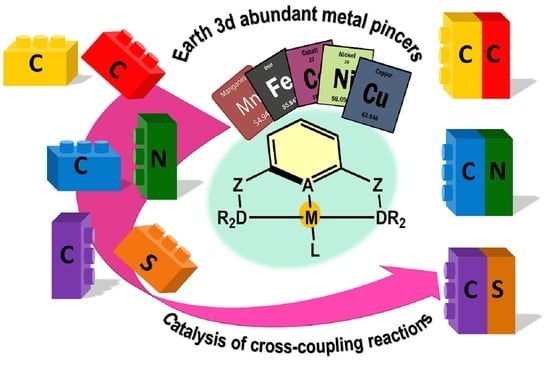
Advances in Cross-Coupling Reactions Catalyzed by Aromatic Pincer Complexes Based on Earth-Abundant 3d Metals (Mn, Fe, Co, Ni, Cu)
Abstract
:1. Introduction
2. Pincer Complexes: An Overview
3. Carbon–Carbon Cross-Coupling Reactions Catalyzed Using Pincer Complexes
3.1. Kumada–Corriu Cross-Coupling
| Entry | Complex Number | Loading (mol %) | Alkyl-Halide | Grignard Reagent | Reaction Condition | Yield (%) | Ref |
|---|---|---|---|---|---|---|---|
| 1 | a3 | 2 | o-TolCl | PhMgBr | THF, r.t., Ar, 22 h. | 71 | [59] |
| 2 | 4-(methoxy)chlorobenzene | PhMgBr | 69 | ||||
| 3 | a4 | 2 | o-TolCl | PhMgBr | 99 | ||
| 4 | 2 | 4-(methoxy)chlorobenzene | PhMgBr | 98 | |||
| 5 | a5 | 2 | PhCl | o-TolMgCl | THF, 40 °C, 12 h. | 40 | [60] |
| 6 | a6 | 2 | 96 | ||||
| 7 | a7 | 2 | 68 | ||||
| 8 | a8 | 2 | 56 | ||||
| 9 | a9 | 2 | 97 | ||||
| 10 | a10 | 3.5 | PhI | PhMgBr | THF, 0 °C, 24 h | 60 | [62] |
| 11 | 3.5 | PhCl | PhMgBr | 28 | |||
| 12 | a11 | 3.5 | PhCl | PhMgBr | THF, 25 °C, 24 h | 19 | |
| 13 | 3.5 | PhBr | PhMgBr | 51 |
3.2. Suzuki–Miyaura Cross-Coupling
3.3. Sonogashira Cross-Coupling
4. Carbon–Sulfur Cross-Coupling Reactions Catalyzed Using Pincer Complexes
5. Carbon–Nitrogen Bond-Forming Reactions Catalyzed Using Pincer Complexes
5.1. C–N Cross-Coupling Reactions
5.2. Acceptorless Dehydrogenation Coupling (ADC) and Borrowing Hydrogen (BH) Strategy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Song, Y.; Ma, L. Earth-Abundant Metal-Catalyzed Reductive Amination: Recent Advances and Prospect for Future Catalysis. Chem. Asian J. 2021, 16, 2371–2391. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Milstein, D. Homogeneous Catalysis by Cobalt and Manganese Pincer Complexes. ACS Catal. 2018, 8, 11435–11469. [Google Scholar] [CrossRef]
- Ott, J.C.; Bürgy, D.; Guan, H.; Gade, L.H. 3d Metal Complexes in T-Shaped Geometry as a Gateway to Metalloradical Reactivity. Accounts Chem. Res. 2022, 55, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Moulton, C.J.; Shaw, B.L. Transition Metal–Carbon Bonds. Part XLII. Complexes of Nickel, Palladium, Platinum, Rhodium and Iridium with the Tridentate Ligand 2,6-Bis[(Di-t-Butylphosphino)Methyl]Phenyl. J. Chem. Soc. Dalton Trans. 1976, 11, 1020–1024. [Google Scholar] [CrossRef]
- Arora, V.; Narjinari, H.; Nandi, P.G.; Kumar, A. Recent Advances in Pincer-Nickel Catalyzed Reactions. Dalton Trans. 2021, 50, 3394–3428. [Google Scholar] [CrossRef]
- González-Sebastián, L.; Morales-Morales, D. Cross-Coupling Reactions Catalysed by Palladium Pincer Complexes. A Review of Recent Advances. J. Organomet. Chem. 2019, 893, 39–51. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, Z.; Hu, X. Nickamine and Analogous Nickel Pincer Catalysts for Cross-Coupling of Alkyl Halides and Hydrosilylation of Alkenes. Accounts Chem. Res. 2019, 52, 1471–1483. [Google Scholar] [CrossRef]
- Rufino-Felipe, E.; Valdés, H.; Morales-Morales, D. C–S Cross-Coupling Reactions Catalyzed by Well-Defined Copper and Nickel Complexes. Eur. J. Org. Chem. 2022, 2022, e202200654. [Google Scholar] [CrossRef]
- Heravi, M.M.; Hajiabbasi, P. Recent Advances in Kumada-Tamao-Corriu Cross-Coupling Reaction Catalyzed by Different Ligands. Monatshefte Fur Chem. 2012, 143, 1575–1592. [Google Scholar] [CrossRef]
- Selander, N.; Szabó, K.J. Catalysis by Palladium Pincer Complexes. Chem. Rev. 2011, 111, 2048–2076. [Google Scholar] [CrossRef]
- Garbe, M.; Junge, K.; Beller, M. Homogeneous Catalysis by Manganese-Based Pincer Complexes. Eur. J. Org. Chem. 2017, 2017, 4344–4362. [Google Scholar] [CrossRef]
- Junge, K.; Papa, V.; Beller, M. Cobalt–Pincer Complexes in Catalysis. Chem. Eur. J. 2019, 25, 122–143. [Google Scholar] [CrossRef] [PubMed]
- González-Sebastián, L.; Reyes-Sanchez, A.; Morales-Morales, D. Hydrogenation and Cross-Coupling Reactions Catalyzed by Mn, Fe, and Co Aromatic Pincer Complexes. Organometallics 2023, 42, 2426–2446. [Google Scholar] [CrossRef]
- Morales-Morales, D. Pincer Complexes: Applications in Catalysis. ChemInform 2005, 36, 338–346. [Google Scholar] [CrossRef]
- Lawrence, M.A.W.; Green, K.-A.; Nelson, P.N.; Lorraine, S.C. Review: Pincer Ligands—Tunable, Versatile and Applicable. Polyhedron 2018, 143, 11–27. [Google Scholar] [CrossRef]
- Polukeev, A.V.; Wendt, O.F. Iridium Complexes with Aliphatic, Non-Innocent Pincer Ligands. J. Organomet. Chem. 2018, 867, 33–50. [Google Scholar] [CrossRef]
- Azerraf, C.; Gelman, D. New Shapes of PC(Sp3)P Pincer Complexes. Organometallics 2009, 28, 6578–6584. [Google Scholar] [CrossRef]
- Kumar, A.; Milstein, D. Recent Advances in the Applications of Metal-Ligand Cooperation via Dearomatization and Aromatization of Pincer Complexes BT—Metal-Ligand Co-Operativity: Catalysis and the Pincer-Metal Platform; Van Koten, G., Kirchner, K., Moret, M.-E., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–23. ISBN 978-3-030-68916-2. [Google Scholar]
- Ben-Ari, E.; Leitus, G.; Shimon, L.J.W.; Milstein, D. Metal-Ligand Cooperation in C-H and H2 Activation by an Electron-Rich PNP Ir(I) System: Facile Ligand Dearomatization-Aromatization as Key Steps. J. Am. Chem. Soc. 2006, 128, 15390–15391. [Google Scholar] [CrossRef]
- Schwartsburd, L.; Iron, M.A.; Konstantinovski, L.; Ben-Ari, E.; Milstein, D. A Dearomatized Anionic PNP Pincer Rhodium Complex: C-H and H-H Bond Activation by Metal-Ligand Cooperation and Inhibition by Dinitrogen. Organometallics 2011, 30, 2721–2729. [Google Scholar] [CrossRef]
- Castonguay, A.; Spasyuk, D.M.; Madern, N.; Beauchamp, A.L.; Zargarian, D. Regioselective Hydroamination of Acrylonitrile Catalyzed by Cationic Pincer Complexes of Nickel(II). Organometallics 2009, 28, 2134–2141. [Google Scholar] [CrossRef]
- Vabre, B.; Spasyuk, D.M.; Zargarian, D. Impact of Backbone Substituents on POCOP-Ni Pincer Complexes: A Structural, Spectroscopic, and Electrochemical Study. Organometallics 2012, 31, 8561–8570. [Google Scholar] [CrossRef]
- Salah, A.B.; Zargarian, D. The Impact of P-Substituents on the Structures, Spectroscopic Properties, and Reactivities of POCOP-Type Pincer Complexes of Nickel(II). Dalton Trans. 2011, 40, 8977–8985. [Google Scholar] [CrossRef] [PubMed]
- Valdés, H.; Rufino-Felipe, E.; van Koten, G.; Morales-Morales, D. Hybrid POCZP Aryl Pincer Metal Complexes and Their Catalytic Applications. Eur. J. Inorg. Chem. 2020, 2020, 4418–4424. [Google Scholar] [CrossRef]
- Peris, E.; Crabtree, R.H. Key Factors in Pincer Ligand Design. Chem. Soc. Rev. 2018, 47, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Farrell, K.; Albrecht, M. Late Transition Metal Complexes with Pincer Ligands That Comprise N-Heterocyclic Carbene Donor Sites. Privil. Pincer-Metal. Platf. Coord. Chem. Appl. 2015, 54, 45–92. [Google Scholar] [CrossRef]
- Kuwabara, J.; Kanbara, T. Synthesis and Optical Properties of Pincer Palladium and Platinum Complexes Having Thioamide Units. J. Photopolym. Sci. Technol. 2008, 21, 349–353. [Google Scholar] [CrossRef]
- Wang, C.H.; Ma, N.N.; Sun, X.X.; Sun, S.L.; Qiu, Y.Q.; Liu, P.J. Modulation of the Second-Order Nonlinear Optical Properties of the Two-Dimensional Pincer Ru(II) Complexes: Substituent Effect and Proton Abstraction Switch. J. Phys. Chem. A 2012, 116, 10496–10506. [Google Scholar] [CrossRef]
- Kocherga, M.; Boyle, K.M.; Merkert, J.; Schmedake, T.A.; Walter, M.G. Exploring the Molecular Electronic Device Applications of Synthetically Versatile Silicon Pincer Complexes as Charge Transport and Electroluminescent Layers. Mater. Adv. 2022, 3, 2373–2379. [Google Scholar] [CrossRef]
- Hegde, V.; Kulkarni, N.V.; Mathew, J. Synthesis and Characterization of Cobalt (II) Pincer Complexes and Their Application as Dyes in Dye-Sensitized Solar Cells. J. Mol. Struct. 2023, 1286, 135508. [Google Scholar] [CrossRef]
- Qu, J.-J.; Bai, P.; Liu, W.-N.; Liu, Z.-L.; Gong, J.-F.; Wang, J.-X.; Zhu, X.; Song, B.; Hao, X.-Q. New NNN Pincer Copper Complexes as Potential Anti-Prostate Cancer Agents. Eur. J. Med. Chem. 2022, 244, 114859. [Google Scholar] [CrossRef]
- El-Sayed, N.M.A.; Elsawy, H.; Adam, M.S.S. Polar and Nonpolar Iron (II) Complexes of Isatin Hydrazone Derivatives as Effective Catalysts in Oxidation Reactions and Their Antimicrobial and Anticancer Activities. Appl. Organomet. Chem. 2022, 36, e6662. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Reyes-Márquez, V.; Cañavera-Buelvas, F.; Parra-Unda, J.R.; Cuenú-Cabezas, F.; Polo-Cerón, D.; Colorado-Peralta, R.; Suárez-Moreno, G.V.; Aguilar-Castillo, B.A.; Morales-Morales, D. Pincer Complexes Derived from Tridentate Schiff Bases for Their Use as Antimicrobial Metallopharmaceuticals. Inorganics 2022, 10, 134. [Google Scholar] [CrossRef]
- Aleksanyan, D.V.; Konovalov, A.V.; Churusova, S.G.; Rybalkina, E.Y.; Peregudov, A.S.; Aksenova, S.A.; Gutsul, E.I.; Klemenkova, Z.S.; Kozlov, V.A. Modulation of the Cytotoxic Properties of Pd(II) Complexes Based on Functionalized Carboxamides Featuring Labile Phosphoryl Coordination Sites. Pharmaceutics 2023, 15, 1088. [Google Scholar] [CrossRef] [PubMed]
- Zell, T.; Milstein, D. Hydrogenation and Dehydrogenation Iron Pincer Catalysts Capable of Metal-Ligand Cooperation by Aromatization/Dearomatization. Accounts Chem. Res. 2015, 48, 1979–1994. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sahoo, B.; Junge, K.; Beller, M. Cobalt Complexes as an Emerging Class of Catalysts for Homogeneous Hydrogenations. Accounts Chem. Res. 2018, 51, 1858–1869. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ward, T.R. Recent Advances in the Palladium Catalyzed Suzuki-Miyaura Cross-Coupling Reaction in Water. Catal. Lett. 2016, 146, 820–840. [Google Scholar] [CrossRef]
- Roy, D.; Uozumi, Y. Recent Advances in Palladium-Catalyzed Cross-Coupling Reactions at Ppm to Ppb Molar Catalyst Loadings. Adv. Synth. Catal. 2018, 360, 602–625. [Google Scholar] [CrossRef]
- Christoffel, F.; Ward, T.R. Palladium-Catalyzed Heck Cross-Coupling Reactions in Water: A Comprehensive Review. Catal. Lett. 2018, 148, 489–511. [Google Scholar] [CrossRef]
- Kumbhar, A. Functionalized Nitrogen Ligands (C–N) for Palladium Catalyzed Cross-Coupling Reactions (Part II). J. Organomet. Chem. 2019, 881, 79–129. [Google Scholar] [CrossRef]
- Salih, K.S.M. Modern Development in Copper- and Nickel-Catalyzed Cross-Coupling Reactions: Formation of Carbon-Carbon and Carbon-Heteroatom Bonds under Microwave Irradiation Conditions. Asian J. Org. Chem. 2022, 11, 244–269. [Google Scholar] [CrossRef]
- Andrade, M.A.; Martins, L.M.D.R.S. New Trends in C–C Cross-Coupling Reactions: The Use of Unconventional Conditions. Molecules 2020, 25, 5506. [Google Scholar] [CrossRef] [PubMed]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd Metal Catalysts for Cross-Couplings and Related Reactions in the 21st Century: A Critical Review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.P.; Navarro, O. C–C Bond Formation by Cross-Coupling. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-0-12-409547-2. [Google Scholar]
- Corriu, R.J.P.; Masse, J.P. Activation of Grignard Reagents by Transition-Metal Complexes. A New and Simple Synthesis of Trans-Stilbenes and Polyphenyls. J. Chem. Soc. Chem. Commun. 1972, 7062, 144a. [Google Scholar] [CrossRef]
- Tamao, K.; Sumitani, K.; Kumada, M. Selective Carbon-Carbon Bond Formation by Cross-Coupling of Grignard Reagents with Organic Halides. Catalysis by Nickel-Phosphine Complexes. J. Am. Chem. Soc. 1972, 94, 4374–4376. [Google Scholar] [CrossRef]
- Gartia, Y.; Biswas, A.; Stadler, M.; Nasini, U.B.; Ghosh, A. Cross Coupling Reactions of Multiple CCl Bonds of Polychlorinated Solvents with Grignard Reagent Using a Pincer Nickel Complex. J. Mol. Catal. A Chem. 2012, 363–364, 322–327. [Google Scholar] [CrossRef]
- Frisch, A.C.; Beller, M. Catalysts for Cross-Coupling Reactions with Non-Activated Alkyl Halides. Angew. Chem. Int. Ed. 2005, 44, 674–688. [Google Scholar] [CrossRef]
- Hu, X. Nickel-Catalyzed Cross Coupling of Non-Activated Alkyl Halides: A Mechanistic Perspective. Chem. Sci. 2011, 2, 1867–1886. [Google Scholar] [CrossRef]
- Durandetti, M. Synthetic Applications of Nickel-Catalyzed Cross-Coupling and Cyclisation Reactions. Chem. Rec. 2021, 21, 3746–3757. [Google Scholar] [CrossRef]
- Jana, R.; Pathak, T.P.; Sigman, M.S. Advances in Transition Metal (Pd, Ni, Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-Organometallics as Reaction Partners. Chem. Rev. 2011, 111, 1417–1492. [Google Scholar] [CrossRef]
- Breitenfeld, J.; Ruiz, J.; Wodrich, M.D.; Hu, X. Bimetallic Oxidative Addition Involving Radical Intermediates in Nickel-Catalyzed Alkyl-Alkyl Kumada Coupling Reactions. ChemInform 2013, 135, 12004–12012. [Google Scholar] [CrossRef]
- Perez Garcia, P.M.; Di Franco, T.; Epenoy, A.; Scopelliti, R.; Hu, X. From Dimethylamine to Pyrrolidine: The Development of an Improved Nickel Pincer Complex for Cross-Coupling of Nonactivated Secondary Alkyl Halides. ACS Catal. 2016, 6, 258–261. [Google Scholar] [CrossRef]
- Gartia, Y.; Pulla, S.; Ramidi, P.; Farris, C.C.; Nima, Z.; Jones, D.E.; Biris, A.S.; Ghosh, A. A Novel Iron Complex for Cross-Coupling Reactions of Multiple C–Cl Bonds in Polychlorinated Solvents with Grignard Reagents. Catal. Lett. 2012, 142, 1397–1404. [Google Scholar] [CrossRef]
- Pandiri, H.; Gonnade, R.G.; Punji, B. Synthesis of Quinolinyl-Based Pincer Copper(II) Complexes: An Efficient Catalyst System for Kumada Coupling of Alkyl Chlorides and Bromides with Alkyl Grignard Reagents. Dalton Trans. 2018, 47, 16747–16754. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.G. Palladium-Catalysed Coupling Reactions; Beller, M., Blaser, H.-U., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–34. ISBN 978-3-642-32833-6. [Google Scholar]
- Ayogu, J.I.; Onoabedje, E.A. Recent Advances in Transition Metal-Catalysed Cross-Coupling of (Hetero)Aryl Halides and Analogues under Ligand-Free Conditions. Catal. Sci. Technol. 2019, 9, 5233–5255. [Google Scholar] [CrossRef]
- Zhang, W.; Dai, J.; Xu, H. Recent Advances of Transition Metal-Catalyzed C(Sp3)-C(Sp3) Cross-Coupling Reactions of Non-Activated Alkyl Halides. Chin. J. Org. Chem. 2015, 35, 1820–1833. [Google Scholar] [CrossRef]
- Sanford, J.; Dent, C.; Masuda, J.D.; Xia, A. Synthesis, Characterization and Application of Pincer-Type Nickel Iminophosphinite Complexes. Polyhedron 2011, 30, 1091–1094. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Sun, H. Arylation of Non-Activated C–Cl Bond with Grignard Reagents Catalyzed by Pincer [PCP]-Nickel Complexes. Inorganica Chim. Acta 2014, 415, 95–97. [Google Scholar] [CrossRef]
- Mastalir, M.; Glatz, M.; Stöger, B.; Weil, M.; Pittenauer, E.; Allmaier, G.; Kirchner, K. Synthesis, Characterization and Reactivity of Vanadium, Chromium, and Manganese PNP Pincer Complexes. Inorganica Chim. Acta 2017, 455, 707–714. [Google Scholar] [CrossRef]
- Hasche, P.; Joksch, M.; Vlachopoulou, G.; Agarwala, H.; Spannenberg, A.; Beweries, T. Synthesis of Symmetric and Nonsymmetric Ni II Thiophosphinito PECSP (E = S, O) Pincer Complexes and Their Applications in Kumada Coupling under Mild Conditions. Eur. J. Inorg. Chem. 2018, 2018, 676–680. [Google Scholar] [CrossRef]
- Miyaura, N.; Yamada, K.; Suzuki, A. A New Stereospecific Cross-Coupling by the Palladium-Catalyzed Reaction of 1-Alkenylboranes with 1-Alkenyl or 1-Alkynyl Halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Han, F.-S. Transition-Metal-Catalyzed Suzuki–Miyaura Cross-Coupling Reactions: A Remarkable Advance from Palladium to Nickel Catalysts. Chem. Soc. Rev. 2013, 42, 5270–5298. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.K.; Leonard, D.K.; Bajo, S.; Burton, P.M.; Nelson, D.J. Aldehydes and Ketones Influence Reactivity and Selectivity in Nickel-Catalysed Suzuki-Miyaura Reactions. Chem. Sci. 2020, 11, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Estudiante-Negrete, F.; Hernández-Ortega, S.; Morales-Morales, D. Ni(II)–POCOP Pincer Compound [NiCl{C10H5-2,10-(OPPh2)2}] an Efficient and Robust Nickel Catalyst for the Suzuki-Miyaura Coupling Reactions. Inorganica Chim. Acta 2012, 387, 58–63. [Google Scholar] [CrossRef]
- Favela-Mendoza, R.; Rufino-Felipe, E.; Valdés, H.; Toscano, R.A.; Hernandez-Ortega, S.; Morales-Morales, D. Synthesis and Characterization of Non-Symmetric Ni(II)- and Pd(II)-POCOP Pincer Complexes Derived from 1,7-Naphthalenediol. Evaluation of Their Catalytic Activity in Suzuki-Miyaura Couplings. Inorganica Chim. Acta 2020, 512, 119920. [Google Scholar] [CrossRef]
- Neely, J.M.; Bezdek, M.J.; Chirik, P.J. Insight into Transmetalation Enables Cobalt-Catalyzed Suzuki-Miyaura Cross Coupling. ACS Cent. Sci. 2016, 2, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.M.; Bhat, B.R. Cobalt Pincer Complex Catalyzed Suzuki-Miyaura Cross Coupling—A Green Approach. J. Organomet. Chem. 2017, 827, 41–48. [Google Scholar] [CrossRef]
- Kumar, L.M.; Bhat, B.R. Iron Pincer Complexes as Catalysts in Cross-Coupling of Aryl Halides and Phenylboronic Acid. Int. J. Eng. Technol. 2018, 7, 428–430. [Google Scholar] [CrossRef]
- Inamoto, K.; Kuroda, J.I.; Sakamoto, T.; Hiroya, K. Catalytic Activities of a Bis(Carbene)-Derived Nickel(II)-Pincer Complex in Kumada-Tamao-Corriu and Suzuki-Miyaura Coupling Reactions for the Synthesis of Biaryl Compounds. Synthesis 2007, 2007, 2853–2861. [Google Scholar] [CrossRef]
- Kuroda, J.I.; Inamoto, K.; Hiroya, K.; Doi, T. N-Heterocyclic Carbene Derived Nickel-Pincer Complexes: Efficient and Applicable Catalysts for Suzuki-Miyaura Coupling Reactions of Aryl/Alkenyl Tosylates and Mesylates. Eur. J. Org. Chem. 2009, 2009, 2251–2261. [Google Scholar] [CrossRef]
- Serrano-Becerra, J.M.; Hernández-Ortega, S.; Morales-Morales, D. Synthesis of a Novel Non-Symmetric Pd(II) Phosphinito-Thiophosphinito PSCOP Pincer Compound. Inorganica Chim. Acta 2010, 363, 1306–1310. [Google Scholar] [CrossRef]
- Duong, H.A.; Wu, W.; Teo, Y.Y. Cobalt-Catalyzed Cross-Coupling Reactions of Arylboronic Esters and Aryl Halides. Organometallics 2017, 36, 4363–4366. [Google Scholar] [CrossRef]
- Keskin, E.; Arslan, H. Palladium Complexes Containing NNN Pincer Type Ligands and Their Activities in Suzuki-Miyaura Cross Coupling Reaction. Heliyon 2023, 9, e17608. [Google Scholar] [CrossRef] [PubMed]
- Bolliger, J.L.; Blacque, O.; Frech, C.M. Short, Facile, and High-Yielding Synthesis of Extremely Efficient Pincer-Type Suzuki Catalysts Bearing Aminophosphine Substituents. Angew. Chem. Int. Ed. 2007, 46, 6514–6517. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, I.; Mujahid, A.; Rasool, N.; Rizwan, K.; Malik, A.; Ahmad, G.; Shah, S.A.A.; Rashid, U.; Nasir, N.M. Palladium and Copper Catalyzed Sonogashira Cross Coupling an Excellent Methodology for C-C Bond Formation over 17 Years: A Review. Catalysts 2020, 10, 443. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, X.; Sun, H. Nickel-catalyzed cross-coupling of primary alkyl halides with phenylethynyl- and trimethylsilyethynyl-lithium reagents. J. Org. Chem. 2011, 696, 3011–3014. [Google Scholar] [CrossRef]
- Gallego, D.; Brück, A.; Irran, E.; Meier, F.; Kaupp, M.; Driess, M.; Hartwig, J.F. From Bis(Silylene) and Bis(Germylene) Pincer-Type Nickel(II) Complexes to Isolable Intermediates of the Nickel-Catalyzed Sonogashira Cross-Coupling Reaction. J. Am. Chem. Soc. 2013, 135, 15617–15626. [Google Scholar] [CrossRef] [PubMed]
- Mastalir, M.; Pittenauer, E.; Stöger, B.; Allmaier, G.; Kirchner, K. Three Different Reactions, One Catalyst: A Cu(I) PNP Pincer Complex as Catalyst for C–C and C–N Cross-Couplings. Org. Lett. 2017, 19, 2178–2181. [Google Scholar] [CrossRef]
- Domyati, D.; Latifi, R.; Tahsini, L. Sonogashira-Type Cross-Coupling Reactions Catalyzed by Copper Complexes of Pincer N-Heterocyclic Carbenes. J. Organomet. Chem. 2018, 860, 98–105. [Google Scholar] [CrossRef]
- Zhang, J.H.; Li, P.; Hu, W.P.; Wang, H.X. Substituent Effect of Diimino-Palladium (II) Pincer Complexes on the Catalysis of Sonogashira Coupling Reaction. Polyhedron 2015, 96, 107–112. [Google Scholar] [CrossRef]
- Zhang, B.S.; Wang, C.; Gong, J.F.; Song, M.P. Facile Synthesis of Achiral and Chiral PCN Pincer Palladium(II) Complexes and Their Application in the Suzuki and Copper-Free Sonogashira Cross-Coupling Reactions. J. Organomet. Chem. 2009, 694, 2555–2561. [Google Scholar] [CrossRef]
- Li, L.; Ding, Y. Recent Advances in the Synthesis of Thioethers. Mini. Rev. Org. Chem. 2017, 14, 407–431. [Google Scholar] [CrossRef]
- Lee, C.F.; Liu, Y.C.; Badsara, S.S. Transition-Metal-Catalyzed C–S Bond Coupling Reaction. Chem. Asian J. 2014, 9, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, P.H. Palladium-Catalyzed Carbon-Sulfur Cross-Coupling Reactions with Indium Tri (Organothiolate) and Its Application to Sequential One-Pot Processes. J. Org. Chem. 2008, 73, 7413–7416. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, P.; Liu, K.C.; Singh Badsara, S.; Lee, C.F. Carbon-Sulfur Bond Constructions: From Transition-Metal Catalysis to Sustainable Catalysis. Chem. Rec. 2021, 21, 3674–3688. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Benítez, V.; Baldovino-Pantaleón, O.; Herrera-Álvarez, C.; Toscano, R.A.; Morales-Morales, D. High Yield Thiolation of Iodobenzene Catalyzed by the Phosphinite Nickel PCP Pincer Complex: [NiCl{C6H3-2,6-(OPPh2)2}]. Tetrahedron Lett. 2006, 47, 5059–5062. [Google Scholar] [CrossRef]
- Serrano-Becerra, J.M.; Valdés, H.; Canseco-González, D.; Gómez-Benítez, V.; Hernández-Ortega, S.; Morales-Morales, D. C-S Cross-Coupling Reactions Catalyzed by a Non-Symmetric Phosphinito-Thiophosphinito PSCOP-Ni(II) Pincer Complex. Tetrahedron Lett. 2018, 59, 3377–3380. [Google Scholar] [CrossRef]
- Valderrama-García, B.X.; Rufino-Felipe, E.; Valdés, H.; Hernandez-Ortega, S.; Aguilar-Castillo, B.A.; Morales-Morales, D. Novel and Facile Procedure for the Synthesis of Ni(II) and Pd(II) PSCOP Pincer Complexes. Evaluation of Their Catalytic Activity on C-S, C–Se and C–C Cross Coupling Reactions. Inorganica Chim. Acta 2020, 502, 119283. [Google Scholar] [CrossRef]
- Garella, D.; Borretto, E.; Di Stilo, A.; Martina, K.; Cravotto, G.; Cintas, P. Microwave-Assisted Synthesis of N-Heterocycles in Medicinal Chemistry. Medchemcomm 2013, 4, 1323–1343. [Google Scholar] [CrossRef]
- Bariwal, J.; Van Der Eycken, E. C-N Bond Forming Cross-Coupling Reactions: An Overview. Chem. Soc. Rev. 2013, 42, 9283–9303. [Google Scholar] [CrossRef]
- Ullmann, F. Ueber Eine Neue Bildungsweise von Diphenylaminderivaten. Eur. J. Inorg. Chem. 1903, 36, 2382–2384. [Google Scholar] [CrossRef]
- Hartwig, J.F. Transition Metal Catalyzed Synthesis of Arylamines and Aryl Ethers from Aryl Halides and Triflates: Scope and Mechanism. Angew. Chem. Int. Ed. 1998, 37, 2046–2067. [Google Scholar] [CrossRef]
- Hartwig, J. Palladium-Catalyzed Amination of Aryl Halides and Sulfonates. Mod. Amin. Methods 2007, 576, 195–262. [Google Scholar] [CrossRef]
- Albkuri, Y.M.; RanguMagar, A.B.; Brandt, A.; Wayland, H.A.; Chhetri, B.P.; Parnell, C.M.; Szwedo, P.; Parameswaran-Thankam, A.; Ghosh, A. C–N Cross-Coupling Reactions of Amines with Aryl Halides Using Amide-Based Pincer Nickel(II) Catalyst. Catal. Lett. 2020, 150, 1669–1678. [Google Scholar] [CrossRef]
- Minnick, J.L.; Domyati, D.; Ammons, R.; Tahsini, L. C–X (X = N, O) Cross-Coupling Reactions Catalyzed by Copper-Pincer Bis(N-Heterocyclic Carbene) Complexes. Front. Chem. 2019, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, W.; Junge, K.; Fei, Z.; Beller, M.; Dyson, P.J. Selective Acceptorless Dehydrogenation of Primary Amines to Imines by Core–Shell Cobalt Nanoparticles. Angew. Chem. Int. Ed. 2020, 59, 7501–7507. [Google Scholar] [CrossRef] [PubMed]
- Reed-Berendt, B.G.; Polidano, K.; Morrill, L.C. Recent Advances in Homogeneous Borrowing Hydrogen Catalysis Using Earth-Abundant First Row Transition Metals. Org. Biomol. Chem. 2019, 17, 1595–1607. [Google Scholar] [CrossRef]
- Bagdi, A.K.; Rahman, M.; Bhattacherjee, D.; Zyryanov, G.V.; Ghosh, S.; Chupakhin, O.N.; Hajra, A. Visible Light Promoted Cross-Dehydrogenative Coupling: A Decade Update. Green Chem. 2020, 22, 6632–6681. [Google Scholar] [CrossRef]
- Waiba, S.; Maji, B. Manganese Catalyzed Acceptorless Dehydrogenative Coupling Reactions. ChemCatChem 2020, 12, 1891–1902. [Google Scholar] [CrossRef]
- Bähn, S.; Imm, S.; Neubert, L.; Zhang, M.; Neumann, H.; Beller, M. The Catalytic Amination of Alcohols. ChemCatChem 2011, 3, 1853–1864. [Google Scholar] [CrossRef]
- Nad, P.; Mukherjee, A. Acceptorless Dehydrogenative Coupling Reactions by Manganese Pincer Complexes. Asian J. Org. Chem. 2021, 10, 1958–1985. [Google Scholar] [CrossRef]
- Mukherjee, A.; Nerush, A.; Leitus, G.; Shimon, L.J.W.; Ben David, Y.; Espinosa Jalapa, N.A.; Milstein, D. Manganese-Catalyzed Environmentally Benign Dehydrogenative Coupling of Alcohols and Amines to Form Aldimines and H2: A Catalytic and Mechanistic Study. J. Am. Chem. Soc. 2016, 138, 4298–4301. [Google Scholar] [CrossRef] [PubMed]
- Mastalir, M.; Glatz, M.; Gorgas, N.; Stöger, B.; Pittenauer, E.; Allmaier, G.; Veiros, L.F.; Kirchner, K. Divergent Coupling of Alcohols and Amines Catalyzed by Isoelectronic Hydride MnIand FeIIPNP Pincer Complexes. Chem. Eur. J. 2016, 22, 12316–12320. [Google Scholar] [CrossRef]
- Mastalir, M.; Pittenauer, E.; Allmaier, G.; Kirchner, K. Manganese-Catalyzed Aminomethylation of Aromatic Compounds with Methanol as a Sustainable C1 Building Block. J. Am. Chem. Soc. 2017, 139, 8812–8815. [Google Scholar] [CrossRef] [PubMed]
- Pelckmans, M.; Renders, T.; Van De Vyver, S.; Sels, B.F. Bio-Based Amines through Sustainable Heterogeneous Catalysis. Green Chem. 2017, 19, 5303–5331. [Google Scholar] [CrossRef]
- Qian, M.; Liauw, M.A.; Emig, G. Formaldehyde Synthesis from Methanol over Silver Catalysts. Appl. Catal. A Gen. 2003, 238, 211–222. [Google Scholar] [CrossRef]
- Lin, W.-H.; Chang, H.-F. A Study of Ethanol Dehydrogenation Reaction in a Palladium Membrane Reactor. Catal. Today 2004, 97, 181–188. [Google Scholar] [CrossRef]
- Huh, K.-T.; Tsuji, Y.; Kobayashi, M.; Okuda, F.; Watanabe, Y. Ruthenium Catalyzed N-Methylation of Aminoaranes Using Methanol. Chem. Lett. 1988, 17, 449–452. [Google Scholar] [CrossRef]
- Naskar, S.; Bhattacharjee, M. Selective N-Monoalkylation of Anilines Catalyzed by a Cationic Ruthenium(II) Compound. Tetrahedron Lett. 2007, 48, 3367–3370. [Google Scholar] [CrossRef]
- Campos, J.; Sharninghausen, L.S.; Manas, M.G.; Crabtree, R.H. Methanol Dehydrogenation by Iridium N-Heterocyclic Carbene Complexes. Inorg. Chem. 2015, 54, 5079–5084. [Google Scholar] [CrossRef]
- Tsarev, V.N.; Morioka, Y.; Caner, J.; Wang, Q.; Ushimaru, R.; Kudo, A.; Naka, H.; Saito, S. N-Methylation of Amines with Methanol at Room Temperature. Org. Lett. 2015, 17, 2530–2533. [Google Scholar] [CrossRef]
- Neumann, J.; Elangovan, S.; Spannenberg, A.; Junge, K.; Beller, M. Improved and General Manganese-Catalyzed N-Methylation of Aromatic Amines Using Methanol. Chem. Eur. J. 2017, 23, 5410–5413. [Google Scholar] [CrossRef] [PubMed]
- Bruneau-Voisine, A.; Wang, D.; Dorcet, V.; Roisnel, T.; Darcel, C.; Sortais, J.B. Mono-N-Methylation of Anilines with Methanol Catalyzed by a Manganese Pincer-Complex. J. Catal. 2017, 347, 57–62. [Google Scholar] [CrossRef]
- Homberg, L.; Roller, A.; Hultzsch, K.C. A Highly Active PN 3 Manganese Pincer Complex Performing N-Alkylation of Amines under Mild Conditions. Org. Lett. 2019, 21, 3142–3147. [Google Scholar] [CrossRef] [PubMed]
- Das, U.K.; Ben-David, Y.; Diskin-Posner, Y.; Milstein, D. N-Substituted Hydrazones by Manganese-Catalyzed Coupling of Alcohols with Hydrazine: Borrowing Hydrogen and Acceptorless Dehydrogenation in One System. Angew. Chem. 2018, 130, 2201–2204. [Google Scholar] [CrossRef]
- Mastalir, M.; Tomsu, G.; Pittenauer, E.; Allmaier, G.; Kirchner, K. Co(II) PCP Pincer Complexes as Catalysts for the Alkylation of Aromatic Amines with Primary Alcohols. Org. Lett. 2016, 18, 3462–3465. [Google Scholar] [CrossRef]
- Singh, A.; Maji, A.; Joshi, M.; Choudhury, A.R.; Ghosh, K. Designed Pincer Ligand Supported Co(II)-Based Catalysts for Dehydrogenative Activation of Alcohols: Studies on N-Alkylation of Amines, α-Alkylation of Ketones and Synthesis of Quinolines. Dalton Trans. 2021, 50, 8567–8587. [Google Scholar] [CrossRef]























| Entry | Complex Number | Loading (mol %) | Alkyl-Halide | Grignard Reagent | Reaction Condition | Yield (%) | Ref |
|---|---|---|---|---|---|---|---|
| 1 | a1 | 0.2 | CH3Cl | EtMgCl | THF, N2, r.t., 5 min | 99.5 | [54] |
| 2 | CH2Cl2 | nBuMgCl | 99.9 | ||||
| 3 | CCl4 | HexMgCl | 97.2 | ||||
| 4 | a2 | 0.4 | CH3Cl | nBuMgCl | THF, N2, r.t., 20 min | 99.9 | [47] |
| 5 | CH2Cl2 | HexMgCl | 97.5 | ||||
| 6 | CCl4 | EtMgCl | 99.9 |
| Entry | Complex Number | Loading (mol %) | Alkyl-Halide | Boronic Acid | Reaction Condition | Yield (%) | Ref |
|---|---|---|---|---|---|---|---|
| 1 | b1 | 1.0 | 4-bromobenzaldehyde | PhB(OH)2 | DMF, Na2CO3, 110 °C, 15 h | 99 | [66] |
| 2 | 4-bromoacetophenone | 99 | |||||
| 3 | 4-bromobenzonitrile | 99 | |||||
| 4 | 4-bromonitrobenzene | 97.1 | |||||
| 5 | 4-bromoaniline | 42.4 | |||||
| 6 | b2 | 1.0 | 4-bromobenzonitrile | PhB(OH)2 | DMF, Na2CO3, 90 °C, 12 h | 1 | [67] |
| 7 | b3 | 1.0 | 3 | ||||
| 8 | b4 | 1.0 | 53 | ||||
| 9 | b5 | 5.0 | 4-(trifluoromethyl)phenyl triflate | Benzofuran-2-boronic acid, pinacol ester | THF, NaOCH(Ph)Me, 60 °C, 24 h | 90 | [68] |
| 5.0 | phenyl triflate | 85 | |||||
| 5.0 | 3-(acetyl)phenyl triflate | 80 | |||||
| 5.0 | 4-(methoxy)phenyl triflate | 75 | |||||
| 10 | b6 | 0.5 | 4-bromobenzonitrile | PhB(OH)2 | MeCN, Cs2CO3, 100 °C, 16 h | 81 | [69] |
| 11 | b7 | 0.5 | 83 | ||||
| 12 | b8 | 0.5 | 89 | ||||
| 13 | b6 | 0.5 | 4-iodobenzonitrile | PhB(OH)2 | MeCN, Cs2CO3, 100 °C 16 h | 87 | |
| 14 | b7 | 0.5 | 90 | ||||
| 15 | b8 | 0.5 | 92 | ||||
| 16 | b9 | 0.4 | 4-bromobenzonitrile | PhB(OH)2 | MeCN, Cs2CO3, 100 °C, 14 h | 85 | [70] |
| 17 | b10 | 0.4 | 89 | ||||
| 18 | b9 | 0.4 | 4-iodobenzonitrile | PhB(OH)2 | MeCN, Cs2CO3, 100 °C, 14 h | 89 | |
| 19 | b10 | 0.4 | 94 |
| Entry | Complex Number | Loading (mol %) | Disulfide | Aryl-Halide | Reaction Condition | Yield (%) | Ref |
|---|---|---|---|---|---|---|---|
| 1 | d2 | 0.3 | diphenyl disulfide | iodobenzene | Zn(0), DMF, 140 °C, 16.5 h | 58 | [90] |
| 2 | diphenyl disulfide | 4-iodoaniline | 69 | ||||
| 3 | diphenyl disulfide | 4-iodoacetophenone | 89 | ||||
| 4 | dimethyl disulfide | 4-iodoacetophenone | 89 | ||||
| 5 | n-dibutyl disulfide | 4-iodoacetophenone | 71 | ||||
| 6 | n-dibutyl disulfide | 4-iodoacetophenone | 50 | ||||
| 7 | d3 | 0.2 | diphenyl disulfide | iodobenzene | Zn(0), DMF, 110 °C, 4 h | 88 | [91] |
| 8 | dimethyl disulfide | iodobenzene | 60 | ||||
| 9 | diisopropyl disulfide | iodobenzene | 20 | ||||
| 10 | n-dibutyl disulfide | iodobenzene | 1 | ||||
| 11 | n-dibutyl disulfide | iodobenzene | 14 | ||||
| 12 | ditertbutyl disulfide | iodobenzene | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Navarro, J.A.; Sánchez-Mora, A.; Serrano-García, J.S.; Amaya-Flórez, A.; Colorado-Peralta, R.; Reyes-Márquez, V.; Morales-Morales, D. Advances in Cross-Coupling Reactions Catalyzed by Aromatic Pincer Complexes Based on Earth-Abundant 3d Metals (Mn, Fe, Co, Ni, Cu). Catalysts 2024, 14, 69. https://doi.org/10.3390/catal14010069
Cruz-Navarro JA, Sánchez-Mora A, Serrano-García JS, Amaya-Flórez A, Colorado-Peralta R, Reyes-Márquez V, Morales-Morales D. Advances in Cross-Coupling Reactions Catalyzed by Aromatic Pincer Complexes Based on Earth-Abundant 3d Metals (Mn, Fe, Co, Ni, Cu). Catalysts. 2024; 14(1):69. https://doi.org/10.3390/catal14010069
Chicago/Turabian StyleCruz-Navarro, Jesús Antonio, Arturo Sánchez-Mora, Juan S. Serrano-García, Andrés Amaya-Flórez, Raúl Colorado-Peralta, Viviana Reyes-Márquez, and David Morales-Morales. 2024. "Advances in Cross-Coupling Reactions Catalyzed by Aromatic Pincer Complexes Based on Earth-Abundant 3d Metals (Mn, Fe, Co, Ni, Cu)" Catalysts 14, no. 1: 69. https://doi.org/10.3390/catal14010069