Electrocatalytic Hydrodechlorination Using Supported Atomically Precise Gold Nanoclusters under Flow-Through Configuration
Abstract
:1. Introduction
2. Results
2.1. Electrochemical 2,4-Dichlorophenol Hydrodechlorination
2.2. H*-Mediated Electrocatalytic Hydrodechlorination Mechanism
2.3. Density Functional Theory (DFT) Calculations
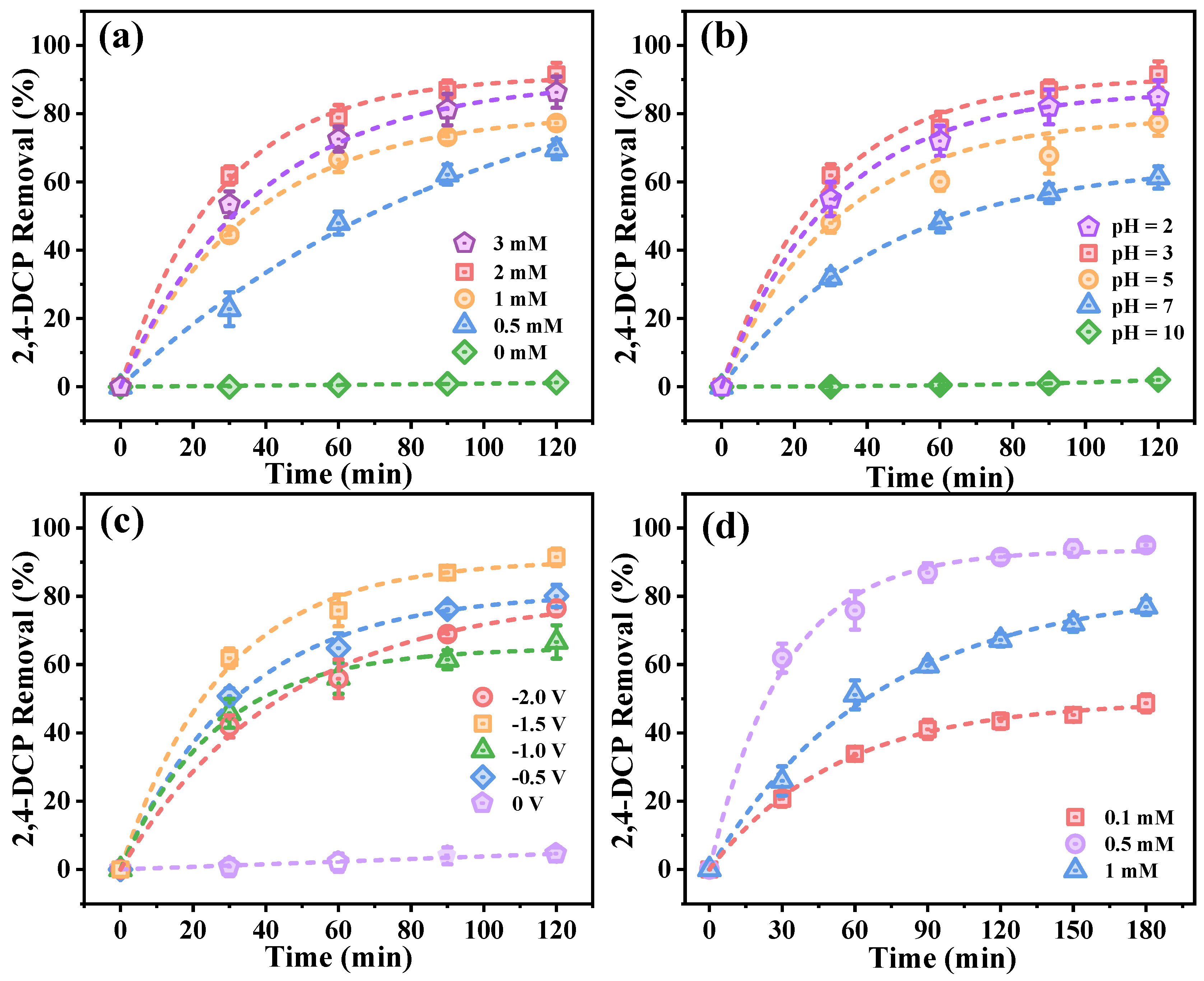
2.4. System Optimization
2.5. System Stability Evaluation
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of AuNC@CNT Nanohybrid Filter
3.3. Characterization
3.4. Electrochemical 2,4-Dichlorophenol Filtration Experiments
3.5. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karci, A. Degradation of chlorophenols and alkylphenol ethoxylates, two representative textile chemicals, in water by advanced oxidation processes: The state of the art on transformation products and toxicity. Chemosphere 2014, 99, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, M.; Pan, Y.; Xu, L. Pre-magnetized Fe0/persulfate for notably enhanced degradation and dechlorination of 2,4-dichlorophenol. Chem. Eng. J. 2017, 307, 1092–1104. [Google Scholar] [CrossRef]
- Wu, P.; Yang, G.-P.; Zhao, X.-K. Sorption behavior of 2,4-dichlorophenol on marine sediment. J. Colloid Interface Sci. 2003, 265, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Y.; Xu, C.; Qiao, C.; Chen, S.; Zhao, C.; Liu, Q.; Zhang, X. Reconstruction of microbiome and functionality accelerated crude oil biodegradation of 2,4-DCP-oil-contaminated soil systems using composite microbial agent B-Cl. J. Hazard. Mater. 2023, 447, 130808. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, R.; Nan, J.; Chen, X.; Huang, C.; Cao, D.; Bai, C.; Han, J.; Liang, B.; Li, Z.; et al. Effective electrocatalytic hydrodechlorination of 2,4,6-trichlorophenol by a novel Pd/MnO2/Ni foam cathode. Chin. Chem. Lett. 2022, 33, 3823–3828. [Google Scholar] [CrossRef]
- Elumalai, P.; Parthipan, P.; Huang, M.; Muthukumar, B.; Cheng, L.; Govarthanan, M.; Rajasekar, A. Enhanced biodegradation of hydrophobic organic pollutants by the bacterial consortium: Impact of enzymes and biosurfactants. Environ. Pollut. 2021, 289, 117956. [Google Scholar] [CrossRef]
- Jiang, P.; Zhou, T.; Bai, J.; Zhang, Y.; Li, J.; Zhou, C.; Zhou, B. Nitrogen-containing wastewater fuel cells for total nitrogen removal and energy recovery based on Cl•/ClO• oxidation of ammonia nitrogen. Water Res. 2023, 235, 119914. [Google Scholar] [CrossRef]
- Bouchoucha, H.; Bekkouche, S.; Merouani, S.; Dehane, A.; Hamdaoui, O. Solar chlorine activation for efficient Rhodamine B removal in strong basic pH: Processing conditions, radicals probing, and TiO2 nanocatalyst effect. Catalysts 2023, 13, 942. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with atomic-level arranged perovskite and oxide layers for advanced oxidation with an enhanced non-free radical pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Sun, M.; Yan, F.; Zhang, R.; Reible, D.D.; Lowry, G.V.; Gregory, K.B. Redox control and hydrogen production in sediment caps using carbon cloth electrodes. Environ. Sci. Technol. 2010, 44, 8209–8215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Liu, F.; Zhang, J.; Liu, Y. Facile synthesis and environmental applications of noble metal-based catalytic membrane reactors. Catalysts 2022, 12, 861. [Google Scholar] [CrossRef]
- Weon, S.; Suh, M.; Chu, C.; Huang, D.; Stavitski, E.; Kim, J. Site-selective loading of single-atom Pt on TiO2 for photocatalytic oxidation and reductive hydrodefluorination. ACS EST Eng. 2021, 1, 512–522. [Google Scholar] [CrossRef]
- Ren, Y.; Zheng, W.; Li, S.; Liu, Y. Atomic H*-mediated electrochemical removal of low concentration antimonite and recovery of antimony from water. J. Hazard. Mater. 2023, 445, 130520. [Google Scholar] [CrossRef]
- Wu, Y.; Gan, L.; Zhang, S.; Song, H.; Lu, C.; Li, W.; Wang, Z.; Jiang, B.; Li, A. Carbon-nanotube-doped Pd-Ni bimetallic three-dimensional electrode for electrocatalytic hydrodechlorination of 4-chlorophenol: Enhanced activity and stability. J. Hazard. Mater. 2018, 356, 17–25. [Google Scholar] [CrossRef]
- Eftekhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Wan, X.; Miao, J.; Zhang, R.; Zhang, J.; Niu, Q.J. Hydrodechlorination of p-chlorophenol on Pd-coated Fe3O4@polypyrrole catalyst with ammonia borane as hydrogen donor. Catal. Lett. 2019, 149, 823–830. [Google Scholar] [CrossRef]
- Mu, D.; Li, Z.; Yu, S.; Liu, S. Hydrodechlorination of chlorophenols with methanol as hydrogen donor over carbon nanotube supported Pd-catalysts. Catal. Today 2022, 405–406, 47–56. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, B.; Luo, Z.; Yao, Q.; Leong, D.T.; Yan, N.; Xie, J. Balancing the rate of cluster growth and etching for gram-scale synthesis of thiolate-protected Au25 nanoclusters with atomic precision. Angew. Chem. Int. Ed. 2014, 53, 4623–4627. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, C.; Wang, W.; Liu, Z.; Li, J.; Liu, C.; Pan, Y.; Liu, Y. Electronic structure engineering of bimetallic Pd-Au alloy nanocatalysts for improving electrocatalytic hydrodechlorination performance. Sep. Purif. Technol. 2022, 289, 120731. [Google Scholar] [CrossRef]
- Xu, J.; White, T.; Li, P.; He, C.; Yu, J.; Yuan, W.; Han, Y.-F. Biphasic Pd–Au alloy catalyst for low-temperature CO oxidation. J. Am. Chem. Soc. 2010, 132, 10398–10406. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Xing, Z.; Zhao, X.; Liu, N.; Chen, F. Monolithic carbon foam-supported Au nanoparticles with excellent catalytic performance in a fixed-bed system. New J. Chem. 2017, 41, 15027–15032. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, T.; Yuan, X.; Xie, J. Toward total synthesis of thiolate-protected metal nanoclusters. Acc. Chem. Res. 2018, 51, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Dharmaratne, A.C.; Krick, T.; Dass, A. Nanocluster size evolution studied by mass spectrometry in room temperature Au25(SR)18 synthesis. J. Am. Chem. Soc. 2009, 131, 13604–13605. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Yao, Q.; Wang, Y.; Fang, X.; Shen, C.; Li, F.; Huang, M.; Wang, Z.; Sand, W.; et al. Supported atomically-precise gold nanoclusters for enhanced flow-through electro-Fenton. Environ. Sci. Technol. 2020, 54, 5913–5921. [Google Scholar] [CrossRef]
- Smirnov, E.; Peljo, P.; Scanlon, M.D.; Girault, H.H. Interfacial redox catalysis on gold nanofilms at soft interfaces. ACS Nano 2015, 9, 6565–6575. [Google Scholar] [CrossRef] [Green Version]
- Priecel, P.; Adekunle Salami, H.; Padilla, R.H.; Zhong, Z.; Lopez-Sanchez, J.A. Anisotropic gold nanoparticles: Preparation and applications in catalysis. Chin. J. Catal 2016, 37, 1619–1650. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yan, B.; Cheng, Y. State-of-the-art review of oxidative dehydrogenation of ethane to ethylene over MoVNbTeOx catalysts. Catalysts 2023, 13, 204. [Google Scholar] [CrossRef]
- Lou, Z.; Yu, C.; Wen, X.; Xu, Y.; Yu, J.; Xu, X. Construction of Pd nanoparticles/two-dimensional Co-MOF nanosheets heterojunction for enhanced electrocatalytic hydrodechlorination. Appl. Catal. B 2022, 317, 121730. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Abdirova, P.; Kanafina, D.; Arkhangelsky, E.; Kyzas, G.Z.; Poulopoulos, S.G. UV and zero-valent iron (ZVI) activated continuous flow persulfate oxidation of municipal wastewater. Catalysts 2023, 13, 25. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, L.; Zhou, C.; Zhou, Y.; Zhou, J.; Xia, S.; Rittmann, B.E. A kinetic model for 2,4-dichlorophenol adsorption and hydrodechlorination over a palladized biofilm. Water Res. 2022, 214, 118201. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Wang, K.; Lv, X.; Fu, H.; Dong, X.; Chen, L.; Zhang, X.; Jiang, G. Palladium nanoparticles assembled on titanium nitride for enhanced electrochemical hydrodechlorination of 2,4-dichlorophenol in water. Chin. J. Catal. 2018, 39, 693–700. [Google Scholar] [CrossRef]
- Jiang, G.; Lan, M.; Zhang, Z.; Lv, X.; Lou, Z.; Xu, X.; Dong, F.; Zhang, S. Identification of active hydrogen species on palladium nanoparticles for an enhanced electrocatalytic hydrodechlorination of 2,4-dichlorophenol in water. Environ. Sci. Technol. 2017, 51, 7599–7605. [Google Scholar] [CrossRef]
- Jiang, G.S.; Li, J.; Shi, X.; Lv, X.; Huang, Y.-X.; Dong, F.; Jiang, G. Identifying the rate-determining step of the electrocatalytic hydrodechlorination reaction on palladium nanoparticles. Nanoscale 2019, 11, 15892–15899. [Google Scholar]
- Shen, Y.; Tong, Y.; Xu, J.; Wang, S.; Wang, J.; Zeng, T.; He, Z.; Yang, W.; Song, S. Ni-based layered metal-organic frameworks with palladium for electrochemical dechlorination. Appl. Catal. B 2020, 264, 118505. [Google Scholar] [CrossRef]
- Zheng, W.; You, S.; Yao, Y.; Jin, L.; Liu, Y. Development of atomic hydrogen-mediated electrocatalytic filtration system for peroxymonosulfate activation towards ultrafast degradation of emerging organic contaminants. Appl. Catal. B 2021, 298, 120593. [Google Scholar] [CrossRef]
- Li, S.; Liu, C.; Lv, W.; Liu, G. Incorporating oxygen atoms in a SnS2 atomic layer to simultaneously stabilize atomic hydrogen and accelerate the generation of hydroxyl radicals for water decontamination. Environ. Sci. Technol. 2022, 56, 4980–4987. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Z.; You, S.; Liu, Y. Electrogenerated quinone intermediates mediated peroxymonosulfate activation toward effective water decontamination and electrode antifouling. Appl. Catal. B 2023, 320, 121980. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Y.; Liu, F.; Wang, Y.; Ren, N.; You, S. Atomic hydrogen in electrocatalytic systems: Generation, identification, and environmental applications. Water Res. 2022, 223, 118994. [Google Scholar] [CrossRef]
- Li, Y.; Ma, J.; Wu, Z.; Wang, Z. Direct electron transfer coordinated by oxygen vacancies boosts selective nitrate reduction to N2 on a Co–CuOx electroactive filter. Environ. Sci. Technol. 2022, 56, 8673–8681. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, W.; Jia, R.; Yu, Y.; Zhang, B. Unveiling the activity origin of a copper-based electrocatalyst for selective nitrate reduction to ammonia. Angew. Chem. Int. Ed. 2020, 59, 5350–5354. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Wu, J.; Wang, Y.; Long, Y.; Fan, G. Active site and adsorption behavior engineering of subsize PdNi nanoparticles for boosting electrocatalytic hydrodechlorination of 4-chlorophenol. Appl. Surf. Sci. 2022, 600, 153988. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, L.; Zhou, C.; Zhou, Y.; Xia, S.; Rittmann, B.E. Co-removal of 2,4-dichlorophenol and nitrate using a palladized biofilm: Denitrification-promoted microbial mineralization following catalytic dechlorination. J. Hazard. Mater. 2022, 422, 126916. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B Condens Matter. 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B Condens Matter. 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Du, B.; Nasaruddin, R.R.; Chen, T.; Xie, J. Golden carbon nanotube membrane for continuous flow catalysis. Ind. Eng. Chem. Res. 2017, 56, 2999–3007. [Google Scholar] [CrossRef] [Green Version]





| Materials | External Energy | Pollutant Concentration | pH | Removal Efficiency | Ref. |
|---|---|---|---|---|---|
| Pd-MBfR | H2 | 0.1 mM | 75% (2 h) | [32] | |
| Pd/C | Electricity | 0.31 mM | 4.0 | 35.7% (2 h) | [33] |
| Pd/TiN | Electricity | 0.31 mM | 4.0 | 60.3% (2 h) | [33] |
| C–Pd | Electricity | 0.31 mM | 6.5 | 47.5% (2 h) | [34] |
| Pd/TiN | Electricity | 0.31 mM | 3.5 | 76.4% (2 h) | [35] |
| Pd-NiMOF/Ni foam | Electricity | 0.045 mM | 4.0 | 82.8% (2 h) | [36] |
| AuNC@CNT | Electricity | 0.5 mM | 3.0 | 91.5% (2 h) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Yan, H.; Liu, F.; Yao, J.; You, S.; Liu, Y. Electrocatalytic Hydrodechlorination Using Supported Atomically Precise Gold Nanoclusters under Flow-Through Configuration. Catalysts 2023, 13, 1045. https://doi.org/10.3390/catal13071045
Zhao Z, Yan H, Liu F, Yao J, You S, Liu Y. Electrocatalytic Hydrodechlorination Using Supported Atomically Precise Gold Nanoclusters under Flow-Through Configuration. Catalysts. 2023; 13(7):1045. https://doi.org/10.3390/catal13071045
Chicago/Turabian StyleZhao, Zhiyuan, Haochen Yan, Fuqiang Liu, Jie Yao, Shijie You, and Yanbiao Liu. 2023. "Electrocatalytic Hydrodechlorination Using Supported Atomically Precise Gold Nanoclusters under Flow-Through Configuration" Catalysts 13, no. 7: 1045. https://doi.org/10.3390/catal13071045





