Enhancing the Activity of Cu-MOR by Water for Oxidation of Methane to Methanol
Abstract
:1. Introduction
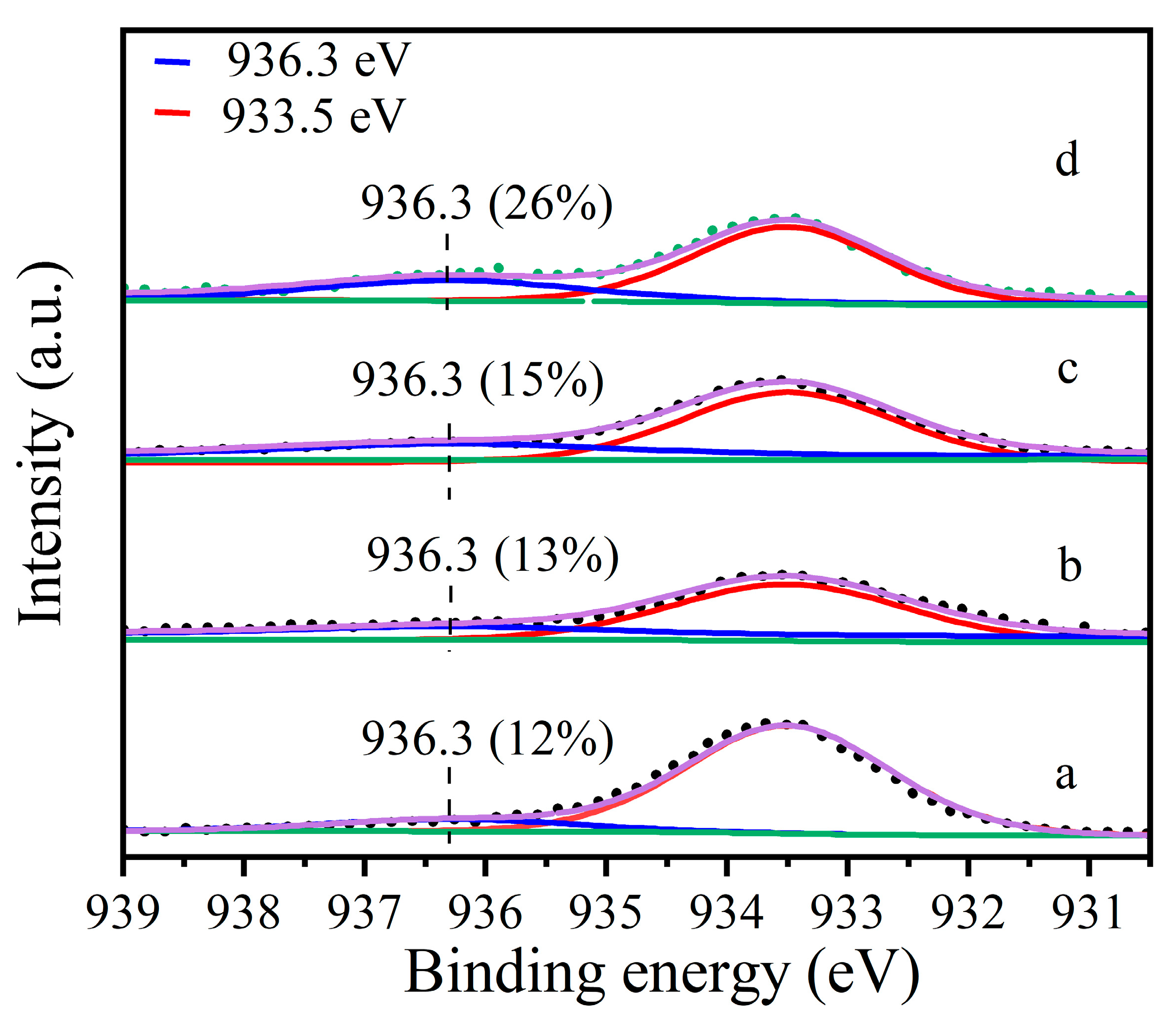
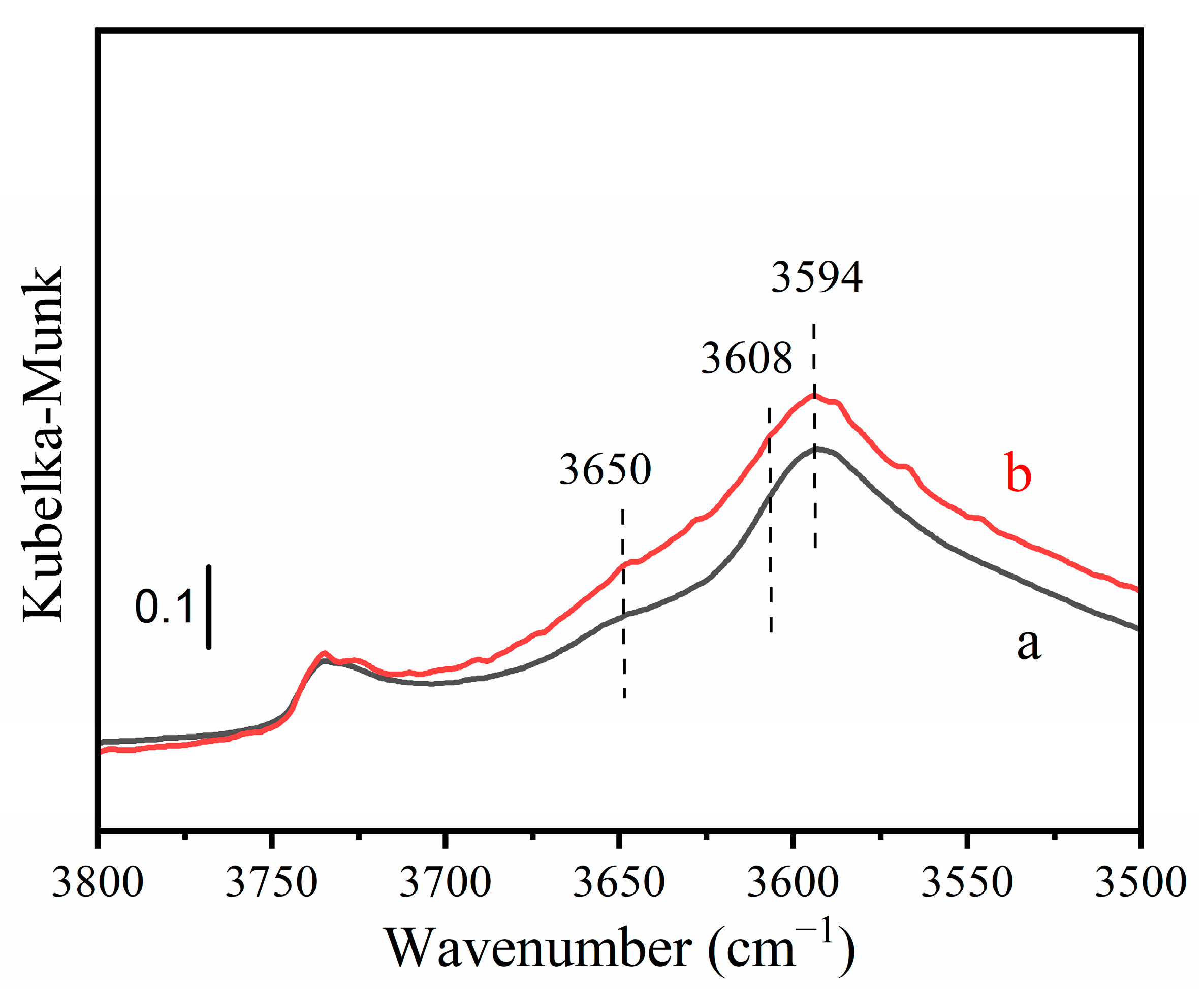
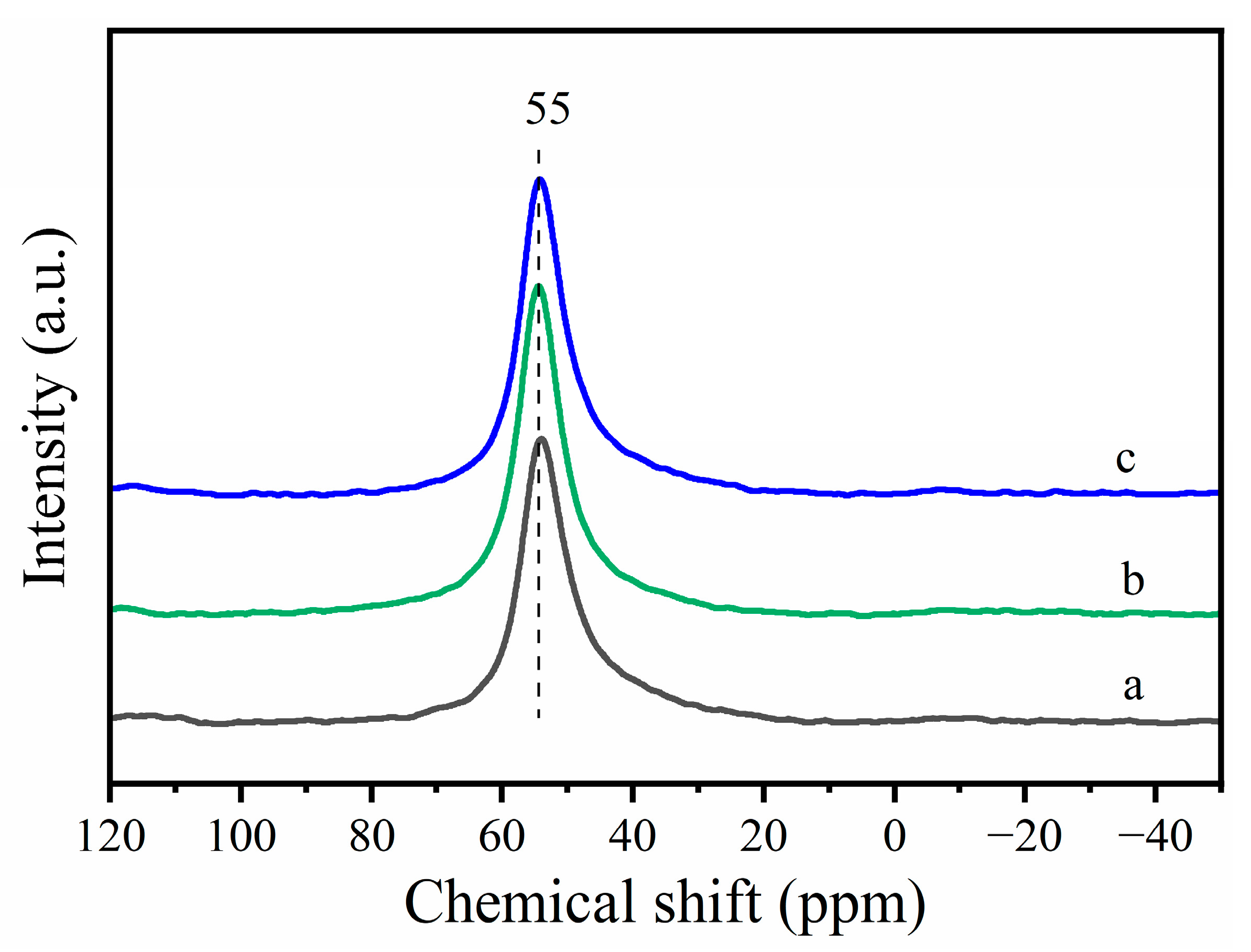
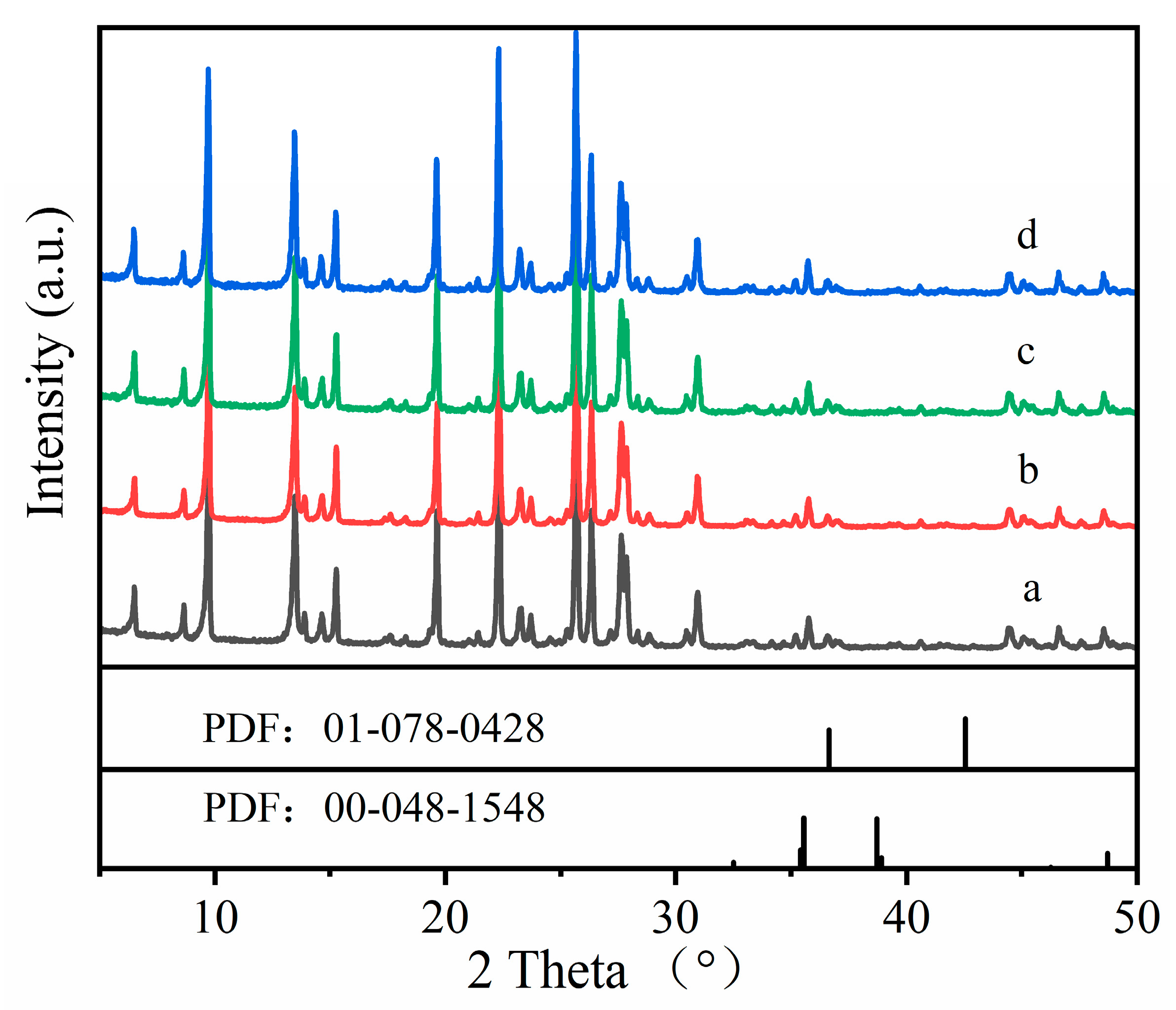
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Characterization of Catalysts
3.4. Testing of Activity for Selective Oxidation of Methane
- Step (1)
- A total of 0.2 g Cu-MOR was heated from ambient temperature to 450 °C at 5 °C/min and activated at 450 °C for 2 h in a flow of air (20 mL/min). The activated catalyst was cooled to room temperature at 5 °C/min in dry air.
- Step (2)
- The activated catalyst was transferred to an autoclave. The autoclave was first purged several times with CH4 and charged with 1 MPa CH4, and then heated to 200 °C for 12 h.
- Step (3)
- After the reaction, the autoclave was cooled to room temperature and excess CH4 was vented. The gas was collected using a gas bag and analyzed by a GC with a TCD detector. The catalyst was then extracted with a solution of 5% H2O/CH3CN for 2 h. Internal standard n-propanol was added to the extraction solution and the product was quantified by gas chromatography (Agilent Technologies, Santa Clara, CA, USA) on an Agilent 7890A (HP-5 column, 30 m, 0.32 mm inner diameter). The yield of methanol was calculated based on the total amount of methanol and dimethyl ether, where each dimethyl ether was calculated as two methanol.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dickens, G.R. The Potential Volume of Oceanic Methane Hydrates with Variable External Conditions. Org. Geochem. 2001, 32, 1179–1193. [Google Scholar] [CrossRef]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X. Sen Review of Natural Gas Hydrates as an Energy Resource: Prospects and Challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- McFarland, E. Unconventional Chemistry for Unconventional Natural Gas. Science 2012, 338, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Albarracín-Suazo, S.; Pagán-Torres, Y.; Nikolla, E. Advances in Methane Conversion Processes. Catal. Today 2017, 285, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, H. Chemistry with Methane: Concepts Rather than Recipes. Angew. Chem.—Int. Ed. 2011, 50, 10096–10115. [Google Scholar] [CrossRef]
- Schwach, P.; Pan, X.; Bao, X. Direct Conversion of Methane to Value-Added Chemicals over Heterogeneous Catalysts: Challenges and Prospects. Chem. Rev. 2017, 117, 8497–8520. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Guan, N.; Li, L. Methane Activation and Utilization: Current Status and Future Challenges. Energy Technol. 2020, 8, 1900826. [Google Scholar] [CrossRef]
- Olivos-Suarez, A.I.; Szécsényi, À.; Hensen, E.J.M.; Ruiz-Martinez, J.; Pidko, E.A.; Gascon, J. Strategies for the Direct Catalytic Valorization of Methane Using Heterogeneous Catalysis: Challenges and Opportunities. ACS Catal. 2016, 6, 2965–2981. [Google Scholar] [CrossRef]
- Zhao, G.; Drewery, M.; Mackie, J.; Oliver, T.; Kennedy, E.M.; Stockenhuber, M. The Catalyzed Conversion of Methane to Value-Added Products. Energy Technol. 2020, 8, 1900665. [Google Scholar] [CrossRef]
- Ravi, M.; Ranocchiari, M.; van Bokhoven, J.A. The Direct Catalytic Oxidation of Methane to Methanol—A Critical Assessment. Angew. Chem.—Int. Ed. 2017, 56, 16464–16483. [Google Scholar] [CrossRef]
- Tabor, E.; Dedecek, J.; Mlekodaj, K.; Sobalik, Z.; Andrikopoulos, P.C.; Sklenak, S. Dioxygen Dissociation over Man-Made System at Room Temperature to Form the Active α-Oxygen for Methane Oxidation. Sci. Adv. 2020, 6, eaaz9776. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Huang, W.; Nguyen, L.; Yu, Y.; Zhang, S.; Li, Y.; Frenkel, A.I.; Tao, F. Conversion of Methane to Methanol with a Bent Mono(μ-Oxo)Dinickel Anchored on the Internal Surfaces of Micropores. Langmuir 2014, 30, 8558–8569. [Google Scholar] [CrossRef] [PubMed]
- Groothaert, M.H.; Smeets, P.J.; Sels, B.F.; Jacobs, P.A.; Schoonheydt, R.A. Selective Oxidation of Methane by the Bis(μ-Oxo)Dicopper Core Stabilized on ZSM-5 and Mordenite Zeolites. J. Am. Chem. Soc. 2005, 127, 1394–1395. [Google Scholar] [CrossRef]
- Grundner, S.; Markovits, M.A.C.; Li, G.; Tromp, M.; Pidko, E.A.; Hensen, E.J.M.; Jentys, A.; Sanchez-Sanchez, M.; Lercher, J.A. Single-Site Trinuclear Copper Oxygen Clusters in Mordenite for Selective Conversion of Methane to Methanol. Nat. Commun. 2015, 6, 7546. [Google Scholar] [CrossRef] [Green Version]
- Pappas, D.K.; Martini, A.; Dyballa, M.; Kvande, K.; Teketel, S.; Lomachenko, K.A.; Baran, R.; Glatzel, P.; Arstad, B.; Berlier, G.; et al. The Nuclearity of the Active Site for Methane to Methanol Conversion in Cu-Mordenite: A Quantitative Assessment. J. Am. Chem. Soc. 2018, 140, 15270–15278. [Google Scholar] [CrossRef]
- Heyer, A.J.; Plessers, D.; Braun, A.; Rhoda, H.M.; Bols, M.L.; Hedman, B.; Hodgson, K.O.; Schoonheydt, R.A.; Sels, B.F.; Solomon, E.I. Methane Activation by a Mononuclear Copper Active Site in the Zeolite Mordenite: Effect of Metal Nuclearity on Reactivity. J. Am. Chem. Soc. 2022, 144, 19305–19316. [Google Scholar] [CrossRef] [PubMed]
- Woertink, J.S.; Smeets, P.J.; Groothaert, M.H.; Vance, M.A.; Sels, B.F.; Schoonheydt, R.A.; Solomon, E.I. A [Cu2O]2+ Core in Cu-ZSM-5, the Active Site in the Oxidation of Methane to Methanol. Proc. Natl. Acad. Sci. USA 2009, 106, 18908–18913. [Google Scholar] [CrossRef] [PubMed]
- Vanelderen, P.; Snyder, B.E.R.; Tsai, M.L.; Hadt, R.G.; Vancauwenbergh, J.; Coussens, O.; Schoonheydt, R.A.; Sels, B.F.; Solomon, E.I. Spectroscopic Definition of the Copper Active Sites in Mordenite: Selective Methane Oxidation. J. Am. Chem. Soc. 2015, 137, 6383–6392. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.; Lee, M.-S.; Tao, L.; Ikuno, T.; Khare, R.; Jentys, A.; Huthwelker, T.; Borca, C.N.; Kalinko, A.; Gutiérrez, O.Y.; et al. Activity of Cu–Al–Oxo Extra-Framework Clusters for Selective Methane Oxidation on Cu-Exchanged Zeolites. JACS Au 2021, 1, 1412–1421. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Palagin, D.; Ranocchiari, M.; Van Bokhoven, J.A. Selective Anaerobic Oxidation of Methane Enables Direct Synthesis of Methanol. Science 2017, 356, 523–527. [Google Scholar] [CrossRef]
- Koishybay, A.; Shantz, D.F. Water Is the Oxygen Source for Methanol Produced in Partial Oxidation of Methane in a Flow Reactor over Cu-SSZ-13. J. Am. Chem. Soc. 2020, 142, 11962–11966. [Google Scholar] [CrossRef]
- Bulánek, R.; Wichterlová, B.; Sobalík, Z.; Tichý, J. Reducibility and Oxidation Activity of Cu Ions in Zeolites Effect of Cu Ion Coordination and Zeolite Framework Composition. Appl. Catal. B Environ. 2001, 31, 13–25. [Google Scholar] [CrossRef]
- Neylon, M.K.; Marshall, C.L.; Kropf, A.J. In Situ EXAFS Analysis of the Temperature-Programmed Reduction of Cu-ZSM-5. J. Am. Chem. Soc. 2002, 124, 5457–5465. [Google Scholar] [CrossRef] [PubMed]
- Le, H.V.; Parishan, S.; Sagaltchik, A.; Göbel, C.; Schlesiger, C.; Malzer, W.; Trunschke, A.; Schomäcker, R.; Thomas, A. Solid-State Ion-Exchanged Cu/Mordenite Catalysts for the Direct Conversion of Methane to Methanol. ACS Catal. 2017, 7, 1403–1412. [Google Scholar] [CrossRef]
- Sainz-Vidal, A.; Balmaseda, J.; Lartundo-Rojas, L.; Reguera, E. Preparation of Cu-Mordenite by Ionic Exchange Reaction under Milling: A Favorable Route to Form the Mono-(μ-Oxo) Dicopper Active Species. Microporous Mesoporous Mater. 2014, 185, 113–120. [Google Scholar] [CrossRef]
- Wang, J.B.; Lin, S.C.; Huang, T.J. Selective CO Oxidation in Rich Hydrogen over CuO/Samaria-Doped Ceria. Appl. Catal. A Gen. 2002, 232, 107–120. [Google Scholar] [CrossRef]
- Contarini, S.; Kevan, L. X-Ray Photoelectron Spectroscopic Study of Copper-Exchanged X- and Y-Type Sodium Zeolites: Resolution of Two Cupric Ion Components and Dependence on Dehydration and X-Irradiation. J. Phys. Chem. 1986, 90, 1630–1632. [Google Scholar] [CrossRef]
- Platzman, I.; Brener, R.; Haick, H.; Tannenbaum, R. Oxidation of Polycrystalline Copper Thin Films at Ambient Conditions. J. Phys. Chem. C. 2008, 112, 1101–1108. [Google Scholar] [CrossRef]
- Shpiro, E.S.; Grünert, W.; Joyner, R.W.; Baeva, G.N. Nature, Distribution and Reactivity of Copper Species in over-Exchanged Cu-ZSM-5 Catalysts: An XPS/XAES Study. Catal. Lett. 1994, 24, 159–169. [Google Scholar] [CrossRef]
- Artiglia, L.; Sushkevich, V.L.; Palagin, D.; Knorpp, A.J.; Roy, K.; van Bokhoven, J.A. In Situ X-Ray Photoelectron Spectroscopy Detects Multiple Active Sites Involved in the Selective Anaerobic Oxidation of Methane in Copper-Exchanged Zeolites. ACS Catal. 2019, 9, 6728–6737. [Google Scholar] [CrossRef]
- Paparazzo, E. On the Curve-Fitting of XPS Ce(3d) Spectra of Cerium Oxides. Mater. Res. Bull. 2011, 46, 323–326. [Google Scholar] [CrossRef]
- Lin, T.C.; Seshadri, G.; Kelber, J.A. A Consistent Method for Quantitative XPS Peak Analysis of Thin Oxide Films on Clean Polycrystalline Iron Surfaces. Appl. Surf. Sci. 1997, 119, 83–92. [Google Scholar] [CrossRef]
- Sunding, M.F.; Hadidi, K.; Diplas, S.; Løvvik, O.M.; Norby, T.E.; Gunnæs, A.E. XPS Characterisation of in Situ Treated Lanthanum Oxide and Hydroxide Using Tailored Charge Referencing and Peak Fitting Procedures. J. Electron Spectros. Relat. Phenom. 2011, 184, 399–409. [Google Scholar] [CrossRef]
- Martini, A.; Borfecchia, E.; Lomachenko, K.A.; Pankin, I.A.; Negri, C.; Berlier, G.; Beato, P.; Falsig, H.; Bordiga, S.; Lamberti, C. Composition-Driven Cu-Speciation and Reducibility in Cu-CHA Zeolite Catalysts: A Multivariate XAS/FTIR Approach to Complexity. Chem. Sci. 2017, 8, 6836–6851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ipek, B.; Wulfers, M.J.; Kim, H.; Göltl, F.; Hermans, I.; Smith, J.P.; Booksh, K.S.; Brown, C.M.; Lobo, R.F. Formation of [Cu2O2]2+ and [Cu2O]2+ toward C-H Bond Activation in Cu-SSZ-13 and Cu-SSZ-39. ACS Catal. 2017, 7, 4291–4303. [Google Scholar] [CrossRef]
- Borfecchia, E.; Lomachenko, K.A.; Giordanino, F.; Falsig, H.; Beato, P.; Soldatov, A.V.; Bordiga, S.; Lamberti, C. Revisiting the Nature of Cu Sites in the Activated Cu-SSZ-13 Catalyst for SCR Reaction. Chem. Sci. 2015, 6, 548–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappas, D.K.; Borfecchia, E.; Dyballa, M.; Pankin, I.A.; Lomachenko, K.A.; Martini, A.; Signorile, M.; Teketel, S.; Arstad, B.; Berlier, G.; et al. Methane to Methanol: Structure-Activity Relationships for Cu-CHA. J. Am. Chem. Soc. 2017, 139, 14961–14975. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Wang, Y.; Lu, J.; Zhang, J.; Zhang, Z.; Xu, S.; Liu, X.; Zhang, T.; Gocyla, M.; Heggen, M.; et al. Acid-Promoter-Free Ethylene Methoxycarbonylation over Ru-Clusters/Ceria: The Catalysis of Interfacial Lewis Acid-Base Pair. J. Am. Chem. Soc. 2018, 140, 4172–4181. [Google Scholar] [CrossRef]
- Xue, H.; Huang, X.; Zhan, E.; Ma, M.; Shen, W. Selective Dealumination of Mordenite for Enhancing Its Stability in Dimethyl Ether Carbonylation. Catal. Commun. 2013, 37, 75–79. [Google Scholar] [CrossRef]
- Maurin, G.; Senet, P.; Devautour, S.; Gaveau, P.; Henn, F.; Van Doren, V.E.; Giuntini, J.C. Combining the Monte Carlo Technique with 29SI NMR Spectroscopy: Simulations of Cation Locations in Zeolites with Various Si/Al Ratios. J. Phys. Chem. B. 2001, 105, 9157–9161. [Google Scholar] [CrossRef]
- Dimitrijevic, R.; Lutz, W.; Ritzmann, A. Hydrothermal Stability of Zeolites: Determination of Extra-Framework Species of H-Y Faujasite-Type Steamed Zeolite. J. Phys. Chem. Solids 2006, 67, 1741–1748. [Google Scholar] [CrossRef]


| Entry | Sample | Methanol Yield (μmol/g) 1 | Efficiency (mol CH3OH/mol Cu) | CH3OH/(CH3OH + DME) |
|---|---|---|---|---|
| 1 | Cu-MOR1 2 | 36 | 0.07 | 100% |
| 2 | Cu-MOR1-water 2 | 92 | 0.17 | 86% |
| 3 | Cu-MOR1-water 3 | 92 | 0.17 | 83% |
| 4 | Cu-MOR1-water 4 | 90 | 0.17 | 83% |
| 5 | Cu-MOR1-water 5 | 26 | 0.05 | 100% |
| 6 | Cu-MOR1-water 6 | 46 | 0.09 | 100% |
| 7 | Cu-MOR2 7 | 50 | 0.06 | 100% |
| 8 | Cu-MOR2-water 7 | 128 | 0.15 | 93% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, X.; Wang, Y.; Liu, X.; Du, H.; Guo, X.; Zhang, Z. Enhancing the Activity of Cu-MOR by Water for Oxidation of Methane to Methanol. Catalysts 2023, 13, 1066. https://doi.org/10.3390/catal13071066
Guan X, Wang Y, Liu X, Du H, Guo X, Zhang Z. Enhancing the Activity of Cu-MOR by Water for Oxidation of Methane to Methanol. Catalysts. 2023; 13(7):1066. https://doi.org/10.3390/catal13071066
Chicago/Turabian StyleGuan, Xi’an, Yehong Wang, Xiumei Liu, Hong Du, Xinwen Guo, and Zongchao Zhang. 2023. "Enhancing the Activity of Cu-MOR by Water for Oxidation of Methane to Methanol" Catalysts 13, no. 7: 1066. https://doi.org/10.3390/catal13071066






