Chiral Porous Organic Frameworks: Synthesis, Chiroptical Properties, and Asymmetric Organocatalytic Applications
Abstract
:1. Introduction
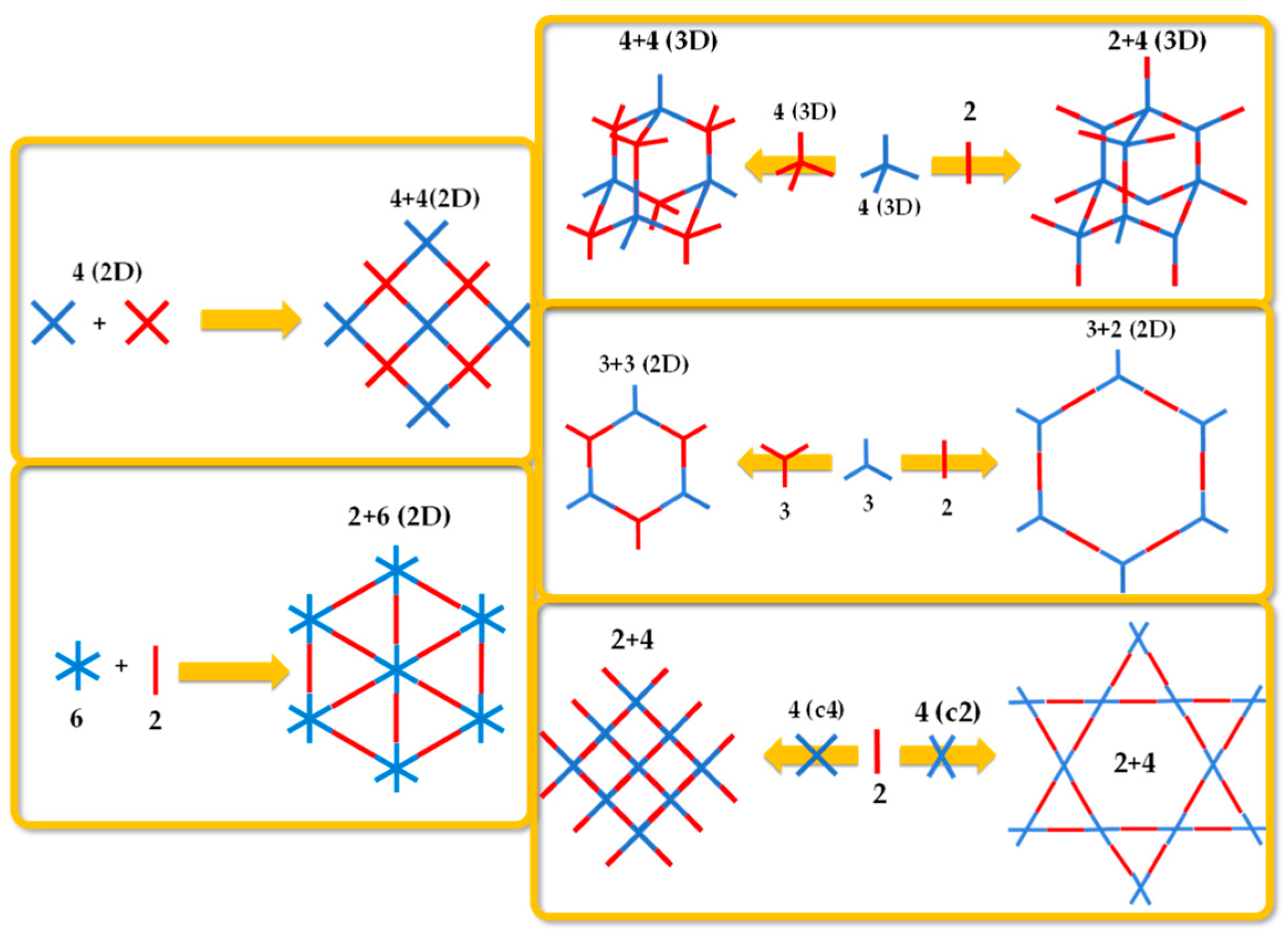
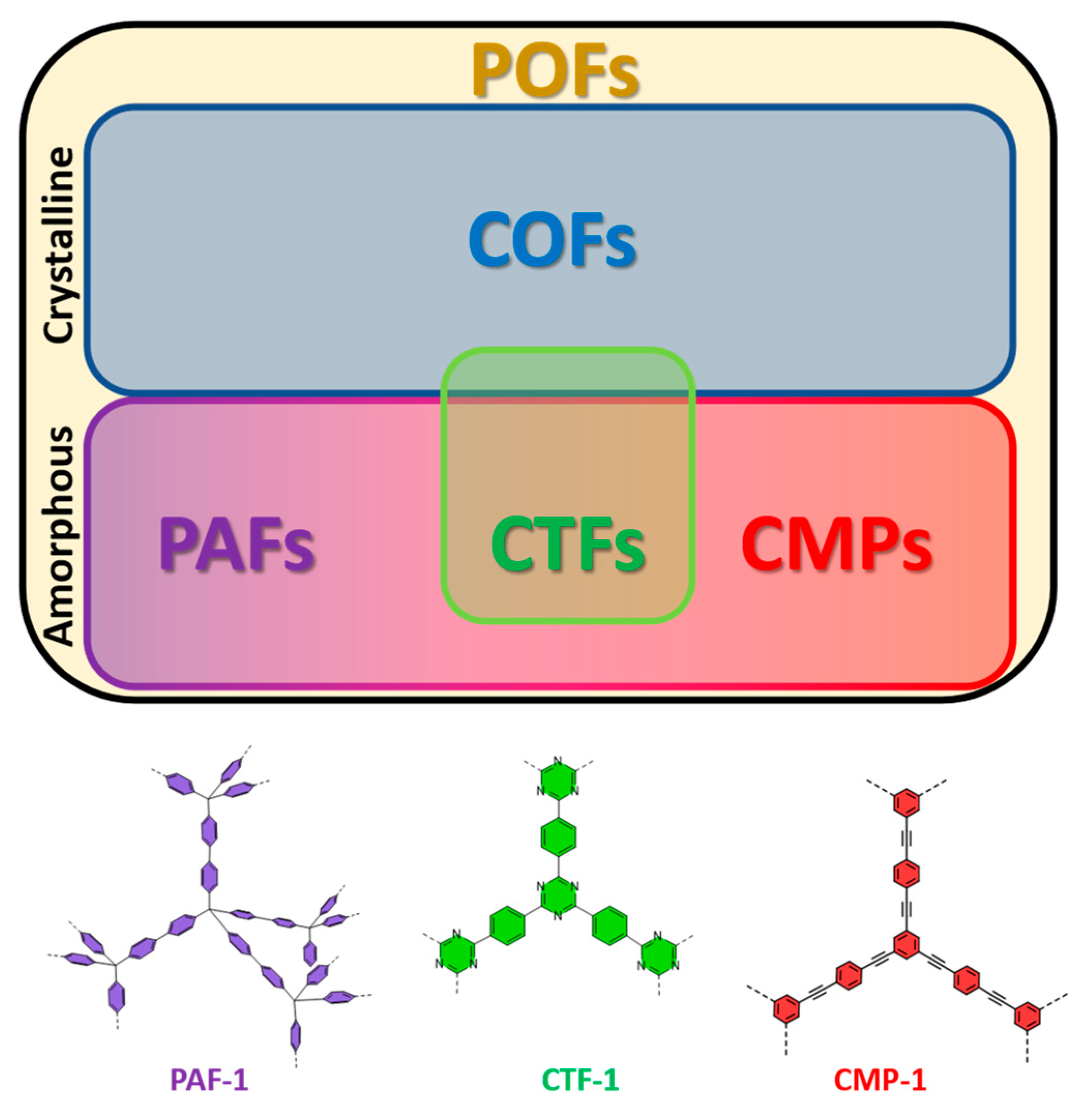
2. Critical Considerations on the Classification of Porous Organic Frameworks
3. Introduction of Chirality into Porous Organic Frameworks
- (i)
- Synthesis using chiral building blocks;
- (ii)
- Post-synthetic modification;
- (iii)
- Asymmetric synthesis;
- (iv)
- External chiral induction.
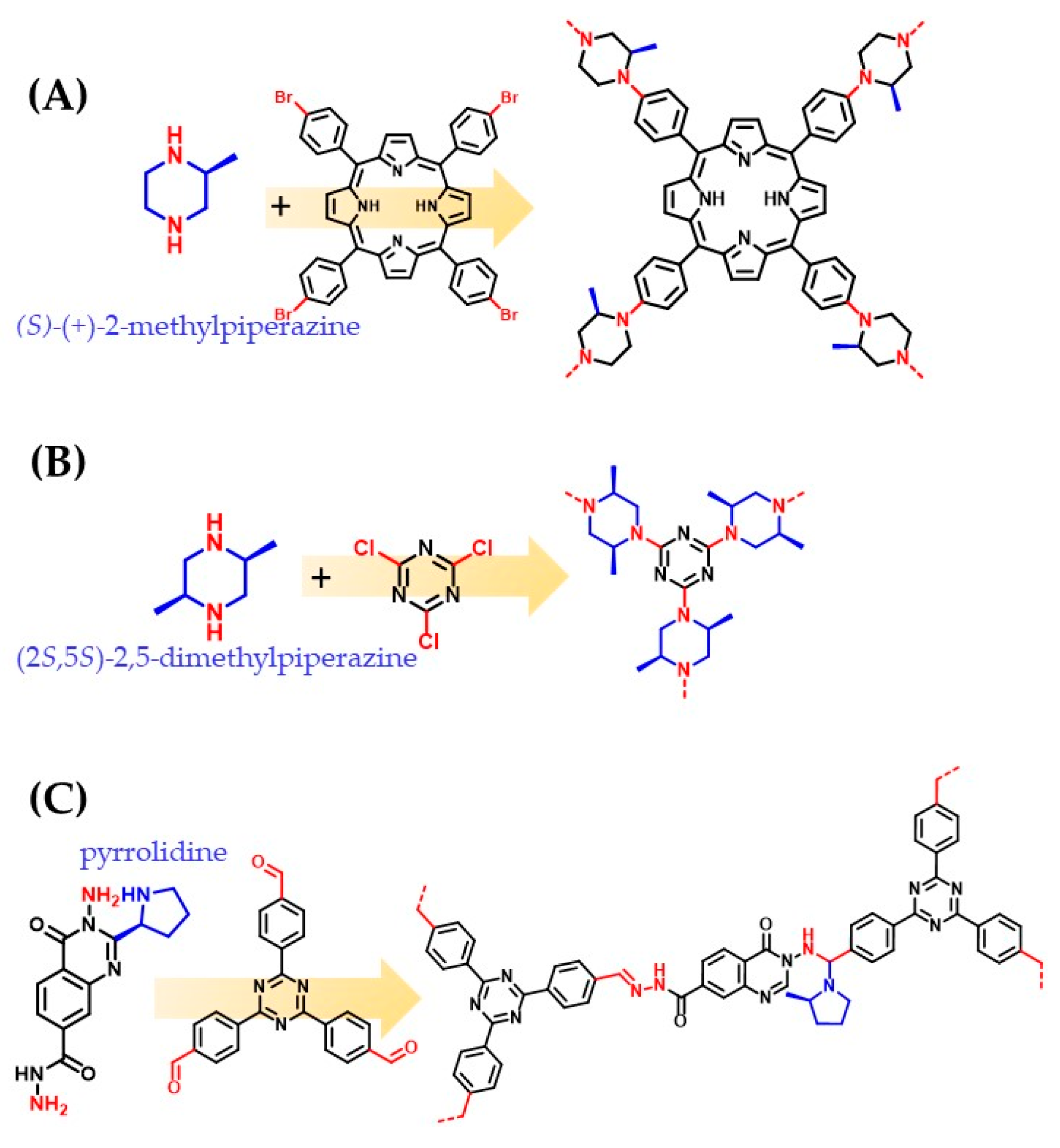
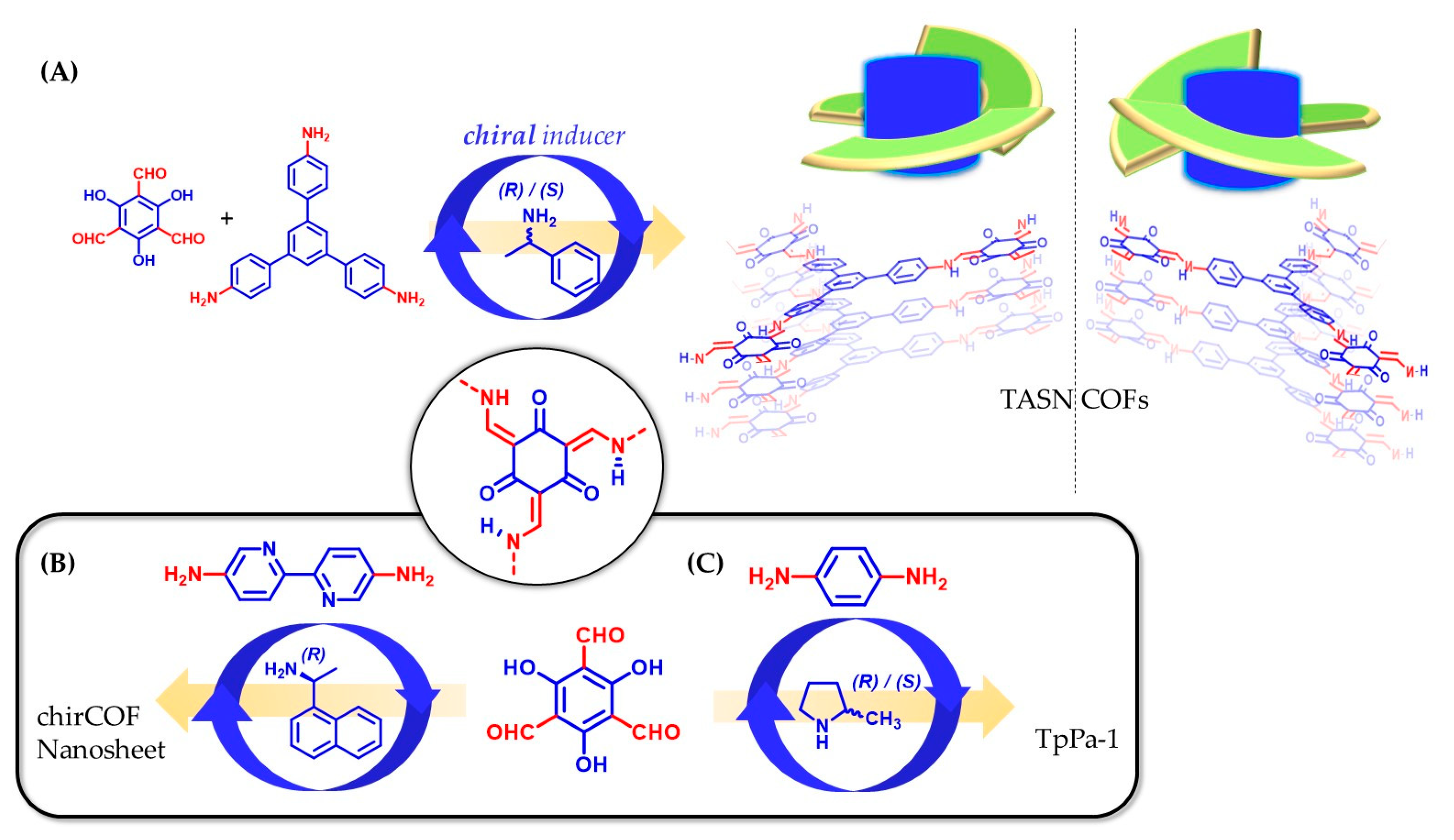
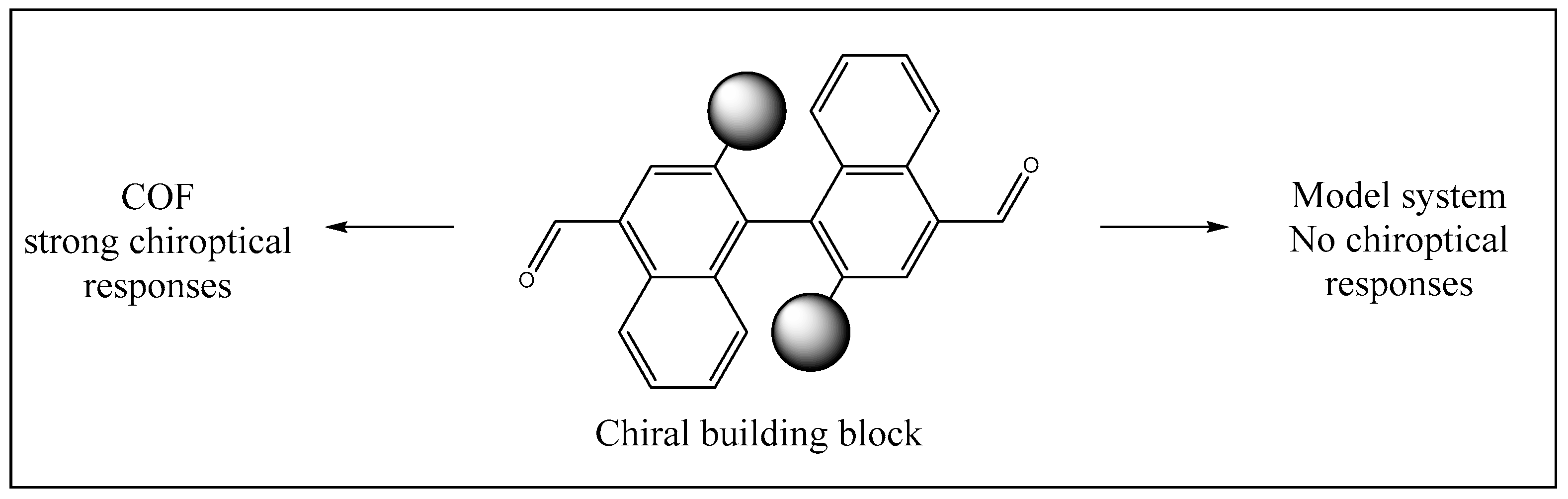
3.1. Chiral Building Blocks
3.2. Post-Synthetic Modification with Chiral Moieties
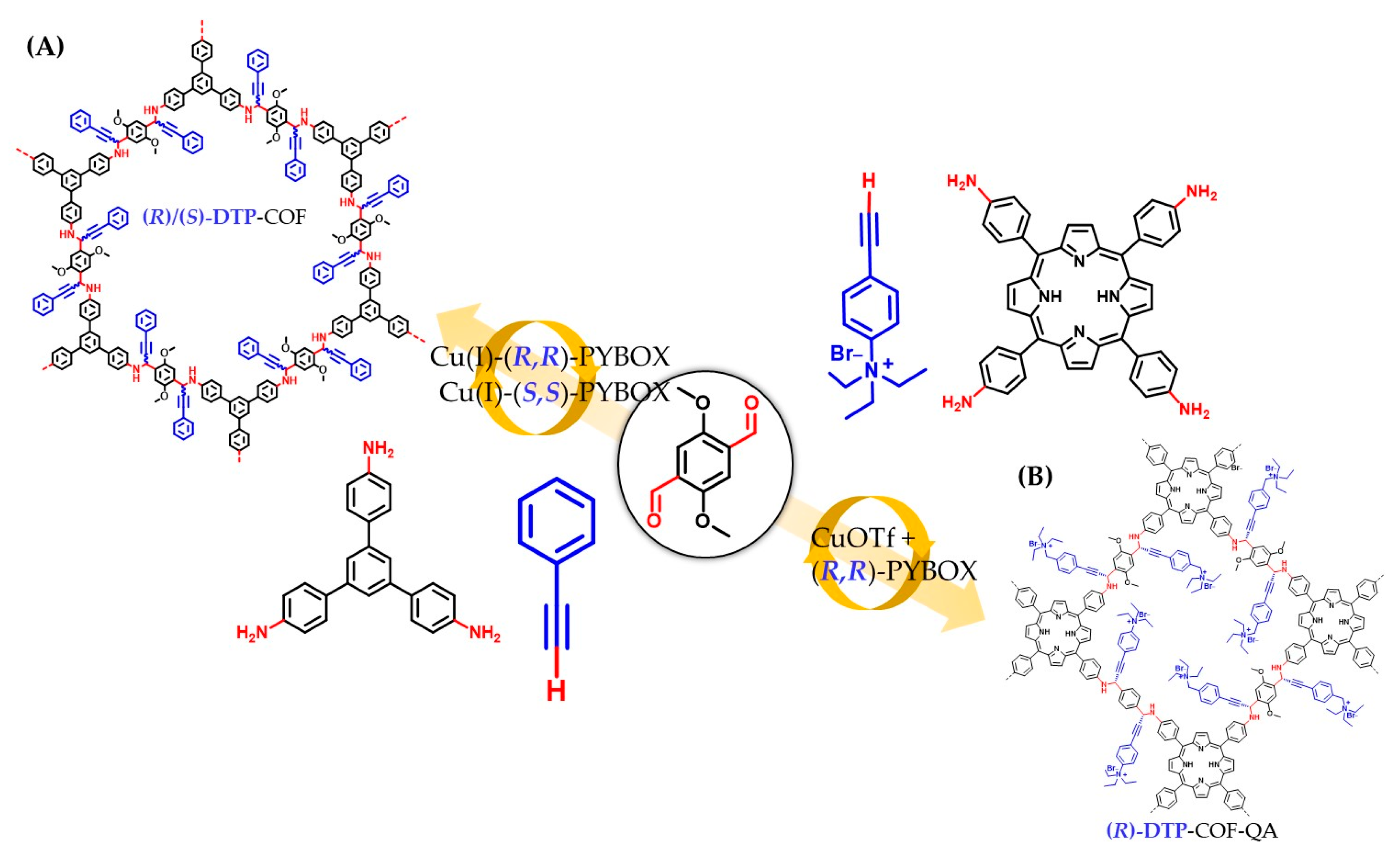
3.3. Asymmetric Catalysis
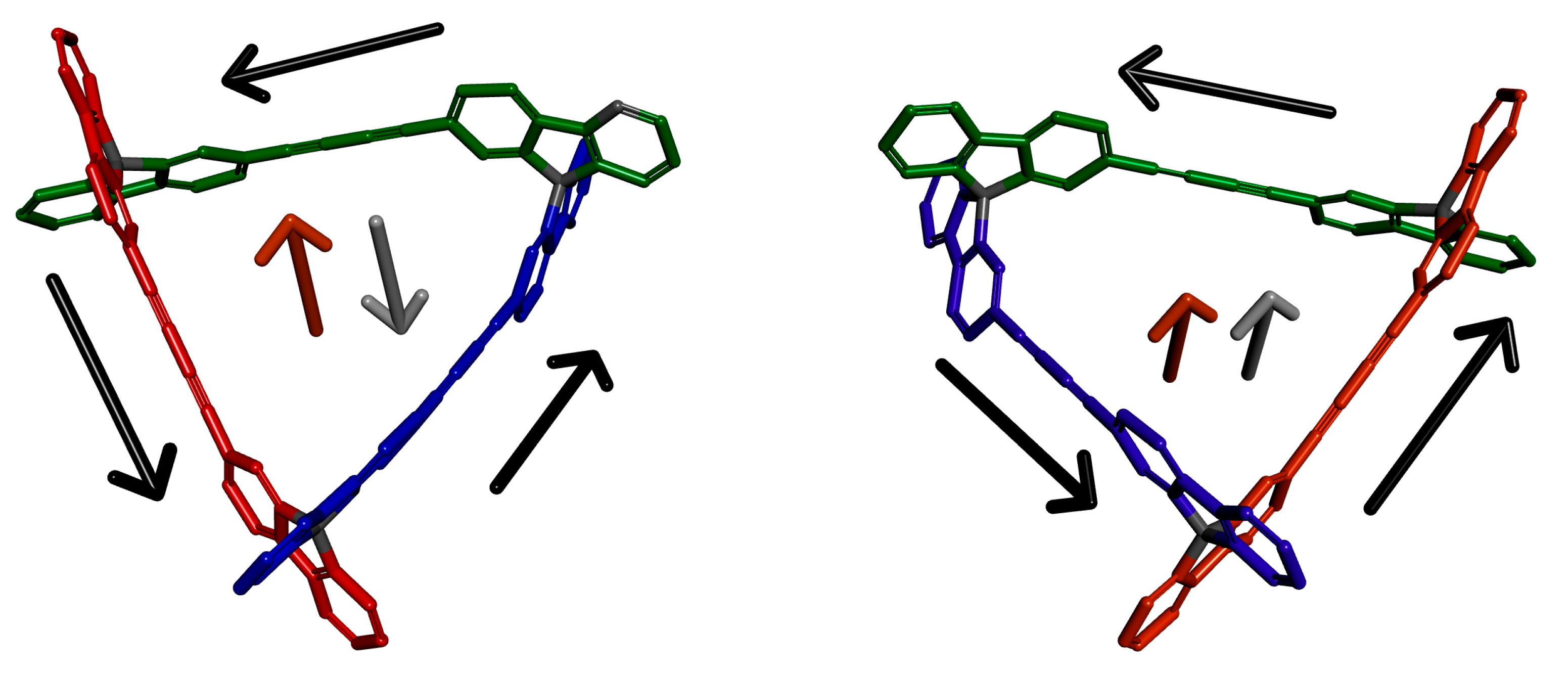
3.4. Chiral Induction
4. Chiroptical Responses
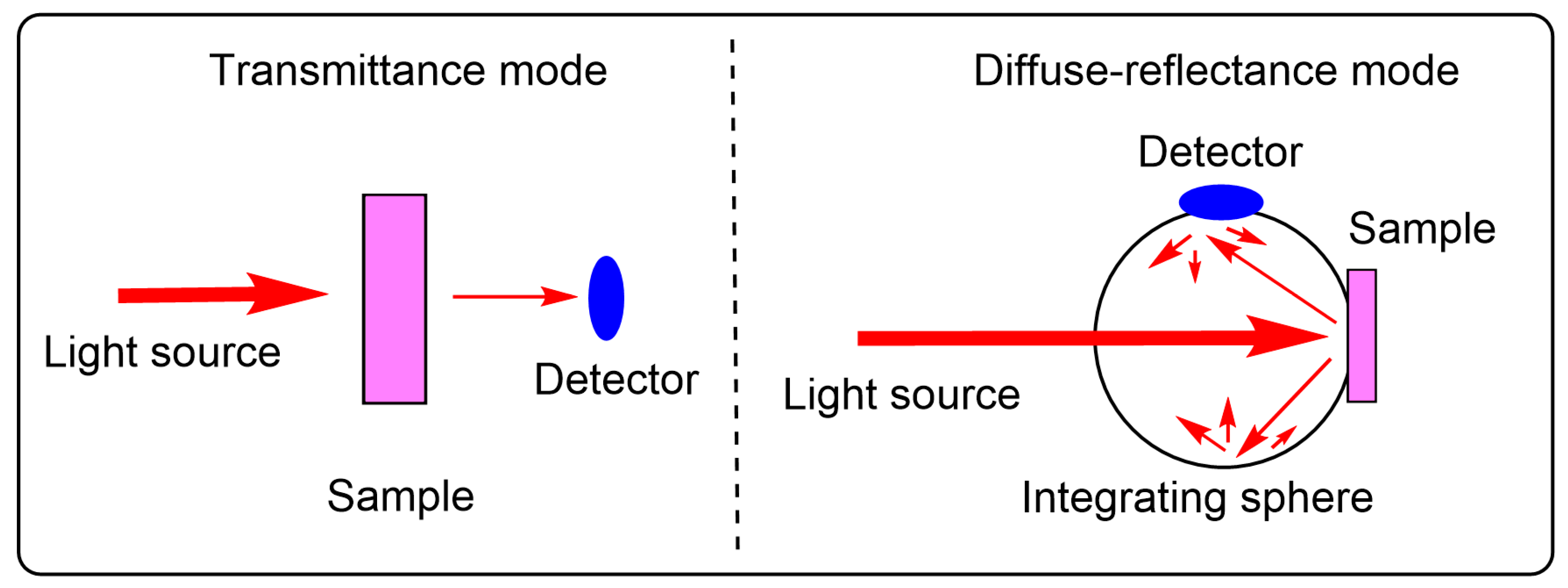
4.1. Sample Preparation and Measurement Modes for Determining Chiroptical Responses of Solid Samples
4.2. Chiroptical Responses in Porous Organic Frameworks
5. Applications in Asymmetric Organocatalysis
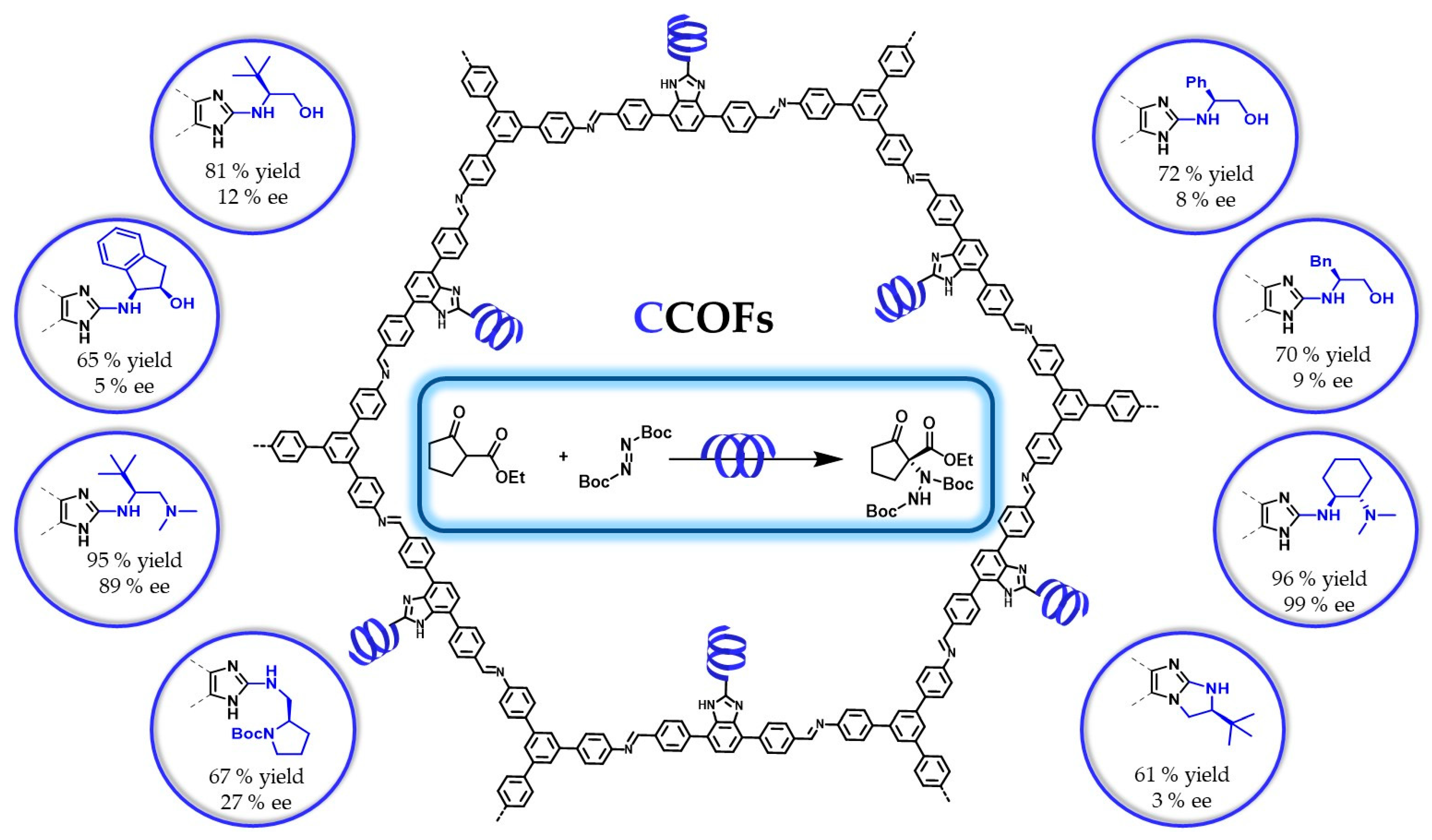
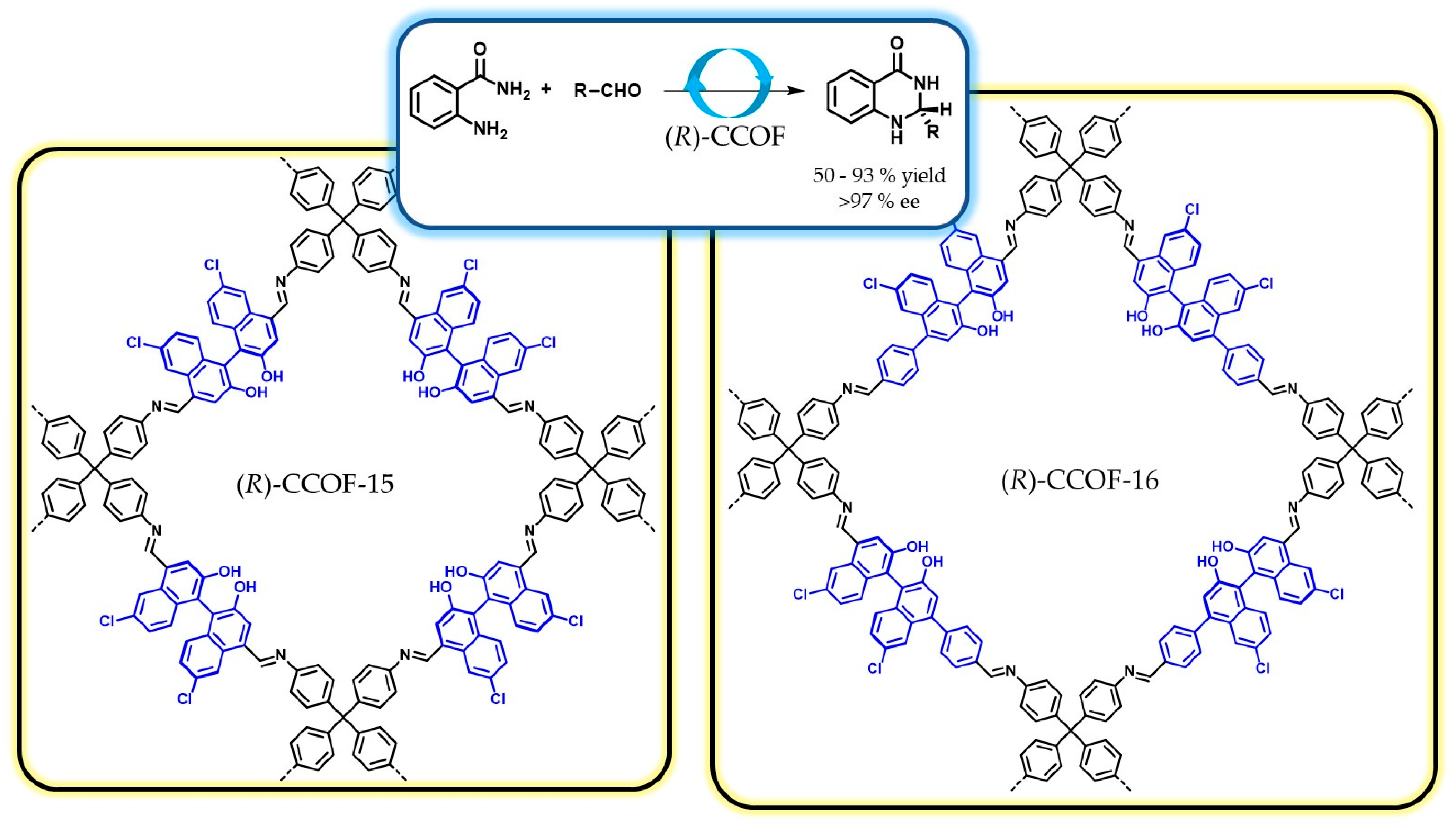
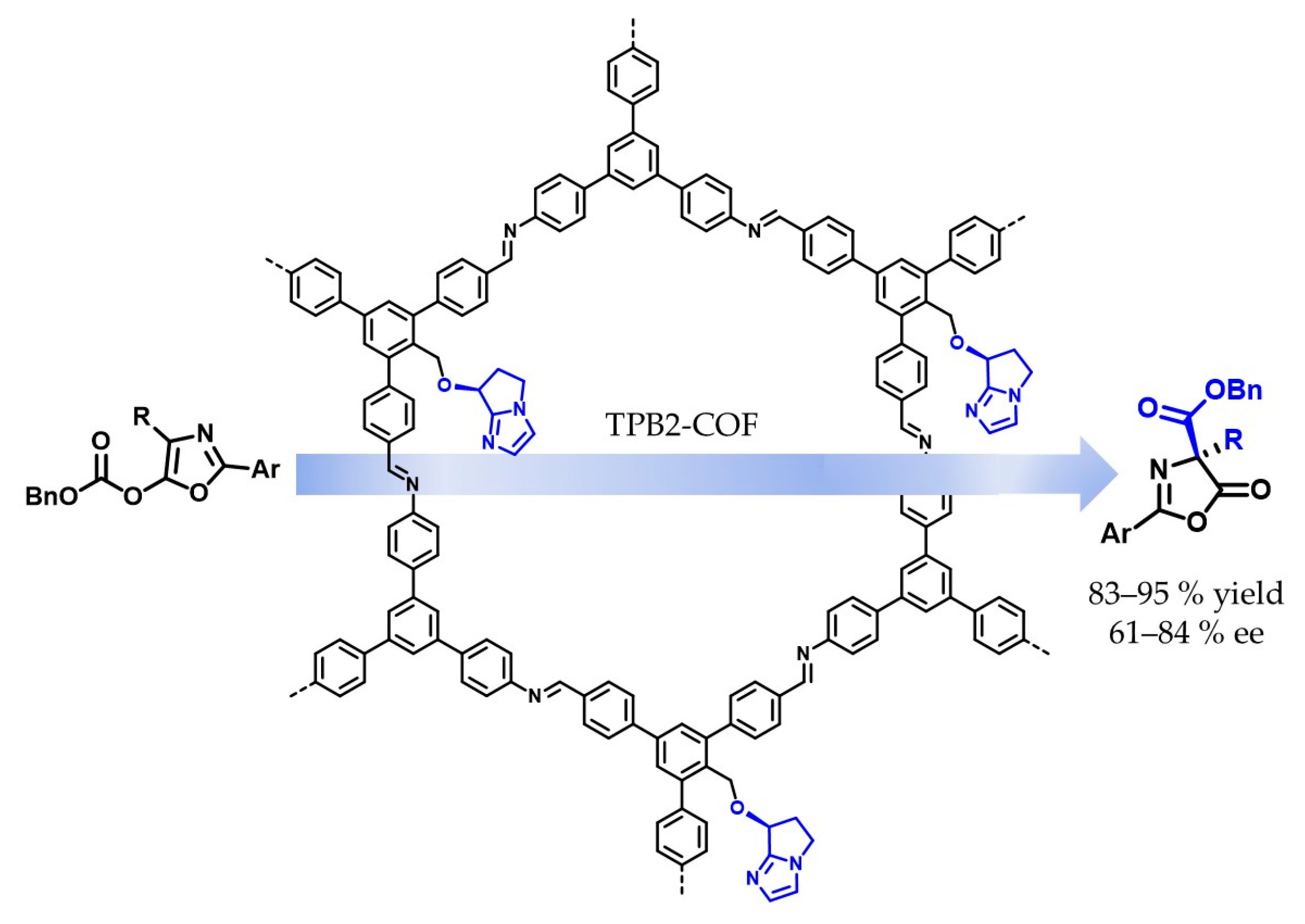
5.1. Covalent Catalysis
5.2. Hydrogen-Bond Catalysis
5.3. Other Types of Organocatalysis
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bonner, W.A. Chirality and Life. Orig. Life Evol. Biosph. 1995, 25, 175–190. [Google Scholar] [CrossRef]
- Inaki, M.; Liu, J.; Matsuno, K. Cell Chirality: Its Origin and Roles in Left–Right Asymmetric Development. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150403. [Google Scholar] [CrossRef] [Green Version]
- Coelho, M.M.; Fernandes, C.; Remião, F.; Tiritan, M.E. Enantioselectivity in Drug Pharmacokinetics and Toxicity: Pharmacological Relevance and Analytical Methods. Molecules 2021, 26, 3113. [Google Scholar] [CrossRef]
- Ma, B.; Bianco, A. Regulation of Biological Processes by Intrinsically Chiral Engineered Materials. Nat. Rev. Mater. 2023, 8, 403–413. [Google Scholar] [CrossRef]
- Tombo, G.M.R.; Belluš, D. Chirality and Crop Protection. Angew. Chem. Int. Ed. Engl. 1991, 30, 1193–1215. [Google Scholar] [CrossRef]
- Abate, A.; Brenna, E.; Fuganti, C.; Gatti, F.G.; Giovenzana, T.; Malpezzi, L.; Serra, S. Chirality and Fragrance Chemistry: Stereoisomers of the Commercial Chiral Odorants Muguesia and Pamplefleur. J. Org. Chem. 2005, 70, 1281–1290. [Google Scholar] [CrossRef]
- Mosandl, A. Chirality in Flavor Chemistry-recent Developments in Synthesis and Analysis. Food Rev. Int. 1988, 4, 1–43. [Google Scholar] [CrossRef]
- Marino, S.T.; Stachurska-Buczek, D.; Huggins, D.A.; Krywult, B.M.; Sheehan, C.S.; Nguyen, T.; Choi, N.; Parsons, J.G.; Griffiths, P.G.; James, I.W.; et al. Synthesis of Chiral Building Blocks for Use in Drug Discovery. Molecules 2004, 9, 405–426. [Google Scholar] [CrossRef] [Green Version]
- List, B. Introduction: Organocatalysis. Chem. Rev. 2007, 107, 5413–5415. [Google Scholar] [CrossRef] [Green Version]
- Varma, R.S. Greener and Sustainable Trends in Synthesis of Organics and Nanomaterials. ACS Sustain. Chem. Eng. 2016, 4, 5866–5878. [Google Scholar] [CrossRef] [PubMed]
- Rajesh Krishnan, G.; Sreekumar, K. Chapter 14—Supported and Reusable Organocatalysts. In New and Future Developments in Catalysis; Suib, S.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 343–364. ISBN 978-0-444-53876-5. [Google Scholar]
- McGuirk, C.M.; Katz, M.J.; Stern, C.L.; Sarjeant, A.A.; Hupp, J.T.; Farha, O.K.; Mirkin, C.A. Turning On Catalysis: Incorporation of a Hydrogen-Bond-Donating Squaramide Moiety into a Zr Metal–Organic Framework. J. Am. Chem. Soc. 2015, 137, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhao, J.; Johnson, J.A. Polymer Networks: From Plastics and Gels to Porous Frameworks. Angew. Chem. Int. Ed. 2020, 59, 5022–5049. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A. Functional Materials: From Hard to Soft Porous Frameworks. Angew. Chem. Int. Ed. 2010, 49, 8328–8344. [Google Scholar] [CrossRef] [PubMed]
- Daliran, S.; Oveisi, A.R.; Peng, Y.; López-Magano, A.; Khajeh, M.; Mas-Ballesté, R.; Alemán, J.; Luque, R.; Garcia, H. Metal–Organic Framework (MOF)-, Covalent-Organic Framework (COF)-, and Porous-Organic Polymers (POP)-Catalyzed Selective C–H Bond Activation and Functionalization Reactions. Chem. Soc. Rev. 2022, 51, 7810–7882. [Google Scholar] [CrossRef]
- Yaghi, O.M. Reticular Chemistry in All Dimensions. ACS Cent. Sci. 2019, 5, 1295–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Chen, L.; Yang, L.; Lan, T.; Wang, H.; Hu, C.; Han, X.; Liu, Q.; Chen, J.; Feng, Z.; et al. Porous Framework Materials for Energy & Environment Relevant Applications: A Systematic Review. Green Energy Environ. 2023; in press. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Wang, W. Covalent Organic Frameworks (COFs): From Design to Applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef]
- Lohse, M.S.; Bein, T. Covalent Organic Frameworks: Structures, Synthesis, and Applications. Adv. Funct. Mater. 2018, 28, 1705553. [Google Scholar] [CrossRef] [Green Version]
- López-Magano, A.; Daliran, S.; Oveisi, A.R.; Mas-Ballesté, R.; Dhakshinamoorthy, A.; Alemán, J.; Garcia, H.; Luque, R. Recent Advances in the Use of Covalent Organic Frameworks as Heterogeneous Photocatalysts in Organic Synthesis. Adv. Mater. 2022, 35, 2209475. [Google Scholar] [CrossRef]
- López-Magano, A.; Jiménez-Almarza, A.; Alemán, J.; Mas-Ballesté, R. Metal–Organic Frameworks (MOFs) and Covalent Organic Frameworks (COFs) Applied to Photocatalytic Organic Transformations. Catalysts 2020, 10, 720. [Google Scholar] [CrossRef]
- Lee, J.-S.M.; Cooper, A.I. Advances in Conjugated Microporous Polymers. Chem. Rev. 2020, 120, 2171–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, L.; Li, M.; Yin, Y.; Chen, J.; Zhong, Q.; Du, R.; Liu, S.; He, Y.; Fu, W.; Zeng, F. Advances in the Synthesis of Covalent Triazine Frameworks. ACS Omega 2023, 8, 4527–4542. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, Covalent Triazine-Based Frameworks Prepared by Ionothermal Synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Bojdys, M.J.; Dawson, R.; Laybourn, A.; Khimyak, Y.Z.; Adams, D.J.; Cooper, A.I. Porous, Fluorescent, Covalent Triazine-Based Frameworks Via Room-Temperature and Microwave-Assisted Synthesis. Adv. Mater. 2012, 24, 2357–2361. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, G. Porous Aromatic Frameworks (PAFs). Chem. Rev. 2020, 120, 8934–8986. [Google Scholar] [CrossRef] [PubMed]
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted Synthesis of a Porous Aromatic Framework with High Stability and Exceptionally High Surface Area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef]
- Meier, C.B.; Sprick, R.S.; Monti, A.; Guiglion, P.; Lee, J.-S.M.; Zwijnenburg, M.A.; Cooper, A.I. Structure-Property Relationships for Covalent Triazine-Based Frameworks: The Effect of Spacer Length on Photocatalytic Hydrogen Evolution from Water. Polymer 2017, 126, 283–290. [Google Scholar] [CrossRef]
- Wu, G.; Chen, X.; Li, Y.; Jia, Z. Preparation of Submicron PAF-56 Particles and Application in Pervaporation. Microporous Mesoporous Mater. 2019, 279, 19–25. [Google Scholar] [CrossRef]
- Meng, S.; Ma, H.; Jiang, L.; Ren, H.; Zhu, G. A Facile Approach to Prepare Porphyrinic Porous Aromatic Frameworks for Small Hydrocarbon Separation. J. Mater. Chem. A 2014, 2, 14536–14541. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Jiang, D. CMPs as Scaffolds for Constructing Porous Catalytic Frameworks: A Built-in Heterogeneous Catalyst with High Activity and Selectivity Based on Nanoporous Metalloporphyrin Polymers. J. Am. Chem. Soc. 2010, 132, 9138–9143. [Google Scholar] [CrossRef]
- Yan, Z.; Ren, H.; Ma, H.; Yuan, R.; Yuan, Y.; Zou, X.; Sun, F.; Zhu, G. Construction and Sorption Properties of Pyrene-Based Porous Aromatic Frameworks. Microporous Mesoporous Mater. 2013, 173, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Yuan, C.; Hou, B.; Liu, L.; Li, H.; Liu, Y.; Cui, Y. Chiral Covalent Organic Frameworks: Design, Synthesis and Property. Chem. Soc. Rev. 2020, 49, 6248–6272. [Google Scholar] [CrossRef]
- Yuan, C.; Fu, S.; Yang, K.; Hou, B.; Liu, Y.; Jiang, J.; Cui, Y. Crystalline C—C and C=C Bond-Linked Chiral Covalent Organic Frameworks. J. Am. Chem. Soc. 2021, 143, 369–381. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Zhang, J.; Wu, X.; Liu, Y.; Cui, Y. Homochiral 2D Porous Covalent Organic Frameworks for Heterogeneous Asymmetric Catalysis. J. Am. Chem. Soc. 2016, 138, 12332–12335. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.-L.; Yang, C.-X.; Yan, X.-P. Bottom-up Synthesis of Chiral Covalent Organic Frameworks and Their Bound Capillaries for Chiral Separation. Nat. Commun. 2016, 7, 12104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.-S.; Ding, S.-Y.; An, W.-K.; Wu, H.; Wang, W. Constructing Crystalline Covalent Organic Frameworks from Chiral Building Blocks. J. Am. Chem. Soc. 2016, 138, 11489–11492. [Google Scholar] [CrossRef]
- Liu, G.; Sheng, J.; Zhao, Y. Chiral Covalent Organic Frameworks for Asymmetric Catalysis and Chiral Separation. Sci. China Chem. 2017, 60, 1015–1022. [Google Scholar] [CrossRef]
- Segura, J.L.; Royuela, S.; Mar Ramos, M. Post-Synthetic Modification of Covalent Organic Frameworks. Chem. Soc. Rev. 2019, 48, 3903–3945. [Google Scholar] [CrossRef]
- Xu, H.; Gao, J.; Jiang, D. Stable, Crystalline, Porous, Covalent Organic Frameworks as a Platform for Chiral Organocatalysts. Nat. Chem. 2015, 7, 905–912. [Google Scholar] [CrossRef]
- Zhuo, S.; Wang, X.; Li, L.; Yang, S.; Ji, Y. Chiral Carboxyl-Functionalized Covalent Organic Framework for Enantioselective Adsorption of Amino Acids. ACS Appl. Mater. Interfaces 2021, 13, 31059–31065. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, J.; Liu, C.; Li, H.; Yang, H.; Feng, Y.; Zhang, B. Construction of Pyridine-Based Chiral Ionic Covalent Organic Frameworks as a Heterogeneous Catalyst for Promoting Asymmetric Henry Reactions. Org. Lett. 2021, 23, 1748–1752. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, H. Asymmetric Organocatalysis. Tetrahedron 2007, 63, 9267–9331. [Google Scholar] [CrossRef]
- Chen, H.; Gu, Z.-G.; Zhang, J. Chiral-Induced Ultrathin Covalent Organic Frameworks Nanosheets with Tunable Circularly Polarized Luminescence. J. Am. Chem. Soc. 2022, 144, 7245–7252. [Google Scholar] [CrossRef]
- Han, X.; Zhang, J.; Huang, J.; Wu, X.; Yuan, D.; Liu, Y.; Cui, Y. Chiral Induction in Covalent Organic Frameworks. Nat. Commun. 2018, 9, 1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.A.; Zhang, Z.K.; Yue, T.; Sun, Y.L.; Wang, L.; Wang, W.D.; Zhang, Y.; Liu, C.; Wang, W. “Bottom-Up” Embedding of the Jørgensen–Hayashi Catalyst into a Chiral Porous Polymer for Highly Efficient Heterogeneous Asymmetric Organocatalysis. Chem. Eur. J. 2012, 18, 6718–6723. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Liu, Y.; Cui, Y. Chiral Porous TADDOL-Embedded Organic Polymers for Asymmetric Diethylzinc Addition to Aldehydes. Bull. Chem. Soc. Jpn. 2014, 87, 435–440. [Google Scholar] [CrossRef] [Green Version]
- An, W.-K.; Han, M.-Y.; Wang, C.-A.; Yu, S.-M.; Zhang, Y.; Bai, S.; Wang, W. Insights into the Asymmetric Heterogeneous Catalysis in Porous Organic Polymers: Constructing A TADDOL-Embedded Chiral Catalyst for Studying the Structure–Activity Relationship. Chem. Eur. J. 2014, 20, 11019–11028. [Google Scholar] [CrossRef]
- Lan, Y.; Yang, C.; Zhang, Y.; An, W.; Xue, H.; Ding, S.; Zhou, P.; Wang, W. Pyrrolidine-Based Chiral Porous Polymers for Heterogeneous Organocatalysis in Water. Polym. Chem. 2019, 10, 3298–3305. [Google Scholar] [CrossRef]
- Wang, C.-A.; Li, Y.-W.; Han, Y.-F.; Zhang, J.-P.; Wu, R.-T.; He, G.-F. The “Bottom-up” Construction of Chiral Porous Organic Polymers for Heterogeneous Asymmetric Organocatalysis: MacMillan Catalyst Built-in Nanoporous Organic Frameworks. Polym. Chem. 2017, 8, 5561–5569. [Google Scholar] [CrossRef]
- Kong, S.; Ullah, M.A.; Qian, X.; Shu, M.; Xiao, W. Asymmetric Hydrogenation of β-Keto Esters Catalyzed by Ruthenium Species Supported on Porous Organic Polymer. Chin. J. Org. Chem. 2018, 38, 656. [Google Scholar] [CrossRef]
- Valverde-González, A.; Borrallo-Aniceto, M.C.; Pintado-Sierra, M.; Sánchez, F.; Arnanz, A.; Boronat, M.; Iglesias, M. BINOL-Containing Chiral Porous Polymers as Platforms for Enantiorecognition. ACS Appl. Mater. Interfaces 2022, 14, 53936–53946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kormos, A.; Zhang, J. Self-Supported BINOL-Derived Phosphoric Acid Based on a Chiral Carbazolic Porous Framework. Org. Lett. 2017, 19, 6072–6075. [Google Scholar] [CrossRef] [PubMed]
- Monterde, C.; Navarro, R.; Iglesias, M.; Sánchez, F. Adamantyl-BINOL as Platform for Chiral Porous Polymer Aromatic Frameworks. Multiple Applications as Recyclable Catalysts. J. Catal. 2019, 377, 609–618. [Google Scholar] [CrossRef]
- Lin, Z.-J.; Lü, J.; Li, L.; Li, H.-F.; Cao, R. Defect Porous Organic Frameworks (DPOFs) Asa Platform for Chiral Organocatalysis. J. Catal. 2017, 355, 131–138. [Google Scholar] [CrossRef]
- Kang, X.; Stephens, E.R.; Spector-Watts, B.M.; Li, Z.; Liu, Y.; Liu, L.; Cui, Y. Challenges and Opportunities for Chiral Covalent Organic Frameworks. Chem. Sci. 2022, 13, 9811–9832. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Han, X.; Xu, Q.; Liu, Y.; Yuan, C.; Yang, S.; Liu, Y.; Jiang, J.; Cui, Y. Chiral BINOL-Based Covalent Organic Frameworks for Enantioselective Sensing. J. Am. Chem. Soc. 2019, 141, 7081–7089. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huang, J.; Yuan, C.; Liu, Y.; Cui, Y. Chiral 3D Covalent Organic Frameworks for High Performance Liquid Chromatographic Enantioseparation. J. Am. Chem. Soc. 2018, 140, 892–895. [Google Scholar] [CrossRef]
- Zheng, Z.; Yuan, C.; Sun, M.; Dong, J.; Liu, Y.; Cui, Y. Construction of Monophosphine–Metal Complexes in Privileged Diphosphine-Based Covalent Organic Frameworks for Catalytic Asymmetric Hydrogenation. J. Am. Chem. Soc. 2023, 145, 6100–6111. [Google Scholar] [CrossRef]
- Ma, H.-C.; Zhao, C.-C.; Chen, G.-J.; Dong, Y.-B. Photothermal Conversion Triggered Thermal Asymmetric Catalysis within Metal Nanoparticles Loaded Homochiral Covalent Organic Framework. Nat. Commun. 2019, 10, 3368. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.-L.; Guo, P.; Yang, Y.-P.; Ran, X.-Y.; Liu, C.; Wang, B.-J.; Zhang, J.-H.; Xie, S.-M.; Yuan, L.-M. Chiral Covalent Triazine Framework CC-DMP CCTF@SiO2 Core–Shell Microspheres Used for HPLC Enantioseparation. New J. Chem. 2023, 47, 5413–5419. [Google Scholar] [CrossRef]
- Tang, X.; Liao, X.; Cai, X.; Wu, J.; Wu, X.; Zhang, Q.; Yan, Y.; Zheng, S.; Jiang, H.; Fan, J.; et al. Self-Assembly of Helical Nanofibrous Chiral Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2023, 62, e202216310. [Google Scholar] [CrossRef]
- Xu, H.; Chen, X.; Gao, J.; Lin, J.; Addicoat, M.; Irle, S.; Jiang, D. Catalytic Covalent Organic Frameworks via Pore Surface Engineering. Chem. Commun. 2014, 50, 1292–1294. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, X.; Gao, R.; Han, X.; Liu, Y.; Long, Y.; Cui, Y. Nanochannels of Covalent Organic Frameworks for Chiral Selective Transmembrane Transport of Amino Acids. J. Am. Chem. Soc. 2019, 141, 20187–20197. [Google Scholar] [CrossRef]
- Wan, M.; Zheng, Y.; Dai, X.; Yang, H.; Zhou, J.; Ou, J.; Yang, Y.; Liao, M.; Xia, Z.; Wang, L. Click Chemistry for the Preparation of β-Cyclodextrin Grafting Uniform Spherical Covalent Organic Framework Materials for Chiral Separation. Chem. Mater. 2023, 35, 609–616. [Google Scholar] [CrossRef]
- Guo, J.-X.; Yang, C.; Yan, X.-P. “Thiol–Ene” Click Synthesis of Chiral Covalent Organic Frameworks for Gas Chromatography. J. Mater. Chem. A 2021, 9, 21151–21157. [Google Scholar] [CrossRef]
- Mitra, S.; Sasmal, H.S.; Kundu, T.; Kandambeth, S.; Illath, K.; Díaz Díaz, D.; Banerjee, R. Targeted Drug Delivery in Covalent Organic Nanosheets (CONs) via Sequential Postsynthetic Modification. J. Am. Chem. Soc. 2017, 139, 4513–4520. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Y.; Ren, J.; Qu, X. A Chiral Covalent Organic Framework (COF) Nanozyme with Ultrahigh Enzymatic Activity. Mater. Horiz. 2020, 7, 3291–3297. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, Y.; An, H.; Aguila, B.; Yang, C.-X.; Dong, Y.; Xie, W.; Cheng, P.; Zhang, Z.; Chen, Y.; et al. Covalent Organic Frameworks with Chirality Enriched by Biomolecules for Efficient Chiral Separation. Angew. Chem. Int. Ed. 2018, 57, 16754–16759. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-C.; Kan, X.; Shang, J.-Y.; Qiao, H.; Dong, Y.-B. Catalytic Asymmetric Synthesis of Chiral Covalent Organic Frameworks from Prochiral Monomers for Heterogeneous Asymmetric Catalysis. J. Am. Chem. Soc. 2020, 142, 16915–16920. [Google Scholar] [CrossRef]
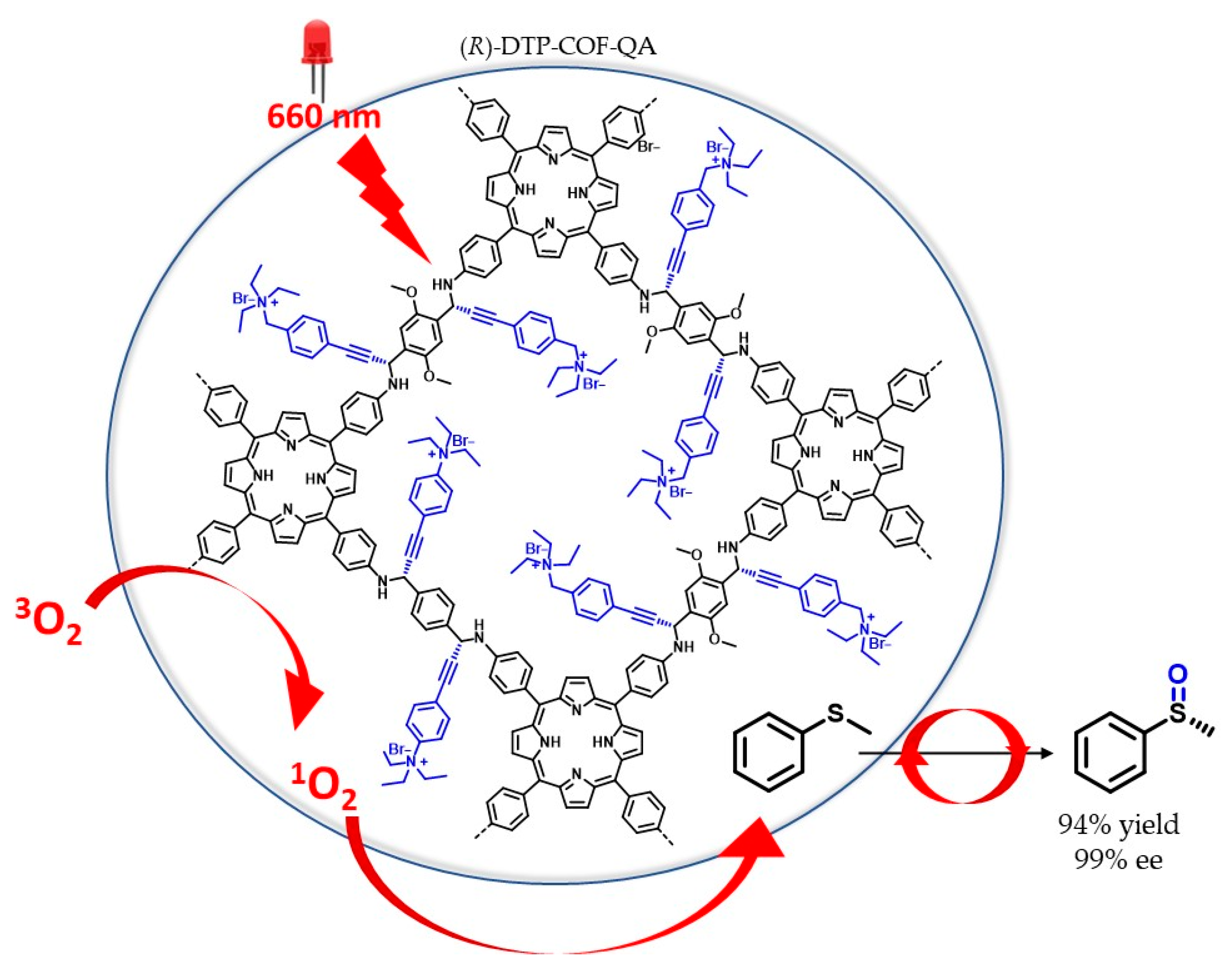
- Kan, X.; Wang, J.-C.; Chen, Z.; Du, J.-Q.; Kan, J.-L.; Li, W.-Y.; Dong, Y.-B. Synthesis of Metal-Free Chiral Covalent Organic Framework for Visible-Light-Mediated Enantioselective Photooxidation in Water. J. Am. Chem. Soc. 2022, 144, 6681–6686. [Google Scholar] [CrossRef]
- Li, F.; Kan, J.-L.; Yao, B.-J.; Dong, Y.-B. Synthesis of Chiral Covalent Organic Frameworks via Asymmetric Organocatalysis for Heterogeneous Asymmetric Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202115044. [Google Scholar] [CrossRef]
- Berova, N.; Woody, R.W.; Polavarapu, P.; Nakanishi, K. Comprehensive Chiroptical Spectroscopy: Volume 1—Instrumentation, Methodologies, and Theoretical Simulations; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Ozcelik, A.; Aranda, D.; Gil-Guerrero, S.; Pola-Otero, X.A.; Talavera, M.; Wang, L.; Behera, S.K.; Gierschner, J.; Peña-Gallego, Á.; Santoro, F.; et al. Distinct Helical Molecular Orbitals through Conformational Lock. Chem. Eur. J. 2020, 26, 17342–17349. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Raghavan, V.; Cimmino, A.; Moeini, A.; Petrovic, A.G.; Santoro, E.; Superchi, S.; Berova, N.; Evidente, A.; Polavarapu, P.L. Absolute Configurations of Chiral Molecules with Multiple Stereogenic Centers without Prior Knowledge of the Relative Configurations: A Case Study of Inuloxin C. Chirality 2018, 30, 1206–1214. [Google Scholar] [CrossRef]
- Petrovic, A.G.; Berova, N.; Alonso-Gómez, J.L. Absolute Configuration and Conformational Analysis of Chiral Compounds via Experimental and Theoretical Prediction of Chiroptical Properties: ORD, ECD, and VCD. In Structure Elucidation in Organic Chemistry; John Wiley & Sons: New York, NY, USA, 2015; pp. 65–104. ISBN 978-3-527-66461-0. [Google Scholar]
- Ouyang, J.; Swartjes, A.; Geerts, M.; Gilissen, P.J.; Wang, D.; Teeuwen, P.C.P.; Tinnemans, P.; Vanthuyne, N.; Chentouf, S.; Rutjes, F.P.J.T.; et al. Absolute Configuration and Host-Guest Binding of Chiral Porphyrin-Cages by a Combined Chiroptical and Theoretical Approach. Nat. Commun. 2020, 11, 4776. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef]
- Laidlaw, B.; Eng, J.; Wade, J.; Shi, X.; Salerno, F.; Fuchter, M.J.; Penfold, T.J. On the Factors Influencing the Chiroptical Response of Conjugated Polymer Thin Films. Chem. Commun. 2021, 57, 9914–9917. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Benson, A.A.; Calvin, M. The Absorption Spectra of Suspensions of Living Micro-Organisms. Biochim. Biophys. Acta 1954, 15, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Castiglioni, E.; Biscarini, P.; Abbate, S. Experimental Aspects of Solid State Circular Dichroism. Chirality 2009, 21, E28–E36. [Google Scholar] [CrossRef]
- Du, C.; Zhu, X.; Yang, C.; Liu, M. Stacked Reticular Frame Boosted Circularly Polarized Luminescence of Chiral Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2022, 61, e202113979. [Google Scholar] [CrossRef]
- Xie, J.-H.; Hou, Y.-M.; Feng, Z.; You, S.-L. Stereodivergent Construction of 1,3-Chiral Centers via Tandem Asymmetric Conjugate Addition and Allylic Substitution Reaction. Angew. Chem. Int. Ed. 2023, 62, e202216396. [Google Scholar] [CrossRef]
- Zhang, J.; Han, X.; Wu, X.; Liu, Y.; Cui, Y. Chiral DHIP- and Pyrrolidine-Based Covalent Organic Frameworks for Asymmetric Catalysis. ACS Sustain. Chem. Eng. 2019, 7, 5065–5071. [Google Scholar] [CrossRef]
- Zhang, J.; Han, X.; Wu, X.; Liu, Y.; Cui, Y. Multivariate Chiral Covalent Organic Frameworks with Controlled Crystallinity and Stability for Asymmetric Catalysis. J. Am. Chem. Soc. 2017, 139, 8277–8285. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-K.; Zhou, J.-J.; Lan, Y.-B.; Ding, S.-Y.; Yu, W.; Wang, W. Divergent Synthesis of Chiral Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2019, 58, 9443–9447. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Yang, S.; Yang, K.; Han, X.; Tang, X.; Liu, Y.; Jiang, J.; Cui, Y. Confinement-Driven Enantioselectivity in 3D Porous Chiral Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2021, 60, 6086–6093. [Google Scholar] [CrossRef]
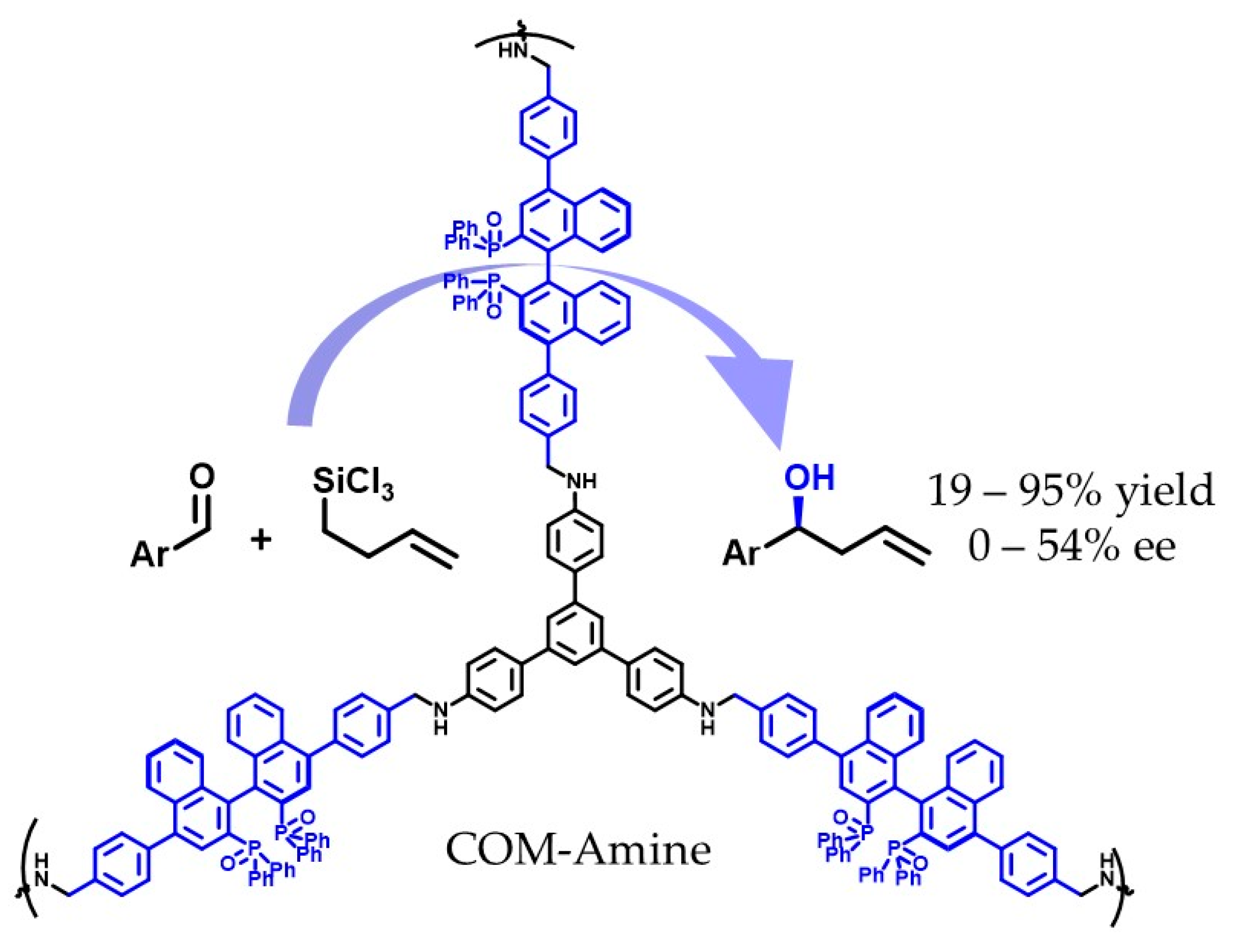
- Sánchez-Fuente, M.; López-Magano, A.; Moya, A.; Mas-Ballesté, R. Stabilized Chiral Organic Material Containing BINAP Oxide Units as a Heterogeneous Asymmetric Organocatalyst for Allylation of Aldehydes. ACS Appl. Mater. Interfaces 2023, 15. [Google Scholar] [CrossRef]
- Padula, D.; Santoro, F.; Pescitelli, G. A Simple Dimeric Model Accounts for the Vibronic ECD Spectra of Chiral Polythiophenes in Their Aggregated States. RSC Adv. 2016, 6, 37938–37943. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Fuente, M.; Alonso-Gómez, J.L.; Salonen, L.M.; Mas-Ballesté, R.; Moya, A. Chiral Porous Organic Frameworks: Synthesis, Chiroptical Properties, and Asymmetric Organocatalytic Applications. Catalysts 2023, 13, 1042. https://doi.org/10.3390/catal13071042
Sanchez-Fuente M, Alonso-Gómez JL, Salonen LM, Mas-Ballesté R, Moya A. Chiral Porous Organic Frameworks: Synthesis, Chiroptical Properties, and Asymmetric Organocatalytic Applications. Catalysts. 2023; 13(7):1042. https://doi.org/10.3390/catal13071042
Chicago/Turabian StyleSanchez-Fuente, Miguel, José Lorenzo Alonso-Gómez, Laura M. Salonen, Ruben Mas-Ballesté, and Alicia Moya. 2023. "Chiral Porous Organic Frameworks: Synthesis, Chiroptical Properties, and Asymmetric Organocatalytic Applications" Catalysts 13, no. 7: 1042. https://doi.org/10.3390/catal13071042