CoFe Amorphous Double Hydroxides Modified Hematite Photoanode with the Synergism of Co and Fe for Enhanced Photoelectrochemical Water Oxidation
Abstract
:1. Introduction
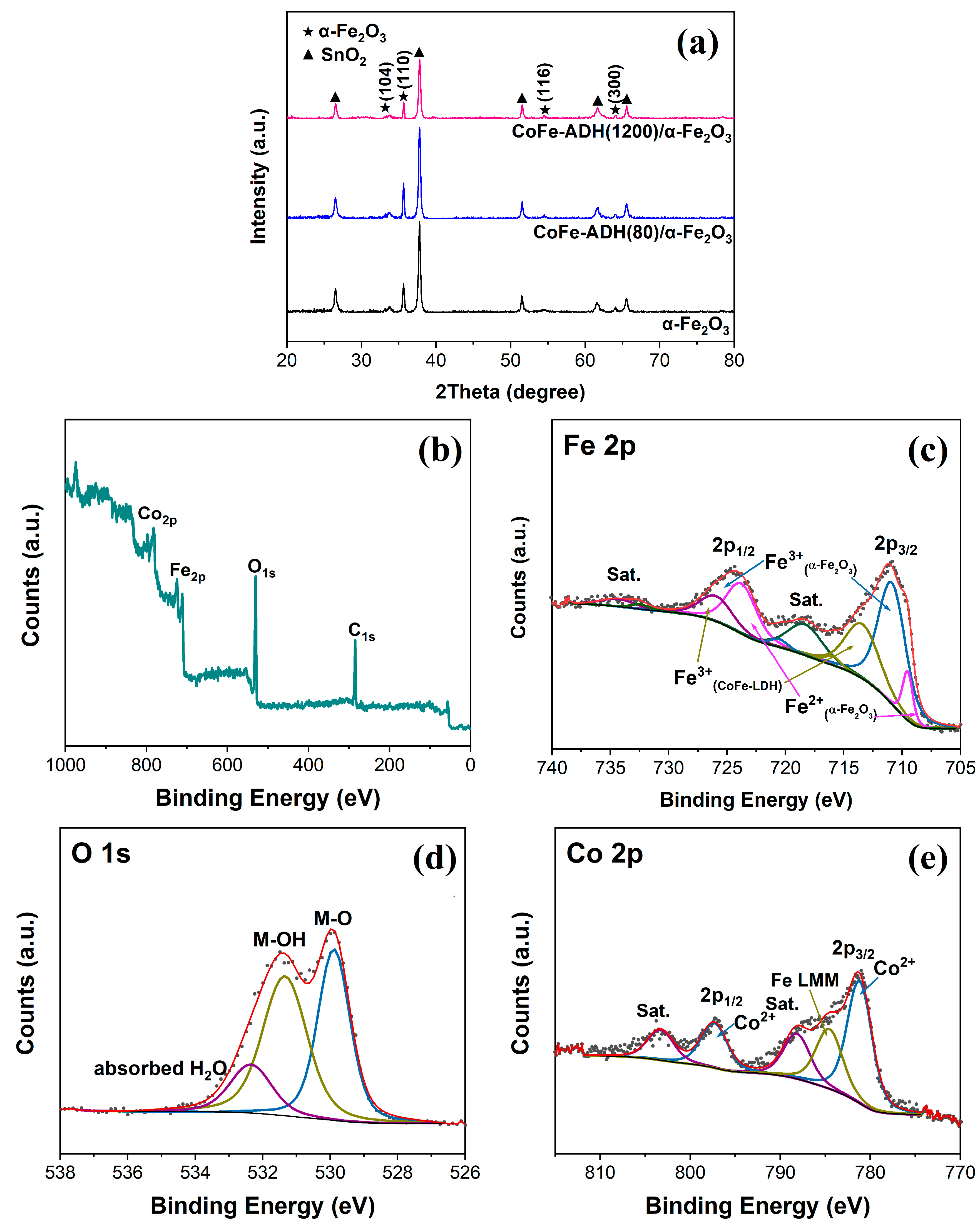
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Photoanodes
3.2. Characterization
3.3. Photoelectrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ta, X.M.C.; Daiyan, R.; Nguyen, T.K.A.; Amal, R.; Tran-Phu, T.; Tricoli, A. Alternatives to Water Photooxidation for Photoelectrochemical Solar Energy Conversion and Green H2 Production. Adv. Energy Mater. 2022, 12, 202201358. [Google Scholar] [CrossRef]
- Tilley, S.D. Recent Advances and Emerging Trends in Photo-Electrochemical Solar Energy Conversion. Adv. Energy Mater. 2019, 9, 1802877. [Google Scholar] [CrossRef]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 0003. [Google Scholar] [CrossRef]
- Xiao, M.; Luo, B.; Wang, Z.L.; Wang, S.C.; Wang, L.Z. Recent Advances of Metal-Oxide Photoanodes: Engineering of Charge Separation and Transportation toward Efficient Solar Water Splitting. Sol. RRL 2020, 4, 1900509. [Google Scholar]
- Li, F.; Jian, J.; Wang, S.; Zhang, Z.; Jia, L.; Guan, X.; Xu, Y.; Wang, H. TiO2 passivation layers with laser derived p-n heterojunctions enable boosted photoelectrochemical performance of α-Fe2O3 photoanodes. Chem. Eng. J. 2023, 461, 141872. [Google Scholar] [CrossRef]
- Yang, W.; Prabhakar, R.R.; Tan, J.; Tilley, S.D.; Moon, J. Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting. Chem. Soc. Rev. 2019, 48, 4979–5015. [Google Scholar] [CrossRef]
- Zhang, H.; Li, P.; Zhou, H.; Xu, J.; Jiang, Q.; Hadden, J.H.L.; Wang, Y.; Wang, M.; Chen, S.; Xie, F.; et al. Unravelling the synergy of oxygen vacancies and gold nanostars in hematite for the electrochemical and photoelectrochemical oxygen evolution reaction. Nano Energy 2022, 94, 106968. [Google Scholar] [CrossRef]
- Yi, S.S.; Wang, Z.Y.; Li, H.M.; Zafar, Z.; Zhang, Z.T.; Zhang, L.Y.; Chen, D.L.; Liu, Z.Y.; Yue, X.Z. Coupling effects of indium oxide layer on hematite enabling efficient photoelectrochemical water splitting. Appl. Catal. B Environ. 2021, 283, 119649. [Google Scholar] [CrossRef]
- Cao, X.; Wen, P.; Ma, R.; Liu, Y.; Sun, S.; Ma, Q.; Zhang, P.; Qiu, Y. Ni2P nanocrystals modification on Ta:α-Fe2O3 photoanode for efficient photoelectrochemical water splitting: In situ formation and synergistic catalysis of Ni2P@NiOOH cocatalyst. Chem. Eng. J. 2022, 449, 137792. [Google Scholar] [CrossRef]
- Corby, S.; Rao, R.R.; Steier, L.; Durrant, J.R. The kinetics of metal oxide photoanodes from charge generation to catalysis. Nat. Rev. Mater. 2021, 6, 1136–1155. [Google Scholar] [CrossRef]
- Ahart, C.S.; Rosso, K.M.; Blumberger, J. Electron and Hole Mobilities in Bulk Hematite from Spin-Constrained Density Functional Theory. J. Am. Chem. Soc. 2022, 144, 4623–4632. [Google Scholar] [CrossRef] [PubMed]
- Bedin, K.C.; Muche, D.N.F.; Melo, M.A.; Freitas, A.L.M.; Goncalves, R.V.; Souza, F.L. Role of Cocatalysts on Hematite Photoanodes in Photoelectrocatalytic Water Splitting: Challenges and Future Perspectives. Chemcatchem 2020, 12, 3156–3169. [Google Scholar]
- Hamdani, I.R.; Bhaskarwar, A.N. Recent progress in material selection and device designs for photoelectrochemical water-splitting. Renew. Sust. Energ. Rev. 2021, 138, 110503. [Google Scholar] [CrossRef]
- Ning, X.M.; Du, P.Y.; Han, Z.G.; Chen, J.; Lu, X.Q. Insight into the Transition-Metal Hydroxide Cover Layer for Enhancing Photoelectrochemical Water Oxidation. Angew. Chem. Int. Ed. 2021, 60, 3504–3509. [Google Scholar] [CrossRef]
- Bu, Q.J.; Zhao, Q.F.; Sun, G.H.; Liu, Q.Y.; Xie, T.F. Boosting the photogenerated hole separation and injection of Ti-Fe2O3 by co-modifying carbon quantum dots and NiFe layered double hydroxide. J. Alloys Compd. 2022, 908, 164643. [Google Scholar] [CrossRef]
- Wang, X.; Lei, Y.; Gao, Y.; Yun, X.; Wang, Z.; Fan, F.; Ma, Y. Multi-Function of the Ni Interlayer in the Design of a BiVO4-Based Photoanode for Photoelectrochemical Water Splitting. ACS Appl. Mater. Inter. 2022, 14, 48682–48693. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, B.H.; Lu, C.X.; Cai, Z.; Li, L.D. Hierarchical hollow nanocages of Ni–Co amorphous double hydroxides for high-performance asymmetric supercapacitors. J. Alloys Compd. 2020, 833, 155130. [Google Scholar] [CrossRef]
- Wang, H.; Xia, Y.; Wang, X.; Han, Y.; Jiao, X.; Chen, D. Interfacial Coupling Effect on Electron Transport in Hierarchical TaON/Au/ZnCo-LDH Photoanode with Enhanced Photoelectrochemical Water Oxidation. ACS Appl. Mater. Inter. 2019, 11, 33062–33073. [Google Scholar] [CrossRef]
- Chong, R.; Wang, G.; Du, Y.; Jia, Y.; Wang, X.; Li, C.; Chang, Z.; Zhang, L. Anion engineering of exfoliated CoAl layered double hydroxides on hematite photoanode toward highly efficient photoelectrochemical water splitting. Chem. Eng. J. 2019, 366, 523–530. [Google Scholar] [CrossRef]
- Zhong, Y.; Wu, C.; Jia, X.; Sun, S.; Chen, D.; Yao, W.; Ding, H.; Zhang, J.; Ma, T. Coupling of self-healing atomic layer CoAl-LDH onto Mo:BiVO4 photoanode for fast surface charge transfer toward stable and high-performance water splitting. Chem. Eng. J. 2023, 465, 142893. [Google Scholar] [CrossRef]
- Arif, M.; Yasin, G.; Shakeel, M.; Fang, X.; Gao, R.; Ji, S.; Yan, D. Coupling of Bifunctional CoMn-Layered Double Hydroxide@Graphitic C3N4 Nanohybrids towards Efficient Photoelectrochemical Overall Water Splitting. Chem. Asian. J. 2018, 13, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.Y.; Lopes, P.P.; Martins, P.F.B.D.; He, H.; Kawaguchi, T.; Zapol, P.; You, H.; Tripkovic, D.; Strmcnik, D.; Zhu, Y.; et al. Dynamic stability of active sites in hydr(oxy)oxides for the oxygen evolution reaction. Nat. Energy 2020, 5, 222–230. [Google Scholar] [CrossRef]
- Hung, S.F.; Chan, Y.T.; Chang, C.C.; Tsai, M.K.; Liao, Y.F.; Hiraoka, N.; Hsu, C.S.; Chen, H.M. Identification of Stabilizing High-Valent Active Sites by Operando High-Energy Resolution Fluorescence-Detected X-ray Absorption Spectroscopy for High-Efficiency Water Oxidation. J. Am. Chem. Soc. 2018, 140, 17263–17270. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Mao, C.; Zhang, R.; Shao, M.; Wei, M.; Feng, P. Reduced titania@layered double hydroxide hybrid photoanodes for enhanced photoelectrochemical water oxidation. J. Mater. Chem. A 2017, 5, 11016–11025. [Google Scholar] [CrossRef]
- Liu, W.; Liu, H.; Dang, L.; Zhang, H.; Wu, X.; Yang, B.; Li, Z.; Zhang, X.; Lei, L.; Jin, S. Amorphous Cobalt–Iron Hydroxide Nanosheet Electrocatalyst for Efficient Electrochemical and Photo-Electrochemical Oxygen Evolution. Adv. Funct. Mater. 2017, 27, 1603904. [Google Scholar] [CrossRef]
- Wei, P.; Wen, Y.; Lin, K.; Li, X. Turning off the “shunt channel” by coating with CoFe layered double hydroxide nanocrystals for efficient photoelectrocatalytic water splitting. Inorg. Chem. Front. 2022, 9, 4685–4694. [Google Scholar] [CrossRef]
- Xu, X.; Jin, S.; Yang, C.; Pan, J.; Du, W.; Hu, J.; Zeng, H.; Zhou, Y. Engineering Interfaces to Steer Hole Dynamics of BiVO4 Photoanodes for Solar Water Oxidation. Sol. RRL 2019, 3, 1900115. [Google Scholar] [CrossRef]
- Bhowmik, T.; Kundu, M.K.; Barman, S. CoFe Layered Double Hydroxide Supported on Graphitic Carbon Nitrides: An Efficient and Durable Bifunctional Electrocatalyst for Oxygen Evolution and Hydrogen Evolution Reactions. Acs Appl. Energy Mater. 2018, 1, 1200–1209. [Google Scholar]
- Arif, M.; Yasin, G.; Shakeel, M.; Mushtaq, M.A.; Ye, W.; Fang, X.; Ji, S.; Yan, D. Hierarchical CoFe-layered double hydroxide and g-C3N4 heterostructures with enhanced bifunctional photo/electrocatalytic activity towards overall water splitting. Mater. Chem. Front. 2019, 3, 520–531. [Google Scholar] [CrossRef]
- Tang, R.; Ying, M.; Zhang, X.; Zheng, R.; Huang, J. Interfacial Heterojunction-Engineered Fe2O3/CoFe-Layered Double Hydroxide Catalyst for the Electrocatalytic Oxygen Evolution Reaction. Energy Fuels 2022, 36, 11584–11590. [Google Scholar] [CrossRef]
- Annal, T.G.H.; Vishnu, K.P. Electrochemical Synthesis of Metal Oxides and Hydroxides. Chem. Mater. 2000, 12, 1195–1204. [Google Scholar]
- Ding, P.; Luo, F.; Wang, P.; Xia, W.; Xu, X.; Hu, J.; Zeng, H. Photo-induced charge kinetic acceleration in ultrathin layered double hydroxide nanosheets boosts the oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 1105–1112. [Google Scholar] [CrossRef]
- Yi, S.S.; Wulan, B.R.; Yan, J.-M.; Jiang, Q. Highly Efficient Photoelectrochemical Water Splitting: Surface Modification of Cobalt-Phosphate-Loaded Co3O4/Fe2O3 p–n Heterojunction Nanorod Arrays. Adv. Funct. Mater. 2019, 29, 1801902. [Google Scholar] [CrossRef]
- Bai, S.; Chu, H.; Xiang, X.; Luo, R.; He, J.; Chen, A. Fabricating of Fe2O3/BiVO4 heterojunction based photoanode modified with NiFe-LDH nanosheets for efficient solar water splitting. Chem. Eng. J. 2018, 350, 148–156. [Google Scholar] [CrossRef]
- Gao, L.; Wang, P.; Chai, H.; Li, S.; Jin, J.; Ma, J. Expediting hole transfer via surface states in hematite-based composite photoanodes. Nanoscale 2022, 14, 17044–17052. [Google Scholar] [CrossRef]
- Lv, J.; Liu, P.; Li, R.; Wang, L.; Zhang, K.; Zhou, P.; Huang, X.; Wang, G. Constructing accelerated charge transfer channels along V-Co-Fe via introduction of V into CoFe-layered double hydroxides for overall water splitting. Appl. Catal. B Environ. 2021, 298, 120587. [Google Scholar] [CrossRef]
- Zhang, J.; García-Rodríguez, R.; Cameron, P.; Eslava, S. Role of cobalt-iron (oxy)hydroxide (CoFeOx) as oxygen evolution catalyst on hematite photoanodes. Energy Environ. Sci. 2018, 11, 2972–2984. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Zhang, Y.; Ding, Y.; Bi, Y. Ultrathin FeOOH Nanolayers with Abundant Oxygen Vacancies on BiVO4 Photoanodes for Efficient Water Oxidation. Angew. Chem. Int. Ed. 2018, 57, 2248–2252. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.L.; Yang, L.C.; Gao, Q.S. Recent Advances of Two-Dimensional CoFe Layered-Double-Hydroxides for Electrocatalytic Water Oxidation, Chin. Chem. Lett. 2022, 33, 2845–2855. [Google Scholar]
- Rui, Q.; Wang, L.; Zhang, Y.; Feng, C.; Zhang, B.; Fu, S.; Guo, H.; Hu, H.; Bi, Y. Synergistic effects of P-doping and a MnO2 cocatalyst on Fe2O3 nanorod photoanodes for efficient solar water splitting. J. Mater. Chem. A 2018, 6, 7021–7026. [Google Scholar] [CrossRef]
- Yang, G.; Li, Y.; Pang, H.; Chang, K.; Ye, J. Ultrathin Cobalt–Manganese Nanosheets: An Efficient Platform for Enhanced Photoelectrochemical Water Oxidation with Electron-Donating Effect. Adv. Funct. Mater. 2019, 29, 1904622. [Google Scholar] [CrossRef]
- Quang, N.D.; Van, P.C.; Majumder, S.; Jeong, J.-R.; Kim, D.; Kim, C. Rational construction of S-doped FeOOH onto Fe2O3 nanorods for enhanced water oxidation. J. Colloid Interf. Sci. 2022, 616, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Z.; Kandiel, T.A.; Abdel-Azeim, S.; Jahangir, T.N.; Alhooshani, K. Phosphate ions interfacial drift layer to improve the performance of CoFe-Prussian blue hematite photoanode toward water splitting. Appl. Catal. B Environ. 2022, 304, 121014. [Google Scholar] [CrossRef]
- Zhe, X.; Wang, H.Y.; Wen, Y.Z.; Li, W.C.; Sun, C.Y.; He, Y.T.; Shi, Z.; Pei, L.; Chen, Y.D.; Yan, S.C.; et al. Balancing Catalytic Activity and Interface Energetics of Electrocatalyst-Coated Photoanodes for Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2022, 10, 3624–3633. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Han, M.; Wang, Y.; Ding, Y.; Huang, F. CoFe Amorphous Double Hydroxides Modified Hematite Photoanode with the Synergism of Co and Fe for Enhanced Photoelectrochemical Water Oxidation. Catalysts 2023, 13, 1235. https://doi.org/10.3390/catal13091235
Chang Y, Han M, Wang Y, Ding Y, Huang F. CoFe Amorphous Double Hydroxides Modified Hematite Photoanode with the Synergism of Co and Fe for Enhanced Photoelectrochemical Water Oxidation. Catalysts. 2023; 13(9):1235. https://doi.org/10.3390/catal13091235
Chicago/Turabian StyleChang, Yue, Minmin Han, Yang Wang, Yehui Ding, and Fei Huang. 2023. "CoFe Amorphous Double Hydroxides Modified Hematite Photoanode with the Synergism of Co and Fe for Enhanced Photoelectrochemical Water Oxidation" Catalysts 13, no. 9: 1235. https://doi.org/10.3390/catal13091235





