“Pharaoh’s Snakes” Reaction-Derived Carbon with Favorable Structure and Composition as Metal-Free Oxygen Reduction Reaction Electrocatalyst
Abstract
:1. Introduction
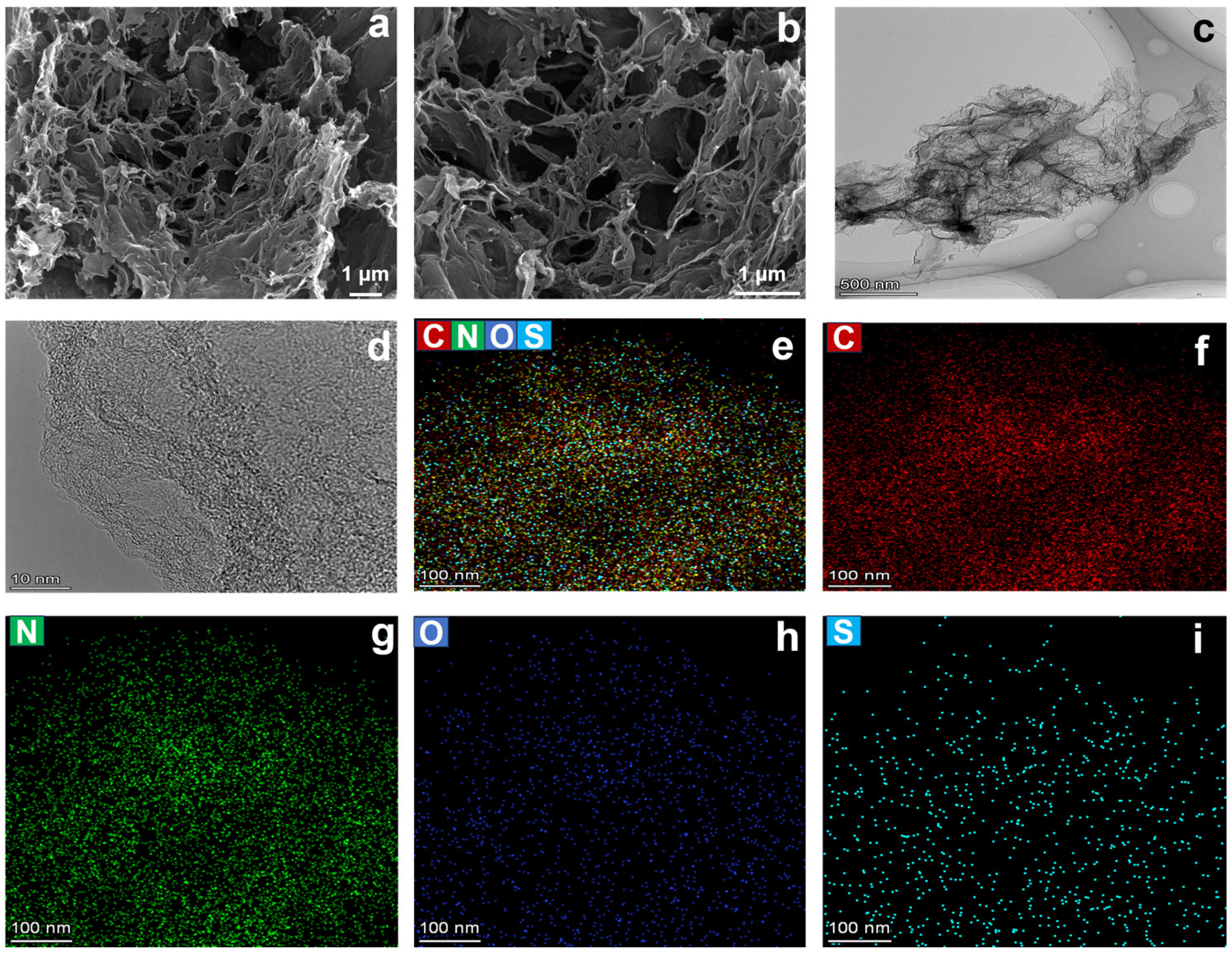
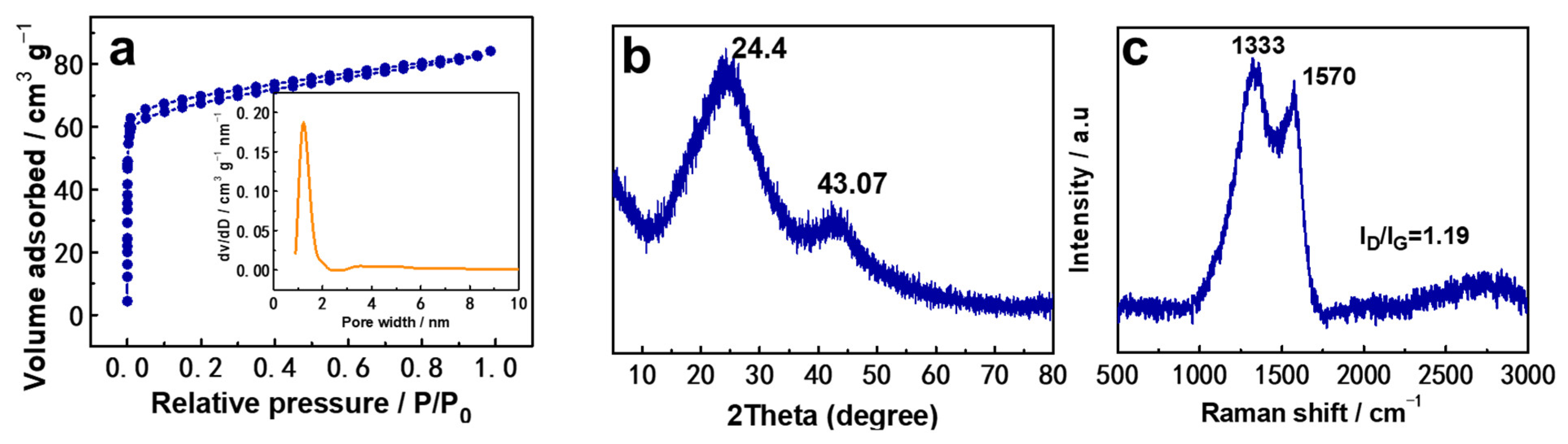
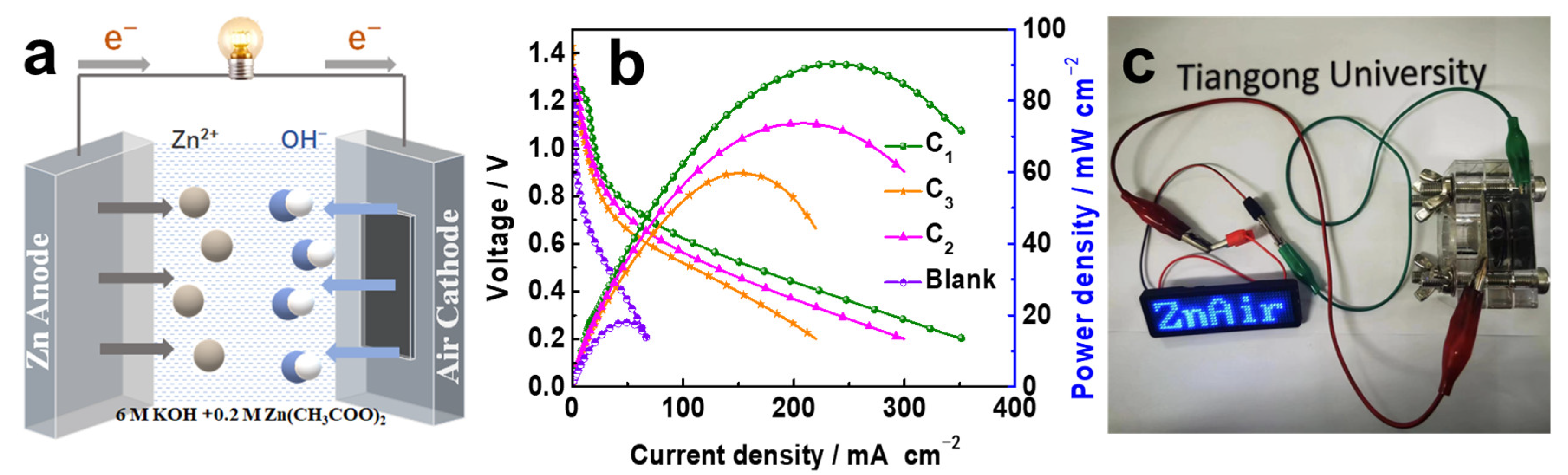
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, G.; Wang, X.L.; Fu, J.; Li, J.D.; Park, M.G.; Zhang, Y.N.; Lui, G.; Chen, Z.W. Pomegranate-inspired design of highly active and durable bifunctional electrocatalysts for rechargeable metal-air batteries. Angew. Chem. Int. Ed. 2016, 55, 4977–4982. [Google Scholar] [CrossRef] [PubMed]
- Borghei, M.; Laocharoen, N.; Kibena-Põldsepp, E.; Johansson, L.-S.; Campbell, J.; Kauppinen, E.; Tammeveski, K.; Rojas, O.J. Porous N, P-doped carbon from coconut shells with high electrocatalytic activity for oxygen reduction: Alternative to Pt-C for alkaline fuel cells. Appl. Catal. B-Environ. 2017, 204, 394–402. [Google Scholar] [CrossRef]
- Adith, R.V.; Madasamy, K.; Ebenezer, J.; Mohanapriya, N.; Kosame, S.; Ramesh, B.K.; Kathiresan, M.; Kumar, S.S.; Chandrasekaran, N. Molecularly engineered oxygen deficient magnetite decorated carbon as electrocatalysts for oxygen reduction reaction. Mol. Catal. 2021, 514, 11837–11847. [Google Scholar] [CrossRef]
- Yin, Z.; Lin, L.; Ma, D. Construction of Pd-based nanocatalysts for fuel cells: Opportunities and challenges. Catal. Sci. Technol. 2014, 4, 4116–4128. [Google Scholar] [CrossRef]
- Gao, J.; Ma, N.; Zhai, J.; Li, T.; Qin, W.; Zhang, T.; Yin, Z. Polymerizable ionic liquid as nitrogen-doping precursor for Co-N-C catalyst with enhanced oxygen reduction activity. Ind. Eng. Chem. Res. 2015, 54, 7984–7989. [Google Scholar] [CrossRef]
- Gao, J.; Ma, N.; Zheng, Y.; Zhang, J.; Gui, J.; Guo, C.; An, H.; Tan, X.; Yin, Z.; Ma, D. Cobalt/nitrogen-doped porous carbon nanosheets derived from polymerizable ionic liquids as bifunctional electrocatalyst for oxygen evolution and oxygen reduction reaction. ChemCatChem 2017, 9, 1601–1609. [Google Scholar] [CrossRef]
- Zhuang, Q.; Ma, N.; Yin, Z.; Yang, X.; Yin, Z.; Gao, J.; Xu, Y.; Gao, Z.; Wang, H.; Kang, J. Rich surface oxygen vacancies of MnO2 for enhancing electrocatalytic oxygen reduction and oxygen evolution reactions. Adv. Energy Sustain. Res. 2021, 2, 2100030. [Google Scholar] [CrossRef]
- Yin, Z.; Chi, M.; Zhu, Q.; Ma, D.; Sun, J.; Bao, X. Supported bimetallic PdAu nanoparticles with superior electrocatalytic activity towards methanol oxidation. J. Mater. Chem. A 2013, 1, 9157–9163. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction. ACS Nano 2012, 6, 205–211. [Google Scholar] [CrossRef]
- Yang, W.G.; Gong, Z.W.; Chen, Y.N.; Chen, R.R.; Meng, D.L.; Cao, M.N. Nitrogen Doped Carbon as Efficient Catalyst toward Oxygen Reduction Reaction. Chin. J. Struc. Chem. 2020, 39, 287–293. [Google Scholar]
- Gao, J.; Wang, Y.; Wu, H.; Liu, X.; Wang, L.; Yu, Q.; Li, A.; Wang, H.; Song, C.; Gao, Z.; et al. Construction of a sp3/sp2 carbon interface in 3D N-doped nanocarbons for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2019, 58, 15089–15097. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jin, C.; Yang, Z.; Tian, J.; Yang, R. Synthesis of phosphorus-doped carbon hollow spheres as efficient metal-free electrocatalysts for oxygen reduction. Carbon 2015, 82, 562–571. [Google Scholar] [CrossRef]
- He, B.; Shen, J.; Ma, D.; Lu, Z.; Yang, Z. Boron-Doped C3N Monolayer as a Promising Metal-Free Oxygen Reduction Reaction Catalyst: A Theoretical Insight. J. Phys. Chem. C 2018, 122, 20312–20322. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, Z.; Shao, Y.; Chen, Z.; Wei, X.; Wu, Z. Single-precursor design and solvent-free nanocasting synthesis of N/S/O-doped ordered mesoporous carbons with trimodal pores for excellent oxygen reduction. Carbon 2021, 183, 390–403. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Najam, T.; Nazir, M.A.; Wu, Y.Y.; Ali, H.; Rehman, A.U.; Rahman, M.M.; Imran, M.; Javed, M.S. Salt-assisted gas-liquid interfacial fluorine doping: Metal-free defect-induced electrocatalyst for oxygen reduction reaction. Mol. Catal. 2021, 514, 11878–11888. [Google Scholar] [CrossRef]
- Gao, J.; He, C.; Liu, J.; Ren, P.; Lu, H.; Feng, J.; Zou, Z.; Yin, Z.; Wen, X.; Tan, X. Polymerizable ionic liquid as a precursor for N, P co-doped carbon toward the oxygen reduction reaction. Catal. Sci. Technol. 2018, 8, 1142–1150. [Google Scholar] [CrossRef]
- Cao, S.; Shang, W.; Li, G.-L.; Lu, Z.-F.; Wang, X.; Yan, Y.; Hao, C.; Wang, S.; Sun, G. Defect-rich and metal-free N, S co-doped 3D interconnected mesoporous carbon material as an advanced electrocatalyst towards oxygen reduction reaction. Carbon 2021, 184, 127–135. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Tang, Y.; Li, S.; Dong, H.; Li, K.; Han, M.; Lan, Y.-Q.; Bao, J.; Dai, Z. Metal–organic framework templated nitrogen and sulfur co-doped porous carbons as highly efficient metal-free electrocatalysts for oxygen reduction reactions. J. Mater. Chem. A 2014, 2, 6316–6319. [Google Scholar] [CrossRef]
- Bera, R.K.; Park, H.; Ryoo, R. Co3O4 nanosheets on zeolite-templated carbon as an efficient oxygen electrocatalyst for a zinc–air battery. J. Mater. Chem. A 2019, 7, 9988–9996. [Google Scholar] [CrossRef]
- Yang, J.; Xiang, F.; Guo, H.; Wang, L.; Niu, X. Honeycomb-like porous carbon with N and S dual-doping as metal-free catalyst for the oxygen reduction reaction. Carbon 2020, 156, 514–522. [Google Scholar] [CrossRef]
- Zhu, Q.; Lian, J.; Chen, X.; Zhao, J.; Gao, Y.; Wang, X. Dual optimization strategy to construct hierarchical reticulated porous framework with enriched Fe-NX active species for the highly efficient oxygen reduction reaction. Int. J. Hydrogen Energy 2022, 47, 16840–16851. [Google Scholar] [CrossRef]
- Jalili, A.H.; Shokouhi, M.; Maurer, G.; Hosseini-Jenab, M. Solubility of CO2 and H2S in the ionic liquid 1-ethyl-3-methylimidazolium tris(pentafluoroethyl) trifluorophosphate. J. Chem. Thermodyn. 2014, 74, 286. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, M.; Wang, X.; Wang, H.; Yin, Z.; Tan, X.; Li, Y. Preparing Co/N-Doped Carbon as Electrocatalyst toward Oxygen Reduction Reaction via the Ancient “Pharaoh’s Snakes” Reaction. Batteries 2022, 8, 150. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Dai, L. Nitrogen, phosphorus, and fluorine tri-doped graphene as a multifunctional catalyst for self-powered electrochemical water splitting. Angew. Chem. Int. Ed. 2016, 55, 13296–13300. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Guo, C.X.; Zheng, Y.; Qiao, S.Z. Surface and interface engineering of noble-metal-free electrocatalysts for efficient energy conversion processes. Acc. Chem. Res. 2017, 50, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Qiu, H.J.; Fujita, T.; Tanabe, Y.; Tanigaki, K.; Chen, M.W. Bicontinuous Nanoporous N-doped Graphene for the Oxygen Reduction Reaction. Adv. Mater. 2014, 26, 4145–4150. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.J.; Baek, J.B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Tran, T.S.; Balu, R.; Nguyen, C.K.; Mata, J.; Truong, V.K.; Dutta, N.K.; Choudhury, N.R. Graphene Nanosheets Stabilized by P3HT Nanoparticles for Printable Metal-Free Electrocatalysts for Oxygen Reduction. ACS Appl. Nano Mater. 2023, 6, 908–917. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wang, P.; Lu, R.; Feng, S.; Yuan, G.; Wang, C. Design and synthesis of hollow Ce/Zr-UiO-66 nanoreactors for synergistic and efficient catalysis. J. Solid State Chem. 2022, 312, 123306. [Google Scholar] [CrossRef]
- Patel, P.P.; Datta, M.K.; Velikokhatnyi, O.I.; Kuruba, R.; Damodaran, K.; Jampani, P.; Gattu, B.; Shanthi, P.M.; Damle, S.S.; Kumta, P.N. Noble metal-free bifunctional oxygen evolution and oxygen reduction acidic media electro-catalysts. Sci. Rep. 2016, 6, 28367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavel, C.C.; Cecconi, F.; Emiliani, C.; Santiccioli, S.; Scaffidi, A.; Catanorchi, S.; Comotti, M. Highly efficient platinum group metal free based membrane-electrode assembly for anion exchange membrane water electrolysis. Angew. Chem. Int. Ed. 2014, 53, 1378–1381. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Cui, X.; Yang, G.; Zheng, W. Amorphous carbon enriched with pyridinic nitrogen as an efficient metal-free electrocatalyst for oxygen reduction reaction. Chem. Commun. 2014, 50, 557–559. [Google Scholar] [CrossRef]
- Cao, L.; Lin, Z.; Huang, J.; Yu, X.; Wu, X.; Zhang, B.; Zhan, Y.; Xie, F.; Zhang, W.; Chen, J. Nitrogen doped amorphous carbon as metal free electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2017, 42, 876–885. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-Doped Graphene by Chemical Vapor Deposition and Its Electrical Properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar]
- Wang, J.; Gao, D.; Wang, G.; Miao, S.; Wu, H.; Li, J.; Bao, X. Cobalt nanoparticles encapsulated in nitrogen-doped carbon as a bifunctional catalyst for water electrolysis. J. Mater. Chem. A 2014, 2, 20067–20074. [Google Scholar]
- Zhimin, L.; Dongliang, Y.; Guangqin, Q.; Jingzhi, S.; Huanping, Y.; Yanlong, W.; Lihui, Y.; Ting, Y.; Wei, H.; Lianhui, W. Microwave-assisted solvothermal preparation of nitrogen and sulfur co-doped reduced graphene oxide and graphene quantum dots hybrids for highly efficient oxygen reduction. J. Mater. Chem. A 2014, 2, 20605–20611. [Google Scholar]
- Wu, H.; Shi, L.; Lei, J.; Liu, D.; Qu, D.; Xie, Z.; Du, X.; Yang, P.; Hu, X.; Li, J. Nitrogen and sulfur co-doped carbon with three-dimensional ordered macroporosity: An efficient metal-free oxygen reduction catalyst derived from ionic liquid. J. Power Sources 2016, 323, 90–96. [Google Scholar] [CrossRef]
- Chen, X.; Xu, X.; Yang, Z.; Liu, Z.; Zhang, L.; Xu, X.; Chen, Y.; Huang, S. Sulfur-doped porous reduced graphene oxide hollow nanosphere frameworks as metal-free electrocatalysts for oxygen reduction reaction and as supercapacitor electrode materials. Nanoscale 2014, 6, 13740–13747. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Jia, Y.; Chen, F.; Zhu, Z.; Yao, X. Defective-activated-carbon-supported Mn-Co nanoparticles as a highly efficient electrocatalyst for oxygen reduction. Adv. Mater. 2016, 28, 8771–8778. [Google Scholar] [CrossRef]
- Yan, X.; Jia, Y.; Odedairo, T.; Zhao, X.; Jin, Z.; Zhu, Z.; Yao, X. Activated carbon becomes active for oxygen reduction and hydrogen evolution reactions. Chem. Commun. 2016, 52, 8156–8159. [Google Scholar] [CrossRef]
- Barik, R.; Raulo, A.; Jha, S.; Nandan, B.; Ingole, P.P. Polymer-derived electrospun Co3O4@ C porous nanofiber network for flexible, high-performance, and stable supercapacitors. ACS. Appl. Energy Mater. 2020, 3, 11002–11014. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, X.; Lv, S.; Li, Y.; Ren, J.; Huang, Y. Hollow NH2-MIL-101@ TA derived electrocatalyst for enhanced oxygen reduction reaction and oxygen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 38692–38700. [Google Scholar] [CrossRef]
- Wang, S.; Dong, S.; Wang, J.; Zhang, L.; Han, P.; Zhang, C.; Wang, X.; Zhang, K.; Lan, Z.; Cui, G. Oxygen-enriched carbon material for catalyzing oxygen reduction towards hybrid electrolyte Li-air battery. J. Mater. Chem. 2012, 22, 21051–21056. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Highly porous S-doped carbons. Micropo. Mesopor. Mat. 2012, 158, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Ruan, C.; Chen, Y.; Jiang, C.; Ai, K.; Lu, L. Covalent entrapment of cobalt–iron sulfides in N-doped mesoporous carbon: Extraordinary bifunctional electrocatalysts for oxygen reduction and evolution reactions. ACS Appl. Mater. Interfaces 2015, 7, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Kurak, K.A.; Anderson, A.B. Nitrogen-treated graphite and oxygen electroreduction on pyridinic edge sites. J. Phys. Chem. C 2009, 113, 6730–6734. [Google Scholar] [CrossRef]
- Zhu, J.; He, C.; Li, Y.; Kang, S.; Shen, P.K. One-step synthesis of boron and nitrogen-dual-self-doped graphene sheets as non-metal catalysts for oxygen reduction reaction. J. Mater. Chem. A 2013, 1, 14700–14705. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Z.; Jia, L.; Xiao, Z. Origin of the catalytic activity of graphite nitride for the electrochemical reduction of oxygen: Geometric factors vs. electronic factors. Phys. Chem. Chem. Phys. 2009, 11, 2730–2740. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wu, H.; Niu, T.; Wang, S.; Fu, G.; Jin, W.; Ma, T. Sulfurated metal–organic framework-derived nanocomposites for efficient bifunctional oxygen electrocatalysis and rechargeable Zn–air battery. ACS Sustain. Chem. Eng. 2020, 8, 9226–9234. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, J. Facile synthesis of multi-walled carbon nanotubes/Co9S8 composites with enhanced performances for sodium-ion battery. Mater. Lett. 2017, 195, 26–30. [Google Scholar] [CrossRef]
- Wang, A.; Liang, H.; Chen, F.; Tian, X.; Yin, S.; Jing, S.; Tsiakaras, P. Facile synthesis of C3N4/NiIn2S4 heterostructure with novel solar steam evaporation efficiency and photocatalytic H2O2 production performance. Appl. Catal. B-Environ. 2022, 310, 121336. [Google Scholar] [CrossRef]
- Ingavale, S.B.; Patil, I.M.; Parse, H.B.; Ramgir, N.; Kakade, B.; Swami, A. B, N, S tri-doped reduced graphite oxide–cobalt oxide composite: A bifunctional electrocatalyst for enhanced oxygen reduction and oxygen evolution reactions. New J. Chem. 2018, 42, 12908–12917. [Google Scholar] [CrossRef]
- Mahmood, J.; Li, F.; Kim, C.; Choi, H.-J.; Gwon, O.; Jung, S.-M.; Seo, J.-M.; Cho, S.-J.; Ju, Y.-W.; Jeong, H.Y. Fe@ C2N: A highly-efficient indirect-contact oxygen reduction catalyst. Nano Energy 2018, 44, 304–310. [Google Scholar] [CrossRef]
- Zhang, D.; Han, M.; Li, Y.; Lei, L.; Shang, Y.; Wang, K.; Wang, Y.; Zhang, Z.; Zhang, X.; Feng, H. Phosphorus and sulfur dual doped hierarchic porous carbons with superior supercapacitance performance. Electrochim. Acta 2016, 222, 141–148. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Liu, D.; Chen, T.; Jia, S.; Yang, F.; Zhang, X. Carboxyl functionalized double-walled carbon nanotubes for oxygen evolution reaction. Electrochim. Acta 2022, 419, 140395. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Z. Mechanisms of Oxygen Reduction Reaction on Nitrogen-Doped Graphene for Fuel Cells. J. Phys. Chem. C 2011, 115, 11170–11176. [Google Scholar] [CrossRef]
- Sun, J.; Lowe, S.E.; Zhang, L.; Wang, Y.; Pang, K.; Wang, Y.; Zhong, Y.; Liu, P.; Zhao, K.; Tang, Z. Ultrathin nitrogen-doped holey carbon@ graphene bifunctional electrocatalyst for oxygen reduction and evolution reactions in alkaline and acidic media. Angew. Chem. Int. Ed. 2018, 57, 16511–16515. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, L.; Zhu, Z.; Chen, S.; Dou, Y.; Liu, P.; Wang, Y.; Yin, H.; Tang, Z.; Zhao, H. Wet-chemistry grafted active pyridinic nitrogen sites on holey graphene edges as high performance ORR electrocatalyst for Zn-Air batteries. Mater. Today Energy 2019, 11, 24–29. [Google Scholar] [CrossRef]
- Fan, H.; Wang, Y.; Gao, F.; Yang, L.; Liu, M.; Du, X.; Wang, P.; Yang, L.; Wu, Q.; Wang, X. Hierarchical sulfur and nitrogen co-doped carbon nanocages as efficient bifunctional oxygen electrocatalysts for rechargeable Zn-air battery. J. Energy Chem. 2019, 34, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Paraknowitsch, J.P.; Thomas, A.; Antonietti, M. A detailed view on the polycondensation of ionic liquid monomers towards nitrogen doped carbon materials. J. Mater. Chem. 2010, 20, 6746–6758. [Google Scholar] [CrossRef]
- Flyagina, I.; Hughes, K.; Pourkashanian, M.; Ingham, D. A theoretical study of molecular oxygen chemisorption on N, B, or O doped carbon edge sites. Fuel Cells 2014, 14, 709–719. [Google Scholar] [CrossRef]
- Wang, S.; Yu, D.; Dai, L. Polyelectrolyte functionalized carbon nanotubes as efficient metal-free electrocatalysts for oxygen reduction. J. Am. Chem. Soc. 2011, 133, 5182–5185. [Google Scholar] [CrossRef]
- Subramanian, P.; Schechter, A. Electrochemical oxygen reduction activity of cobalt-nitrogen-carbon composite catalyst prepared by single precursor pyrolysis under autogenic pressure. J. Electrochem. Soc. 2016, 163, F428–F436. [Google Scholar] [CrossRef]
- Wang, J.; Pan, J.; Zeng, X.; Tang, G.; Cai, J.; Khan, A.; Sun, Y.; Liu, X. A facile preparation of nano-Ag4Bi2O5/MnOx on wrinkled rGO as greatly enhanced ternary catalyst for oxygen reduction reaction in alkaline electrolyte. J. Solid State Electrochem. 2019, 23, 2737–2746. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karaman, C.; Karaman, O.; Karimi, F.; Vasseghian, Y.; Fu, L.; Baghayeri, M.; Rouhi, J.; Senthil Kumar, P.; Show, P.-L. Nanochemistry approach for the fabrication of Fe and N co-decorated biomass-derived activated carbon frameworks: A promising oxygen reduction reaction electrocatalyst in neutral media. J. Nanostructure Chem. 2022, 12, 429–439. [Google Scholar] [CrossRef]
- Goswami, C.; Saikia, H.; Tada, K.; Tanaka, S.; Sudarsanam, P.; Bhargava, S.K.; Bharali, P. Bimetallic palladium–nickel nanoparticles anchored on carbon as high-performance electrocatalysts for oxygen reduction and formic acid oxidation reactions. ACS. Appl. Energy Mater. 2020, 3, 9285–9295. [Google Scholar] [CrossRef]
- Tran, T.S.; Balu, R.; de Campo, L.; Dutta, N.K.; Choudhury, N.R. Sulfonated polythiophene-interfaced graphene for water-redispersible graphene powder with high conductivity and electrocatalytic activity. Energy Adv. 2023, 2, 365–374. [Google Scholar] [CrossRef]
- Gan, R.; Song, Y.; Ma, C.; Shi, J. In situ growth of N-doped carbon nanotubes in Fe-Nx/Fe2O3/Fe3O4-encapsulated carbon sheets for efficient bifunctional oxygen catalysis. Appl. Catal. B-Environ. 2023, 327, 122443–122452. [Google Scholar] [CrossRef]
- Tang, C.; Wang, B.; Wang, H.F.; Zhang, Q. Defect engineering toward atomic Co–Nx–C in hierarchical graphene for rechargeable flexible solid Zn-air batteries. Adv. Mater. 2017, 29, 1703185–1703194. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Zhou, W.; Wang, H.L.; Xie, L.M.; Liang, Y.Y.; Wei, F.; Idrobo, J.C.; Pennycook, S.J.; Dai, H.J. An oxygen reduction electrocatalyst based on carbon nanotube-graphene complexes. Nat. Nanotechnol. 2012, 7, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yu, J.; Wei, Z.; Li, G.; Cao, L.; Zhou, W.; Chen, S. Co-N-doped MoO2 nanowires as efficient electrocatalysts for the oxygen reduction reaction and hydrogen evolution reaction. Nano Energy 2017, 41, 772–779. [Google Scholar] [CrossRef]



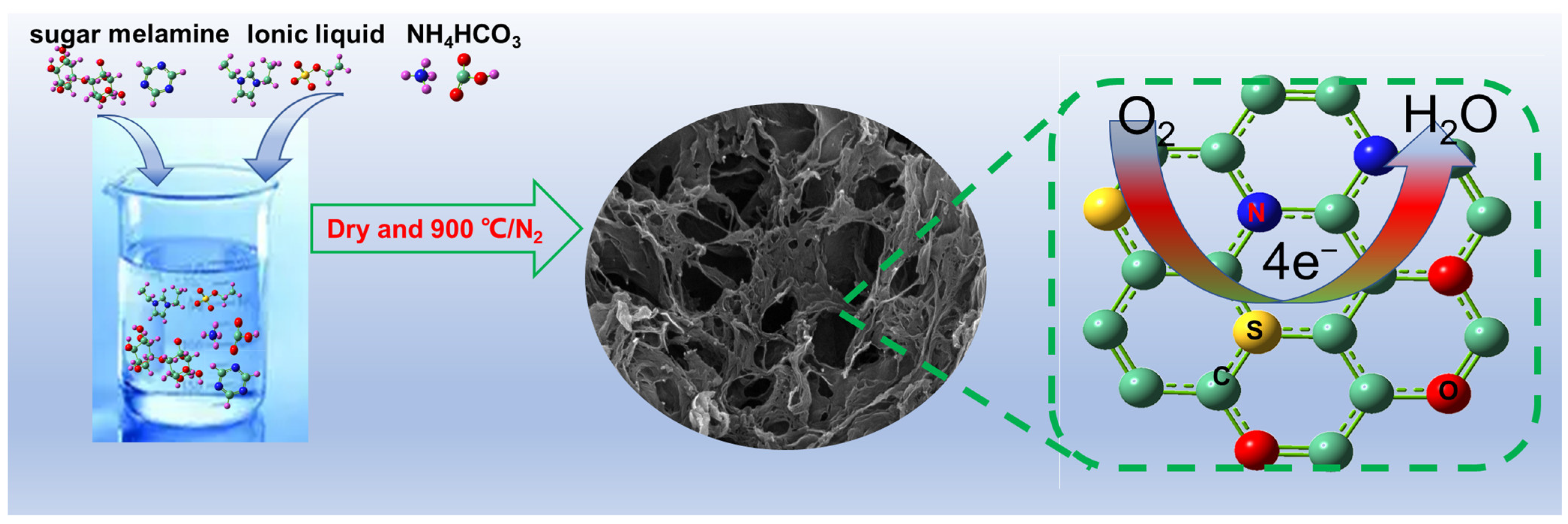
| Sample | Precursor | Composition |
|---|---|---|
| C1 | Sugar, NH4HCO3, melamine, [Etvim]EtSO4 | C, N, S, O |
| C2 | sugar + melamine + NH4HCO3 | C, N, O |
| C3 | [Etvim]EtSO4 + NH4HCO3 | C, N, S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, X.; Wang, H.; Tan, X.; Liu, D.; Gui, J.; Gao, J.; Yin, Z.; Ma, N.; Wang, Y. “Pharaoh’s Snakes” Reaction-Derived Carbon with Favorable Structure and Composition as Metal-Free Oxygen Reduction Reaction Electrocatalyst. Catalysts 2023, 13, 1059. https://doi.org/10.3390/catal13071059
Li Y, Wang X, Wang H, Tan X, Liu D, Gui J, Gao J, Yin Z, Ma N, Wang Y. “Pharaoh’s Snakes” Reaction-Derived Carbon with Favorable Structure and Composition as Metal-Free Oxygen Reduction Reaction Electrocatalyst. Catalysts. 2023; 13(7):1059. https://doi.org/10.3390/catal13071059
Chicago/Turabian StyleLi, Yuan, Xinyao Wang, Hong Wang, Xiaoyao Tan, Dan Liu, Jianzhou Gui, Jian Gao, Zhen Yin, Na Ma, and Yun Wang. 2023. "“Pharaoh’s Snakes” Reaction-Derived Carbon with Favorable Structure and Composition as Metal-Free Oxygen Reduction Reaction Electrocatalyst" Catalysts 13, no. 7: 1059. https://doi.org/10.3390/catal13071059





