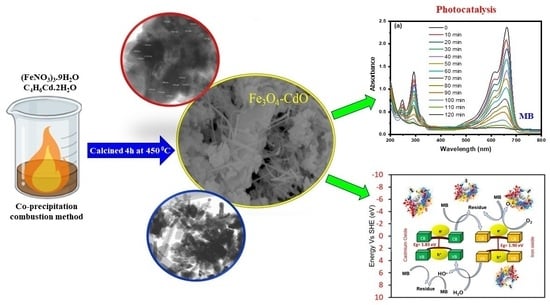
Fe3O4-CdO Nanocomposite for Organic Dye Photocatalytic Degradation: Synthesis and Characterization
Abstract
:1. Introduction
2. Results and Discussion
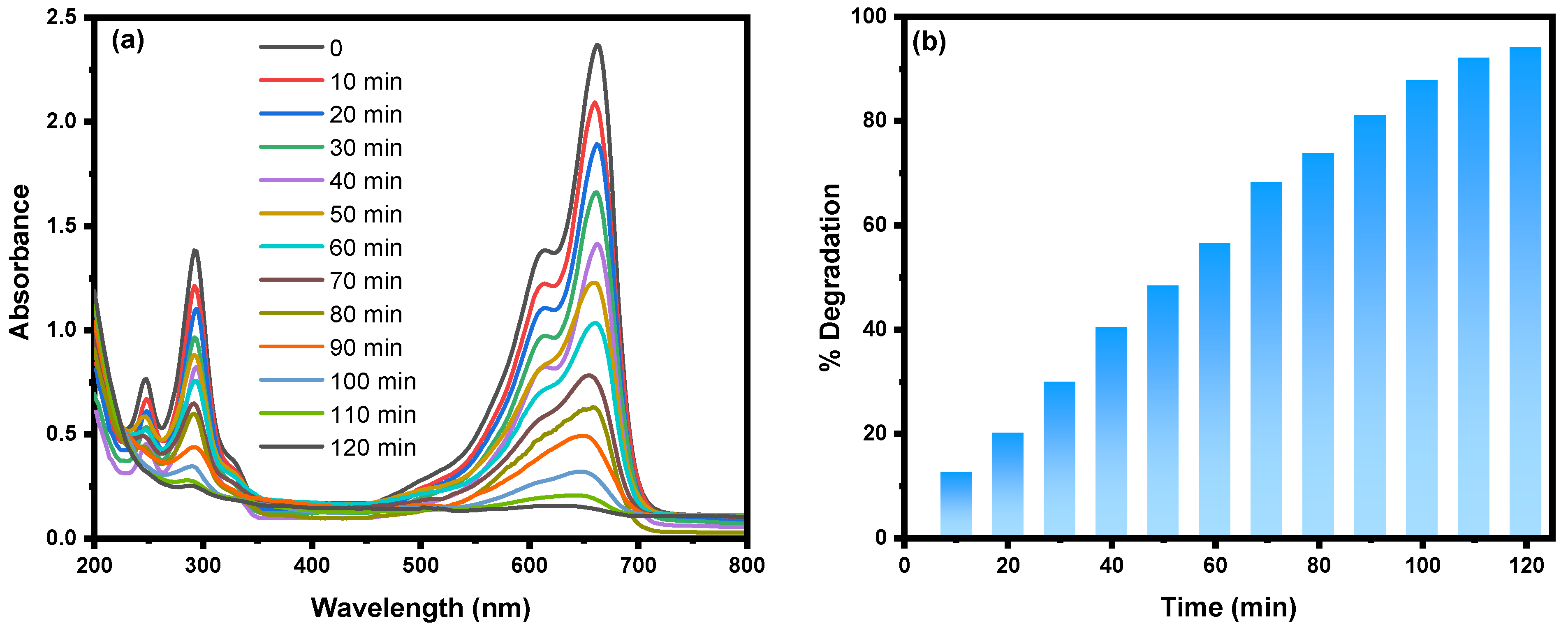
2.1. UV-Visible Spectroscopy Analysis
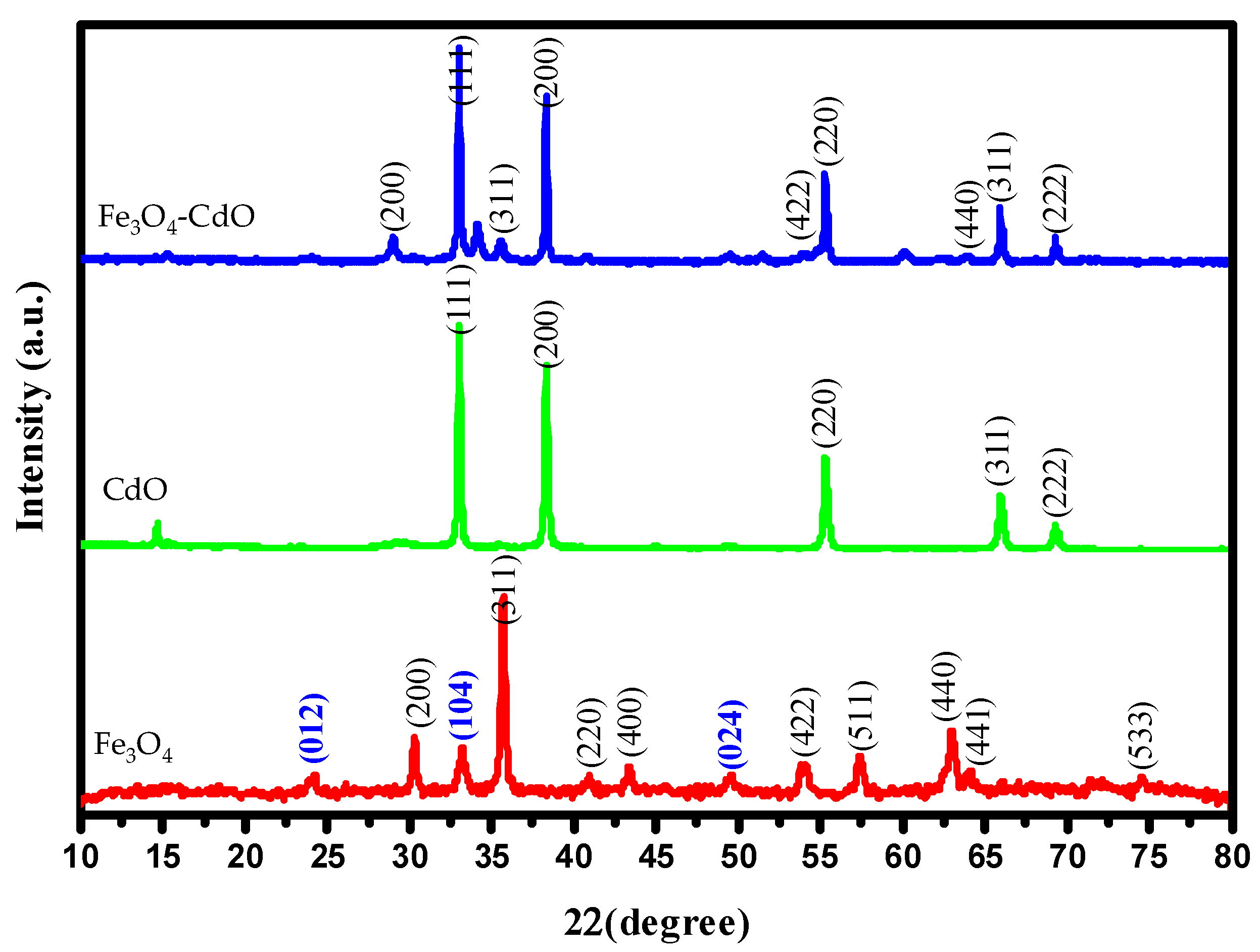
2.2. XRD Analysis
2.3. FTIR Spectroscopy Analysis
2.4. Raman Spectra Analysis
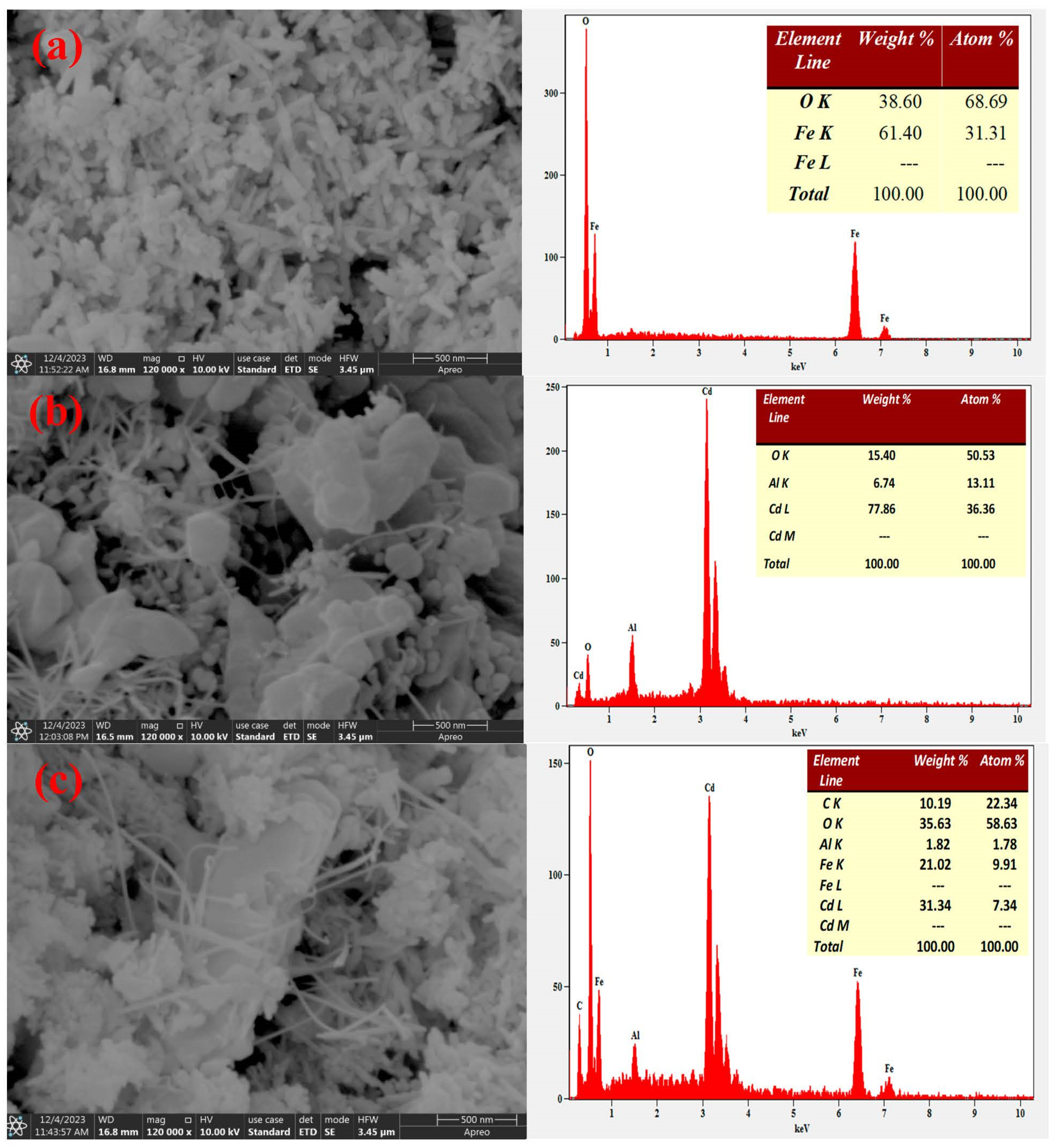
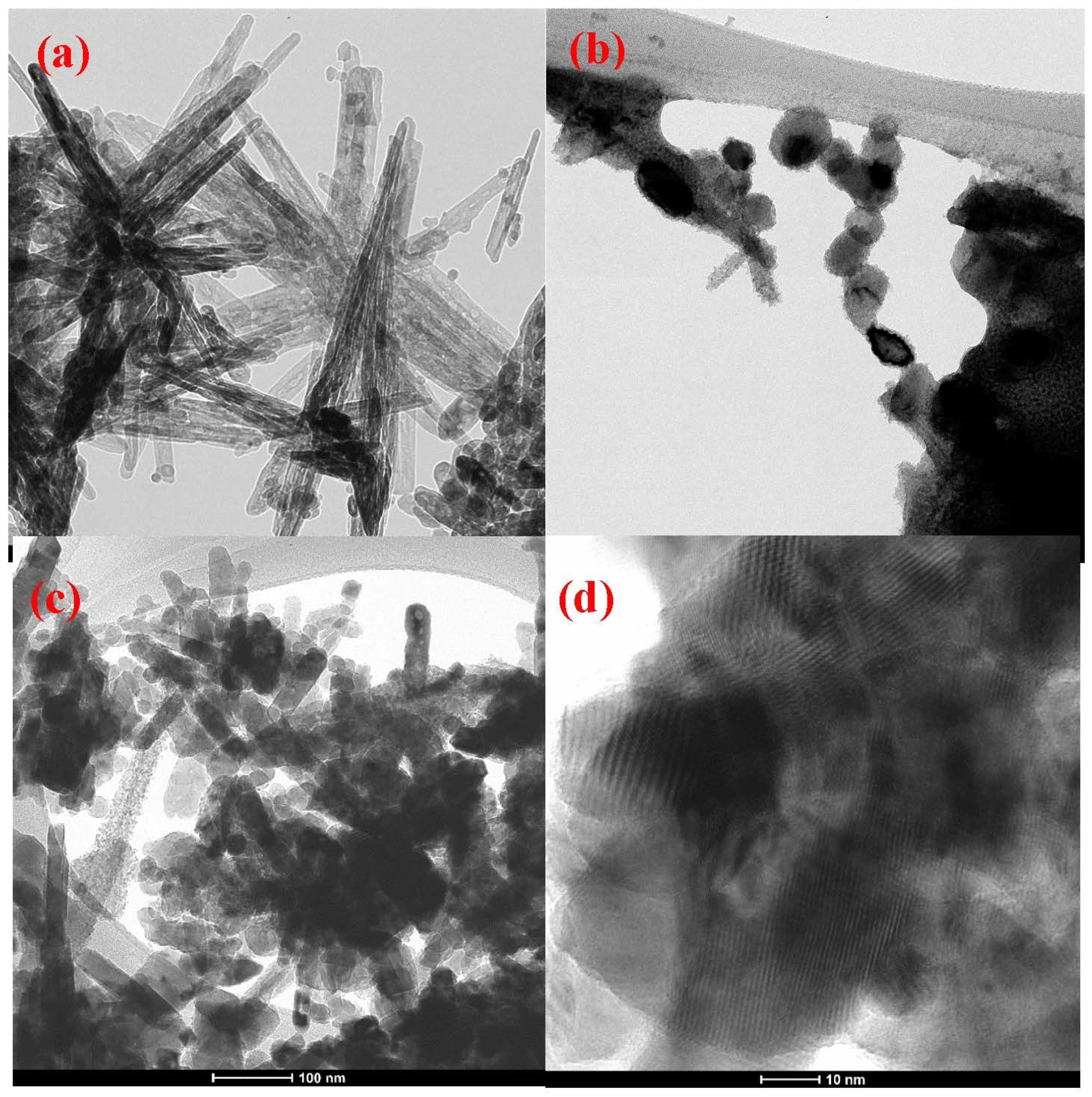
2.5. SEM-EDX and TEM Analysis
2.6. XPS Analysis
2.7. Photocatalytic Activity of Fe3O4-CdO
2.8. Effect Photocatalyst Dosage
2.9. Effect of MB Dye Concentrations
2.10. Effect of pH
2.11. Recyclability Tests
2.12. Photocatalytic Degradation Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation of Fe3O4, CdO and Fe3O4-CdO NPs
3.3. Characterization
3.4. Photocatalytic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rathi, G.; Siddiqui, S.I.; Pham, Q. Nigella Sativa Seeds Based Antibacterial Composites: A Sustainable Technology for Water Cleansing—A Review. Sustain. Chem. Pharm. 2020, 18, 100332. [Google Scholar] [CrossRef]
- Choudhry, A.; Sharma, A.; Siddiqui, S.I.; Ahamad, I.; Sajid, M.; Khan, T.A.; Chaudhry, S.A. Origanum Vulgare Manganese Ferrite Nanocomposite: An Advanced Multifunctional Hybrid Material for Dye Remediation. Environ. Res. 2023, 220, 115193. [Google Scholar] [CrossRef]
- Balcha, A.; Yadav, O.P.; Dey, T. Photocatalytic Degradation of Methylene Blue Dye By Zinc Oxide Nanoparticles Obtained from Precipitation and Sol-Gel Methods. Environ. Sci. Pollut. Res. 2016, 23, 25485–25493. [Google Scholar] [CrossRef]
- Shehnaz; Prasher, I.B.; Ahmad, N.; Ahmed, M.; Raghuwanshi, S.; Kumar, V.; Siddiqui, S.I.; Oh, S. Live Biomass of Rigidoporus Vinctus: A Sustainable Method for Decoloration and Detoxification of Dyes in Water. Microorganisms 2023, 11, 1435. [Google Scholar] [CrossRef]
- Deng, H.; Lu, J.; Li, G.; Zhang, G.; Wang, X. Adsorption of Methylene Blue on Adsorbent Materials Produced from Cotton Stalk. Chem. Eng. J. 2011, 172, 326–334. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Siddiqui, S.I.; Maeng, S.K.; Oh, S. Biological detoxification of oxytetracycline using Achromobacter-immobilized bioremediation system. J. Water Process Eng. 2023, 52, 103491. [Google Scholar] [CrossRef]
- Fatima, B.; Siddiqui, S.; Ahmed, R.; Chaudhry, S.A. Preparation of functionalized CuO nanoparticles using Brassica rapa leave extract for water purification. Desalination Water Treat. 2019, 164, 192–205. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Y.; Sun, B.; Chao, S.; Li, X.; Wang, C.; Zhu, G. Highly Flexible Magnesium Silicate Nanofibrous Membranes for Effective Removal of Methylene Blue from Aqueous Solution. Chem. Eng. J. 2019, 359, 1603–1616. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, Q.; Zhou, M.; Li, C.; Sun, J.; Shi, W.; Ai, S. Degradation of Methylene Blue by a Heterogeneous Fenton Reaction Catalyzed by FeCo2O4-N-C Nanocomposites Derived by ZIFs. Powder Technol. 2021, 383, 212–219. [Google Scholar] [CrossRef]
- Ngullie, R.C.; Alaswad, S.O.; Bhuvaneswari, K.; Shanmugam, P.; Pazhanivel, T.; Arunachalam, P. Synthesis and Characterization of E Ffi Cient ZnO/g-C3N4 Nanocomposites Photocatalyst for Photocatalytic Degradation of Methylene Blue. Coatings 2020, 10, 500. [Google Scholar] [CrossRef]
- Nada, A.A.; Nasr, M.; Viter, R.; Miele, P.; Roualdes, S.; Bechelany, M. Mesoporous ZnFe2O4@TiO2 Nanofibers Prepared by Electrospinning Coupled to PECVD as Highly Performing Photocatalytic Materials. J. Phys. Chem. C 2017, 121, 24669–24677. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Guo, X.; Chen, Z.; Zhang, W.; Wang, Y.; Tang, X.; Zhang, Y.; Zhao, Y. Sulfur-Doped G-C3N4/RGO Porous Nanosheets for Highly Efficient Photocatalytic Degradation of Refractory Contaminants. J. Mater. Sci. Technol. 2020, 41, 117–126. [Google Scholar] [CrossRef]
- Atla, S.B.; Lin, W.R.; Chien, T.C.; Tseng, M.J.; Shu, J.C.; Chen, C.C.; Chen, C.Y. Fabrication of Fe3O4/ZnO Magnetite Core Shell and Its Application in Photocatalysis Using Sunlight. Mater. Chem. Phys. 2018, 216, 380–386. [Google Scholar] [CrossRef]
- Xu, P.; Liu, X.; Zhao, Y.; Lan, D.; Shin, I. Study of Graphdiyne Biomimetic Nanomaterials as Fluorescent Sensors of Ciprofloxacin Hydrochloride in Water Environment. Desalin. Water Treat. 2023, 302, 129–137. [Google Scholar] [CrossRef]
- Li, H.; Wu, Y.; Xu, Z.; Wang, Y. In Situ Anchoring Cu Nanoclusters on Cu-MOF: A New Strategy for a Combination of Catalysis and Fluorescence toward the Detection of H2O2 and 2,4-DNP. Chem. Eng. J. 2024, 479, 147508. [Google Scholar] [CrossRef]
- Kang, J.; Liu, G.; Hu, Q.; Huang, Y.; Liu, L.; Dong, L. Parallel Nanosheet Arrays for Industrial Oxygen Production. J. Am. Chem. Soc. 2023, 145, 25143–25149. [Google Scholar] [CrossRef]
- Medhi, R.; Marquez, M.D.; Lee, T.R. Visible-Light-Active Doped Metal Oxide Nanoparticles: Review of Their Synthesis, Properties, and Applications. ACS Appl. Nano Mater. 2020, 3, 6156–6185. [Google Scholar] [CrossRef]
- Liu, W.; Huang, F.; Liao, Y.; Zhang, J.; Ren, G.; Zhuang, Z.; Zhen, J.; Lin, Z.; Wang, C. Treatment of CrVI-Containing Mg(OH)2 Nanowaste. Angew. Chem. (Int. Ed.) 2008, 47, 5619–5622. [Google Scholar] [CrossRef]
- Narasimharao, K.; Al-Thabaiti, S.; Rajor, H.K.; Mokhtar, M.; Alsheshri, M.; Alfaifi, S.Y.; Siddiqui, S.I.; Abdulla, N.K. Fe3O4@Date Seeds Powder: A Sustainable Nanocomposite Material for Wastewater Treatment. J. Mater. Res. Technol. 2022, 18, 3581–3597. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, T.; Li, Z.; Zhu, W.; Li, L. Enhanced photocatalytic performance of S-scheme CdMoO4/CdO nanosphere photocatalyst. J. Mater. Sci. Technol. 2024, 179, 198–207. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Hadia, N.M.A.; Diab, A.K.; Hakeem, A.M.A. Synthesis, Photoluminescence and Optical Constants Evaluations of Ultralong CdO Nanowires Prepared by Vapor Transport Method. J. Alloys Compd. 2014, 609, 68–72. [Google Scholar] [CrossRef]
- Rajesh, N.; Kannan, J.C.; Leonardi, S.G.; Neri, G.; Krishnakumar, T. Investigation of CdO Nanostructures Synthesized by Microwave Assisted Irradiation Technique for NO2 Gas Detection. J. Alloys Compd. 2014, 607, 54–60. [Google Scholar] [CrossRef]
- Shahzadi, I.; Aqeel, M.; Haider, A.; Naz, S.; Imran, M.; Nabgan, W.; Al-Shanini, A.; Shahzadi, A.; Alshahrani, T.; Ikram, M. Hydrothermal Synthesis of Fe-Doped Cadmium Oxide Showed Bactericidal Behavior and Highly Efficient Visible Light Photocatalysis. ACS Omega 2023, 8, 30681–30693. [Google Scholar] [CrossRef]
- Nallendran, R.; Selvan, G.; Balu, A.R. Cdo-Fe3O4 Nanocomposite with Enhanced Magnetic and Photocatalytic Properties. Mater. Sci.-Pol. 2019, 37, 100–107. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, M.; Xue, C.; Huang, J.; Zhao, N.; Kong, L. All-Solid-State Z-Scheme Nanojunction PW12/Ag/Zno Photocatalyst: Effective Carriers Transfer Promotion and Enhanced Visible Light Driven. J. Mol. Str. 2024, 1300, 137272. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Hemamalini, R.; Rajendran, S.; Qin, J.; Yola, M.L.; Atar, N.; Gracia, F. Nanosized Fe3O4 Incorporated on a TiO2 Surface for the Enhanced Photocatalytic Degradation of Organic Pollutants. J. Mol. Liq. 2019, 287, 110967. [Google Scholar] [CrossRef]
- Ahmad, J.; Majid, K. Enhanced Visible Light Driven Photocatalytic Activity of CdO–Graphene Oxide Heterostructures for the Degradation of Organic Pollutants. New J. Chem. 2018, 42, 3246–3259. [Google Scholar] [CrossRef]
- Hamad, H.; Elsenety, M.M.; Sadik, W.; Ghaffar, A.; Demerdash, E. The Superior Photocatalytic Performance and DFT Insights of S-scheme CuO@TiO2 Heterojunction Composites for Simultaneous Degradation of Organics. Sci. Rep. 2022, 21, 2217. [Google Scholar] [CrossRef]
- May, H.; Zhan, W.; Song, S.; Jia, F.; Zhou, J. Visible-Light Photocatalytic Degradation Pathway of Tetracycline Hydrochloride with Cubic Structured ZnO/SnO2 Heterojunction Nanocatalyst. Chem. Phys. Lett. 2019, 736, 136806. [Google Scholar]
- Sharma, A.; Kanth, S.; Xu, S.; Han, N.; Lei, Z.; Fan, L.; Liu, C.; Zhang, Q. Visible Light Driven G-C3N4/Bi4NbO8X (X = Cl, Br) Heterojunction Photocatalyst for the Degradation of Organic Pollutants. J. Alloys Compd. 2021, 895, 162576. [Google Scholar] [CrossRef]
- Althabaiti, S.A.; Malik, M.A.; Kumar Khanna, M.; Bawaked, S.M.; Narasimharao, K.; Al-Sheheri, S.Z.; Fatima, B.; Siddiqui, S.I. One-Pot Facile Synthesis of CuO–CdWO4 Nanocomposite for Photocatalytic Hydrogen Production. Nanomaterials 2022, 12, 4472. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Zaki, Z.I. Degradation of Imazapyr Herbicide Using Visible Light-Active CdO–TiO2 Heterojunction Photocatalyst. J. Environ. Chem. Eng. 2021, 9, 104732. [Google Scholar] [CrossRef]
- Fatima, B.; Alwan, B.A.; Siddiqui, S.I.; Ahmad, R.; Almesfer, M.; Khanna, M.K.; Mishra, R.; Ravi, R.; Oh, S. Facile Synthesis of Cu-Zn Binary Oxide Coupled Cadmium Tungstate (Cu-ZnBO-Cp-CT) with Enhanced Performance of Dye Adsorption. Water 2021, 13, 3287. [Google Scholar] [CrossRef]
- Fatima, B.; Siddiqui, S.I.; Ahmad, R.; Linh, N.T.; Thai, V.N. CuO-ZnO-CdWO4: A Sustainable and Environmentally Benign Photocatalytic System for Water Cleansing. Environ. Sci. Pollut. Res. 2021, 28, 53793–53803. [Google Scholar] [CrossRef]
- Qin, M.-Z.; Fu, W.-X.; Guo, H.; Niu, C.-G.; Huang, D.-W.; Liang, C.; Yang, Y.-Y.; Liu, H.-Y.; Tang, N.; Fan, Q.-Q. 2D/2D Heterojunction Systems for the Removal of Organic Pollutants: A Review. Adv. Colloid Interface Sci. 2021, 297, 102540. [Google Scholar] [CrossRef]
- Ghanbarnezhad, S.; Baghshahi, S.; Nemati, A.; Mahmoodi, M. Preparation, Magnetic Properties, and Photocatalytic Performance under Natural Daylight Irradiation of Fe3O4-ZnO Core/Shell Nanoparticles Designed on Reduced GO Platelet. Mater. Sci. Semicond. Process. 2017, 72, 85–92. [Google Scholar] [CrossRef]
- Fu, J.R.; Zheng, J.; Fang, W.J.; Chen, C.; Cheng, C.; Yan, R.W.; Huang, S.G.; Wang, C.C. Synthesis of Porous Magnetic Fe3O4/Fe@ZnO Core-Shell Heterostructure with Superior Capability for Water Treatment. J. Alloys Compd. 2015, 650, 463–469. [Google Scholar] [CrossRef]
- Karunakaran, C.; Vinayagamoorthy, P.; Jayabharathi, J. Nonquenching of Charge Carriers by Fe3O4 Core in Fe3O/ZnO Nanosheet Photocatalyst. Langmuir 2014, 30, 15031–15039. [Google Scholar] [CrossRef]
- Qamar, M.T.; Aslam, M.; Ismail, I.M.I.; Salah, N.; Hameed, A. The Assessment of the Photocatalytic Activity of Magnetically Retrievable ZnO Coated γ-Fe2O3 in Sunlight Exposure. Chem. Eng. J. 2016, 283, 656–667. [Google Scholar] [CrossRef]
- Xia, J.; Wang, A.; Liu, X.; Su, Z. Preparation and Characterization of Bifunctional, Fe3O4/ZnO Nanocomposites and Their Use as Photocatalysts. Appl. Surf. Sci. 2011, 257, 9724–9732. [Google Scholar] [CrossRef]
- Vinosel, V.M.; Asisi, S.A.M.; Pauline, J.S.; Praveena, S.D.P. Enhanced Photocatalytic Activity of Fe3O4/SnO2 Magnetic Nanocomposite for the Degradation of Organic Dye. J. Mater. Sci. Mater. Electron. 2019, 30, 9663–9677. [Google Scholar] [CrossRef]
- Dwivedi, P.; Jatrana, I.; Khan, A.U.; Khan, A.A.; Satiya, H.; Khan, M.; Moon, I.S.; Alam, M. Photoremediation of Methylene Blue by Biosynthesized ZnO/Fe3O4 Nanocomposites Using Callistemon Viminalis Leaves Aqueous Extract: A Comparative Study. Nanotechnol. Rev. 2021, 10, 1912–1925. [Google Scholar] [CrossRef]
- Kong, L.; Liu, Y.; Dong, L.; Zhang, L.; Qiao, L.; Wang, W.; You, H. Enhanced red luminescence in CaAl12O19:Mn4+ via doping Ga3+ for plant growth lighting. Dalton Trans. 2020, 49, 1947–1954. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhang, W.; Zhao, Y.; Shen, H.; Lin, H.; Liang, J. Preparation and Characterization of Fe3O4 Particles with Novel Nanosheets Morphology and Magnetochromatic Property by a Modified Solvothermal Method. Sci. Rep. 2015, 5, 9320. [Google Scholar] [CrossRef]
- Somasundaram, G.; Rajan, J.; Sangaiya, P.; Dilip, R. Hydrothermal Synthesis of CdO Nanoparticles for Photocatalytic and Antimicrobial Activities. Results Mater. 2019, 4, 100044. [Google Scholar] [CrossRef]
- Guo, D.; Li, H.; Xu, Z.; Nie, Y. Development of Pyrene-Based Mofs Probe for Water Content and Investigations on Their Mechanochromism and Acidochromism. J. Alloys Compds. 2023, 968, 172004. [Google Scholar] [CrossRef]
- Xitian, H.; Li, Z.; Xu, W.; Yan, P. Review on near-field detection technology in the biomedical field. Adv. Photonics 2023, 2, 44002. [Google Scholar]
- Hai, N.H.; Phu, N.D.; Luong, N.H.; Chau, N.; Chinh, H.D.; Hoang, L.H.; Leslie-Pelecky, D.L. Mechanism for Sustainable Magnetic Nanoparticles under Ambient Conditions. J. Korean Phys. Soc. 2008, 52, 1327–1331. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Bt Ahmad Khairudin, N.B.; Bt Mohamad, S.E.; Hara, H.; Bt Mad Nordin, M.F.; Lee, K.X. An Eco-Friendly Means of Biosynthesis of Superparamagnetic Magnetite Nanoparticles via Marine Polymer. IEEE Trans. Nanotechnol. 2017, 16, 1047–1052. [Google Scholar] [CrossRef]
- Ganesh, V.; Manthrammel, M.A.; Shkir, M.; AlFaify, S. Investigation on Physical Properties of CdO Thin Films Affected by Tb Doping for Optoelectronics. Appl. Phys. A Mater. Sci. Process. 2019, 125, 1–9. [Google Scholar] [CrossRef]
- Anitha, M.; Anitha, N.; Saravanakumar, K.; Kulandaisamy, I.; Amalraj, L. Effect of Zn Doping on Structural, Morphological, Optical and Electrical Properties of Nebulized Spray-Deposited CdO Thin Films. Appl. Phys. A Mater. Sci. Process. 2018, 124, 1–13. [Google Scholar] [CrossRef]
- Kumar, S.; Ojha, A.K. Synthesis, Characterizations and Antimicrobial Activities of Well Dispersed Ultra-Long CdO Nanowires. AIP Adv. 2013, 3, 052109. [Google Scholar] [CrossRef]
- Zargar, R.A. Fabrication and Improved Response of ZnO-CdO Composite Films under Different Laser Irradiation Dose. Sci. Rep. 2022, 12, 10096. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Yang, T.; Liu, X.; Cao, Z.; Gu, J.; Wang, Y. Enabling efficient and economical degradation of PCDD/Fs in MSWIFA via catalysis and dechlorination effect of EMR in synergistic thermal treatment. Chemosphere 2023, 342, 140164. [Google Scholar] [CrossRef]
- Kurnaz Yetim, N.; Kurşun Baysak, F.; Koç, M.M.; Nartop, D. Characterization of Magnetic Fe3O4@SiO2 Nanoparticles with Fluorescent Properties for Potential Multipurpose Imaging and Theranostic Applications. J. Mater. Sci. Mater. Electron. 2020, 31, 18278–18288. [Google Scholar] [CrossRef]
- Ai, Q.; Yuan, Z.; Huang, R.; Yang, C.; Jiang, G.; Xiong, J.; Huang, Z.; Yuan, S. One-Pot Co-Precipitation Synthesis of Fe3O4 Nanoparticles Embedded in 3D Carbonaceous Matrix as Anode for Lithium Ion Batteries. J. Mater. Sci. 2019, 54, 4212–4224. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Xie, S.; Zhai, T.; Yu, M.; Liang, C.; Ouyang, X.; Lu, X.; Li, H.; Tong, Y. Improving the Photoelectrochemical and Photocatalytic Performance of CdO Nanorods with CdS Decoration. CrystEngComm 2013, 15, 4212–4216. [Google Scholar] [CrossRef]
- Alzahrani, S.A.; Al-Thabaiti, S.A.; Al-Arjan, W.S.; Malik, M.A.; Khan, Z. Preparation of Ultra Long α-MnO2 and Ag@MnO2 Nanoparticles by Seedless Approach and Their Photocatalytic Performance. J. Mol. Struct. 2017, 1137, 495–505. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Li, X.; Wang, D.; Wei, B.; Song, H.; Li, X.; Fu, S. Preparation and Photocatalytic Properties of Magnetically Reusable Fe3O4@ZnO Core/Shell Nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2016, 75, 66–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, L.; Yuan, Y.; Zhu, Y.; Jiang, X.; Xiao, J. Applied Catalysis B: Environmental Magnetic Fe3O4@C/Cu and Fe3O4@CuO Core–Shell Composites Constructed from MOF-Based Materials and Their Photocatalytic Properties under Visible Light. Appl. Catal. B Environ. 2014, 144, 863–869. [Google Scholar] [CrossRef]
- Yao, K.F.; Peng, Z.; Liao, Z.H.; Chen, J.J. Preparation and Photocatalytic Property of TiO2-Fe3O4 Core–Shell Nanoparticles. J. Nanosci. Nanotechnol. 2009, 9, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Banerjee, P.; Das, P.; Mukhopadhyay, A. Phytogenic Synthesis of Nanoparticles and Their Application in Photo Catalysis of Dye Rich Effluents. In Photocatalytic Degradation of Dyes; Elsevier: Amsterdam, The Netherlands, 2021; pp. 647–694. [Google Scholar] [CrossRef]
- Senthil, S.; Srinivasan, S.; Thangeeswari, T.; Ratchagar, V. Enrichment of Optical, Magnetic and Photocatalytic Properties in PVP Capped CdO/SnO2 Nanocomposites Synthesized by Microwave Irradiation Method. J. Mater. Sci. Mater. Electron. 2019, 30, 19841–19853. [Google Scholar] [CrossRef]
- Liang, H.; Guo, J.; Shi, Y.; Zhao, G.; Sun, S.; Sun, X. Porous Yolk-Shell Fe/Fe3O4 Nanoparticles with Controlled Exposure of Highly Active Fe(0) for Cancer Therapy. Biomaterials 2021, 268, 120530. [Google Scholar] [CrossRef] [PubMed]
- Karthik, K.; Dhanuskodi, S.; Gobinath, C.; Prabukumar, S.; Sivaramakrishnan, S. Photocatalytic and Antibacterial Activities of Hydrothermally Prepared CdO Nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 11420–11429. [Google Scholar] [CrossRef]
- Akyüz, D. RGO-TiO2-CdO-ZnO-Ag Photocatalyst for Enhancing Photocatalytic Degradation of Methylene Blue. Opt. Mater. 2021, 116, 111090. [Google Scholar] [CrossRef]
- Ramesh, A.V.; Rama Devi, D.; Mohan Botsa, S.; Basavaiah, K. Facile Green Synthesis of Fe3O4 Nanoparticles Using Aqueous Leaf Extract of Zanthoxylum armatum DC. for Efficient Adsorption of Methylene Blue. J. Asian Ceram. Soc. 2018, 6, 145–155. [Google Scholar] [CrossRef]
- Maruthupandy, M.; Muneeswaran, T.; Vennila, T. Development of Chitosan Decorated Fe3O4 Nanospheres for Potential Enhancement of Photocatalytic Degradation of Congo Red Dye Molecules. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120511. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Imani, M.; Kerdari, H.; Bijanzad, K.; Khaledi, D.; Rad, M. Preparation of CdO Rhombus-like Nanostructure and Its Photocatalytic Degradation of Azo Dyes from Aqueous Solution. Nanomater. Nanotechnol. 2014, 4, 4–16. [Google Scholar] [CrossRef]
- Dhatshanamurthi, P.; Subash, B.; Shanthi, M. Investigation on UV-A Light Photocatalytic Degradation of an Azo Dye in the Presence of CdO/TiO2 Coupled Semiconductor. Mater. Sci. Semicond. Process. 2015, 35, 22–29. [Google Scholar] [CrossRef]
- Elshypany, R.; Selim, H.; Zakaria, K.; Moustafa, A.H.; Sadeek, S.A.; Sharaa, S.I.; Raynaud, P.; Nada, A.A. Elaboration of Fe3O4/ZnO Nanocomposite with Highly Performance Photocatalytic Activity for Degradation Methylene Blue under Visible Light Irradiation. Environ. Technol. Innov. 2021, 23, 101710. [Google Scholar] [CrossRef]
- Długosz, O.; Szostak, K.; Krupiński, M.; Banach, M. Synthesis of Fe3O4/ZnO Nanoparticles and Their Application for the Photodegradation of Anionic and Cationic Dyes. Int. J. Environ. Sci. Technol. 2021, 18, 561–574. [Google Scholar] [CrossRef]
- Fatima, B.; Siddiqui, S.I.; Rajor, H.K.; Malik, M.A.; Narasimharao, K.; Ahmad, R.; Vikrant, K.; Kim, T.; Kim, K.H. Photocatalytic Removal of Organic Dye Using Green Synthesized Zinc Oxide Coupled Cadmium Tungstate Nanocomposite under Natural Solar Light Irradiation. Environ. Res. 2023, 216, 114534. [Google Scholar] [CrossRef]
- Zaied, M.; Peulon, S.; Bellakhal, N.; Desmazières, B.; Chaussé, A. Studies of N-Demethylation Oxidative and Degradation of Methylene Blue by Thin Layers of Birnessite Electrodeposited onto SnO2. Appl. Catal. B Environ. 2011, 101, 441–450. [Google Scholar] [CrossRef]
- Wang, X.; Liu, P.; Fu, M.; Ma, J.; Ning, P. Novel Sequential Process for Enhanced Dye Synergistic Degradation Based on Nano Zero-Valent Iron and Potassium Permanganate. Chemosphere 2016, 155, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; He, X.; Zhang, W.; Zhang, X.; Lu, C. In Situ Synthesis of MnO2 Coated Cellulose Nanofibers Hybrid for Effective Removal of Methylene Blue. Carbohydr. Polym. 2014, 110, 302–308. [Google Scholar] [CrossRef]
- Zaheer, Z.; Bawazir, W.A.; Alwael, H.; Al-Jefri, F.M.; Salem, M. Decolorization and Mineralization of Methylene Blue by Potassium Permanganate. J. Mol. Liq. 2023, 394, 123794. [Google Scholar] [CrossRef]
- Althabaiti, S.A.; Khan, Z.; Malik, M.A.; Bawaked, S.M.; Al-Sheheri, S.Z.; Mokhtar, M.; Siddiqui, S.I.; Narasimharao, K. Biomass-derived carbon deposited TiO2 nanotube photocatalysts for enhanced hydrogen production. Nanoscale Adv. 2023, 5, 3671–3683. [Google Scholar] [CrossRef]
- Zaidi, Z.; Siddiqui, S.I.; Fatima, B.; Chaudhry, S.A. Synthesis of ZnO nanospheres for water treatment through adsorption and photocatalytic degradation: Modelling and process optimization. Mater. Res. Bull. 2019, 120, 110584. [Google Scholar] [CrossRef]







| Catalyst | Dye | Degradation Time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|
| Fe3O4 | MB | 180 | 71 | [68] |
| Fe3O4 | Congo red | 60 | 77 | [69] |
| CdO | Congo Red | 120 | 100 | [70] |
| Malachite Green | 100 | |||
| Crystal Violet | 240 | 95 | ||
| CdO/TiO2 | Reactive Orange 4 | 60 | 90.9 | [71] |
| CdO-TiO2 | Imazapyr | 180 | 96 | [32] |
| Fe3O4/ZnO | Methyl Orange | 60 | 93.6 | [40] |
| Fe3O4/ZnO | MB | 120 | 88.5 | [72] |
| Fe3O4@ZnO | Rhodamine B | 60 | 99.3 | [60] |
| Fe3O4/ZnO | MB | 60 | 63.02 | [73] |
| Fe3O4-CdO | MB | 120 | 92 | Present work |
| S. No. | Semiconductor | Χ (eV) | Eg (eV) | EVB | ECB |
|---|---|---|---|---|---|
| 1. | CdO | 5.71 | 1.83 | 2.125 | 2.180 |
| 2. | Fe3O4 | 5.73 | 1.90 | 0.295 | 0.280 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albeladi, A.; Khan, Z.; Al-Thabaiti, S.A.; Patel, R.; Malik, M.A.; Mehta, S. Fe3O4-CdO Nanocomposite for Organic Dye Photocatalytic Degradation: Synthesis and Characterization. Catalysts 2024, 14, 71. https://doi.org/10.3390/catal14010071
Albeladi A, Khan Z, Al-Thabaiti SA, Patel R, Malik MA, Mehta S. Fe3O4-CdO Nanocomposite for Organic Dye Photocatalytic Degradation: Synthesis and Characterization. Catalysts. 2024; 14(1):71. https://doi.org/10.3390/catal14010071
Chicago/Turabian StyleAlbeladi, Ahlam, Zaheer Khan, Shaeel Ahmed Al-Thabaiti, Rajan Patel, Maqsood Ahmad Malik, and Shilpa Mehta. 2024. "Fe3O4-CdO Nanocomposite for Organic Dye Photocatalytic Degradation: Synthesis and Characterization" Catalysts 14, no. 1: 71. https://doi.org/10.3390/catal14010071