeαTiβCoγMnζ)O3−δ as Promising Cathodes for Intermediate Temperature Solid Oxide Fuel Cell. Appl. Catal. B 2021, 295, 120264. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Guan, D.; Xu, M.; Ran, R.; Ni, M.; Zhou, W.; O’Hayre, R.; Shao, Z. Thermal-Expansion Offset for High-Performance Fuel Cell Cathodes. Nature 2021, 591, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Cavazzani, J.; Squizzato, E.; Brusamarello, E.; Glisenti, A. Exsolution in Ni-Doped Lanthanum Strontium Titanate: A Perovskite-Based Material for Anode Application in Ammonia-Fed Solid Oxide Fuel Cell. Int. J. Hydrogen Energy 2022, 47, 13921–13932. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Hua, B.; Behnamian, Y.; Li, J.; Cui, S.; Li, J.; Luo, J. Molybdenum Doped Pr0.5Ba0.5MnO3−δ (Mo-PBMO) Double Perovskite as a Potential Solid Oxide Fuel Cell Anode Material. J. Power Sources 2016, 301, 237–241. [Google Scholar] [CrossRef]
- Rathore, S.S.; Biswas, S.; Fini, D.; Kulkarni, A.P.; Giddey, S. Direct Ammonia Solid-Oxide Fuel Cells: A Review of Progress and Prospects. Int. J. Hydrogen Energy 2021, 46, 35365–35384. [Google Scholar] [CrossRef]
- Song, Y.; Li, H.; Xu, M.; Yang, G.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Infiltrated NiCo Alloy Nanoparticle Decorated Perovskite Oxide: A Highly Active, Stable, and Antisintering Anode for Direct-Ammonia Solid Oxide Fuel Cells. Small 2020, 16, 2001859. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, J.; Huang, X.; Zou, D.; Song, Y.; Xu, M.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Slightly Ruthenium Doping Enables Better Alloy Nanoparticle Exsolution of Perovskite Anode for High-Performance Direct-Ammonia Solid Oxide Fuel Cells. J. Mater. Sci. Technol. 2022, 125, 51–58. [Google Scholar] [CrossRef]
- Du, Z.; Zhao, H.; Yi, S.; Xia, Q.; Gong, Y.; Zhang, Y.; Cheng, X.; Li, Y.; Gu, L.; Świerczek, K. High-Performance Anode Material Sr2FeMo0.65Ni0.35O6−δ with in Situ Exsolved Nanoparticle Catalyst. ACS Nano 2016, 10, 8660–8669. [Google Scholar] [CrossRef]
- Huang, Y.H.; Dass, R.I.; Xing, Z.L.; Goodenough, J.B. Double Perovskites as Anode Materials for Solid-Oxide Fuel Cells. Science 2006, 312, 254–257. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, L.; Sun, W.; Ren, R.; Yang, X.; Ma, M.; Qiao, J.; Wang, Z.; Zhen, S.; Sun, K. Co-Improving the Electrocatalytic Performance and H2S Tolerance of a Sr2Fe1.5Mo0.5O6−δ Based Anode for Solid Oxide Fuel Cells. J. Mater. Chem. A 2022, 10, 16280–16289. [Google Scholar] [CrossRef]
- Han, Z.; Dong, H.; Wu, Y.; Yang, Y. Locating the Rate-Limiting Step of Hydrogen Conversion on Sr2Fe1.5Mo0.5O6 (001) Surface: Implications for Efficient SOFC Anode Design. Appl. Surf. Sci. 2022, 595, 153513. [Google Scholar] [CrossRef]
- Osinkin, D.A.; Beresnev, S.M.; Bogdanovich, N.M. Influence of Pr6O11 on Oxygen Electroreduction Kinetics and Electrochemical Performance of Sr2Fe1.5Mo0.5O6−δ Based Cathode. J. Power Sources 2018, 392, 41–47. [Google Scholar] [CrossRef]
- Hou, S.; Alonso, J.A.; Goodenough, J.B. Co-Free, Iron Perovskites as Cathode Materials for Intermediate-Temperature Solid Oxide Fuel Cells. J. Power Sources 2010, 195, 280–284. [Google Scholar] [CrossRef]
- Ge, X.; Chan, S.; Liu, Q.; Sun, Q. Solid Oxide Fuel Cell Anode Materials for Direct Hydrocarbon Utilization. Adv. Energy Mater. 2012, 2, 1156–1181. [Google Scholar] [CrossRef]
- Aguadero, A.; Alonso, J.A.; Martínez-Coronado, R.; Martínez-Lope, M.J.; Fernández-Díaz, M.T. Evaluation of Sr2CoMoO6−δ as Anode Material in Solid-Oxide Fuel Cells: A Neutron Diffraction Study. J. Appl. Phys. 2011, 109, 034907. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Zhong, Y.; Xu, X.; Veder, J.M.; Rowles, M.R.; Saunders, M.; Ran, R.; Shao, Z. Activation-Free Supercapacitor Electrode Based on Surface-Modified Sr2CoMo1−xNixO6−δ Perovskite. Chem. Eng. J. 2020, 390, 124645. [Google Scholar] [CrossRef]
- McCarthy, G.J.; Clarence, E.G. Compound Formation in the System Sr Mo O. J. Inorg. Nucl. Chem. 1973, 35, 2669–2672. [Google Scholar] [CrossRef]
- Kouno, S.; Shirakawa, N.; Nagai, I.; Umeyama, N.; Tokiwa, K.; Watanabe, T. The Synthesis and Characterization of Double-Layered Perovskite Sr3Mo2O7. J. Phys. Soc. Jpn. 2007, 76, 094706. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, G.; Dai, Z.; Zhang, L.; Ma, Y.J. Photocatalytic and Luminescent Properties of SrMoO4 Phosphors Prepared via Hydrothermal Method with Different Stirring Speeds. J. Mater. Sci. Technol. 2017, 33, 23–29. [Google Scholar] [CrossRef]
- Zhu, T.; Troiani, H.E.; Mogni, L.V.; Han, M.; Barnett, S.A. Ni-Substituted Sr(Ti,Fe)O3 SOFC Anodes: Achieving High Performance Via Metal Alloy Nanoparticle Exsolution. Joule 2018, 2, 478–496. [Google Scholar] [CrossRef] [Green Version]
- Atuchin, V.V.; Kesler, V.G.; Zaitsev, A.I.; Molokeev, M.S.; Aleksandrovsky, A.S.; Kuzubov, A.A.; Ignatova, N.Y. Electronic Structure of α-SrB4O7: Experiment and Theory. J. Phys. Condens. Matter 2013, 25, 085503. [Google Scholar] [CrossRef]
- Hengne, A.M.; Samal, A.K.; Enakonda, L.R.; Harb, M.; Gevers, L.E.; Anjum, D.H.; Hedhili, M.N.; Saih, Y.; Huang, K.; Basset, J. Ni-Sn-Supported ZrO2 Catalysts Modified by Indium for Selective CO2 Hydrogenation to Methanol. ACS Omega 2018, 3, 3688–3701. [Google Scholar] [CrossRef] [Green Version]
- Hashinokuchi, M.; Yokochi, R.; Akimoto, W.; Doi, T.; Inaba, M.; Kugai, J. Mechanism and Activity of Ni-Based (Ni-M: M = Fe, Mo, W, Ta) Cermet Anodes for Ammonia Oxidation in SOFCs. ECS Trans. 2015, 68, 2739–2744. [Google Scholar] [CrossRef]
- Zhong, F.; Wang, X.; Wang, L.; Fang, H.; Luo, Y.; Chen, C.; Lin, L.; Wang, D.; Chen, K.; Jiang, L. Tuning Geometry Distortion of Pyrochlore RE2Zr1.95Ni0.05O7+δ Anodes with Rich Oxygen Vacancies for Ammonia-Fed Solid Oxide Fuel Cell. Sep. Purif. Technol. 2023, 312, 123397. [Google Scholar] [CrossRef]
- Fournier, G.G.M.; Cumming, I.W.; Hellgardt, K. High Performance Direct Ammonia Solid Oxide Fuel Cell. J. Power Sources 2006, 162, 198–206. [Google Scholar] [CrossRef]
- Yang, J.; Molouk, A.F.S.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguch, K. Electrochemical and Catalytic Properties of Ni/BaCe0.75Y0.25O3−δ Anode for Direct Ammonia-Fueled Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2015, 7, 7406–7412. [Google Scholar] [CrossRef]
- Kang, B.S.; Matsuda, J.; Ju, Y.W.; Kim, H.H.; Ishihara, T. Nano Strain Induced Double Columnar Oxide as Highly Active Oxygen-Dissociation Electrode for Ni-Fe Metal Supported Solid Oxide Fuel Cells. Nano Energy 2019, 56, 382–390. [Google Scholar] [CrossRef]
- Liu, K.; Lu, F.; Jia, X.; He, H.; Su, J.; Cai, B. A High Performance Thermal Expansion Offset Composite Cathode for IT-SOFCS. J. Mater. Chem. A 2022, 10, 24410–24421. [Google Scholar] [CrossRef]
- Zou, D.; Yi, Y.; Song, Y.; Guan, D.; Xu, M.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. The BaCe0.16Y0.04Fe0.8O3−δ Nanocomposite: A New High-Performance Cobalt-Free Triple-Conducting Cathode for Protonic Ceramic Fuel Cells Operating at Reduced Temperatures. J. Mater. Chem. A 2022, 10, 5381–5390. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Y.; Chen, J.; Xu, M.; Yang, G.; Ran, R.; Zhou, W.; Wang, W.; Shao, Z. Exsolved Nanoparticles Decorated Double Perovskites as High-Performance Anodes for Direct-Ammonia Solid Oxide Fuel Cells. Catalysts 2023, 13, 996. https://doi.org/10.3390/catal13060996
Yi Y, Chen J, Xu M, Yang G, Ran R, Zhou W, Wang W, Shao Z. Exsolved Nanoparticles Decorated Double Perovskites as High-Performance Anodes for Direct-Ammonia Solid Oxide Fuel Cells. Catalysts. 2023; 13(6):996. https://doi.org/10.3390/catal13060996
Chicago/Turabian StyleYi, Yongning, Jiaming Chen, Meigui Xu, Guangming Yang, Ran Ran, Wei Zhou, Wei Wang, and Zongping Shao. 2023. "Exsolved Nanoparticles Decorated Double Perovskites as High-Performance Anodes for Direct-Ammonia Solid Oxide Fuel Cells" Catalysts 13, no. 6: 996. https://doi.org/10.3390/catal13060996