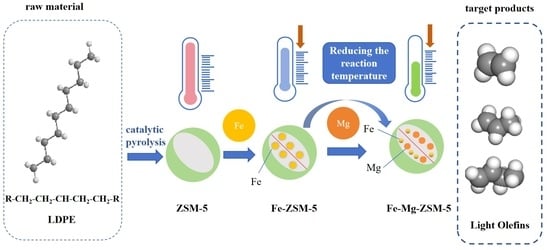
Surface Modification of Fe-ZSM-5 Using Mg for a Reduced Catalytic Pyrolysis Temperature of Low-Density Polyethylene to Produce Light Olefin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.1.1. BET Results
2.1.2. NH3-TPD Results
2.1.3. SEM and TEM Results
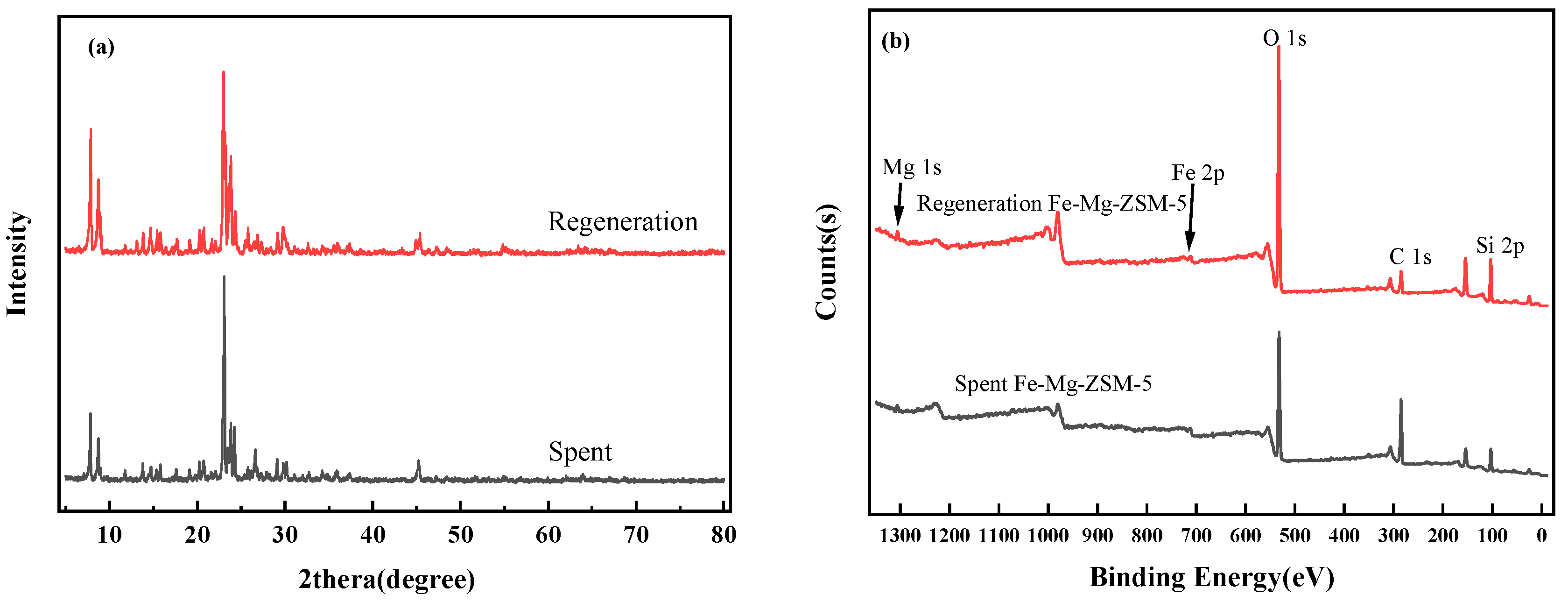
2.1.4. XRD Analysis
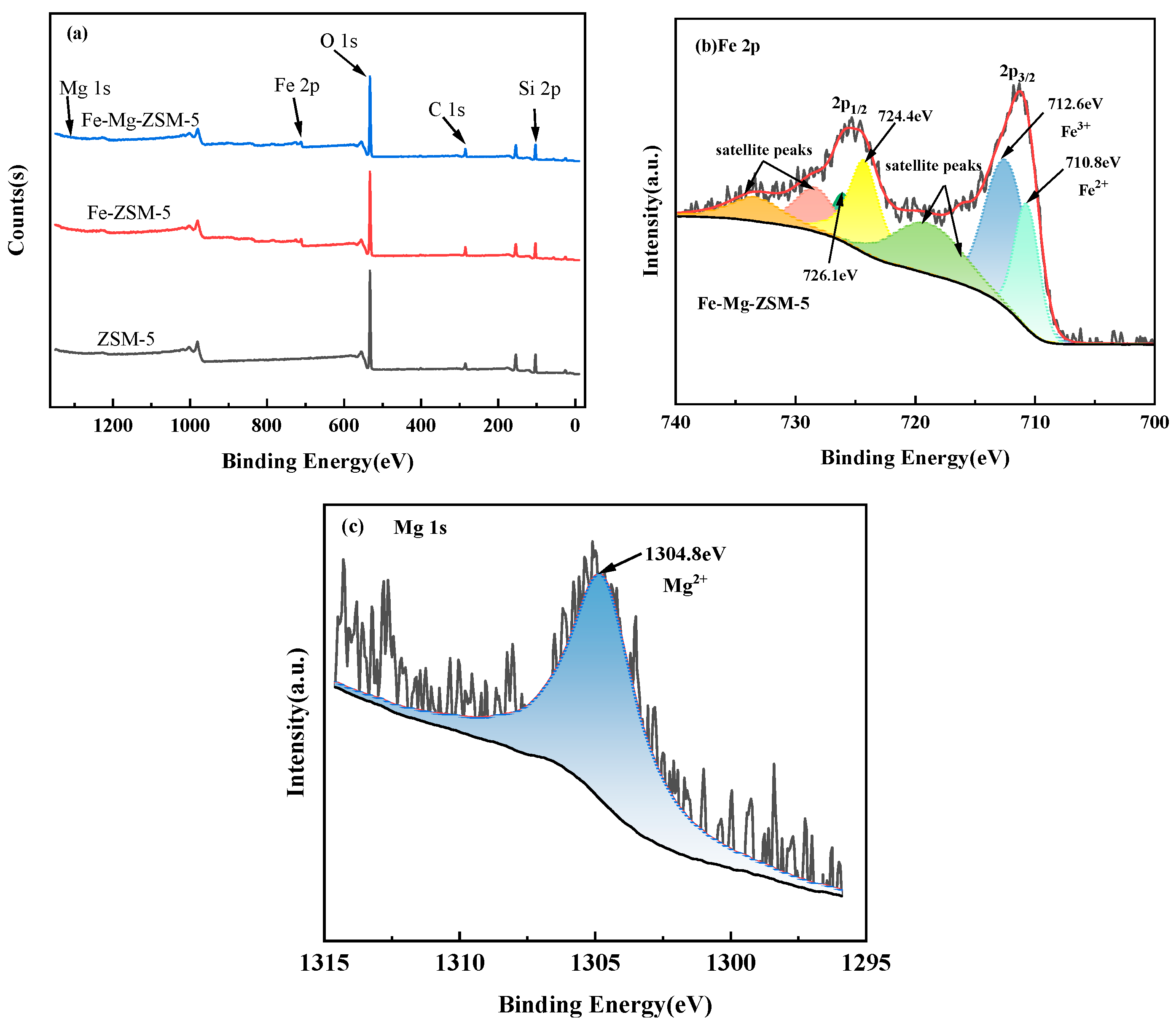
2.1.5. XPS Analysis
2.2. The Catalytic Pyrolysis of LDPE on Different Catalysts
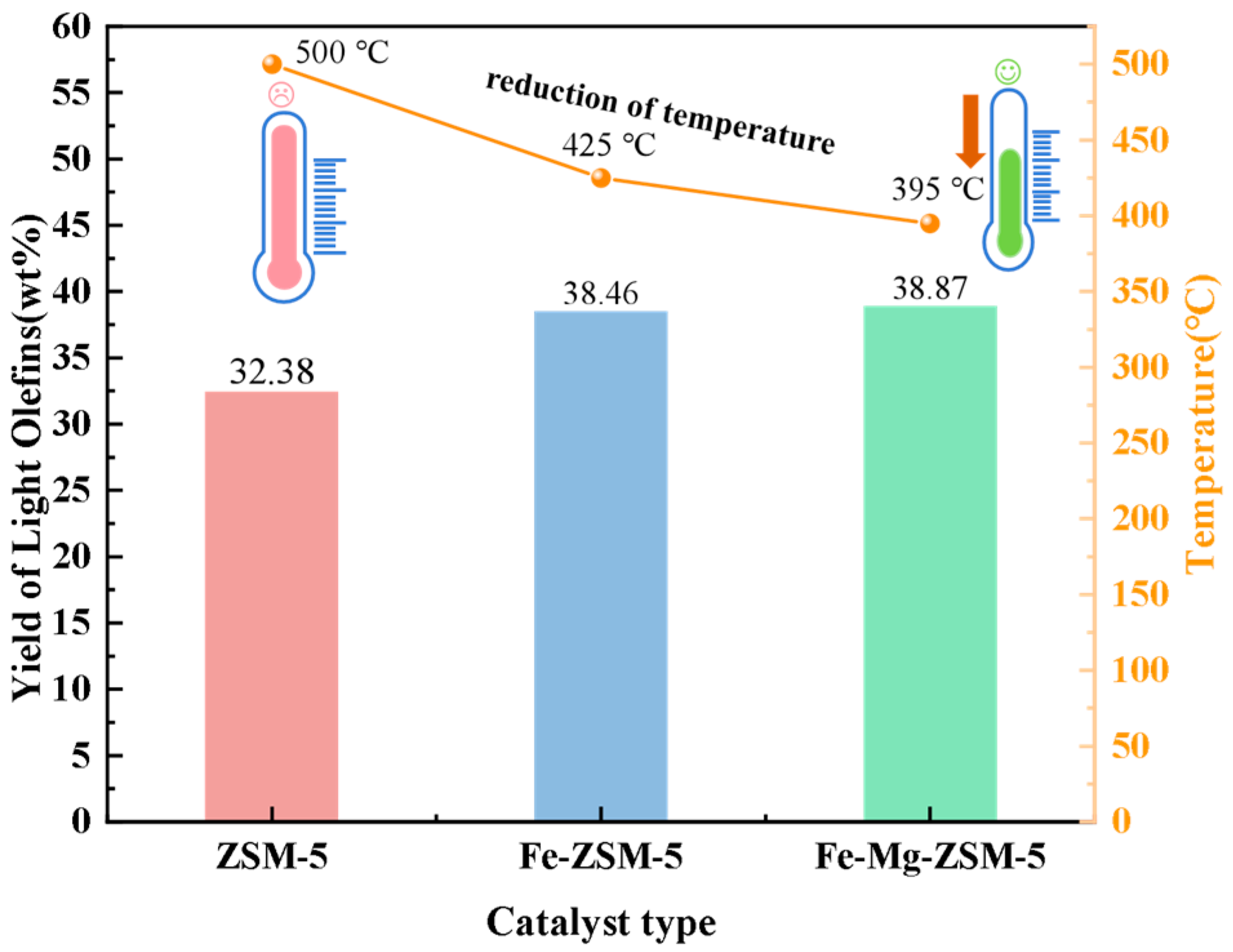
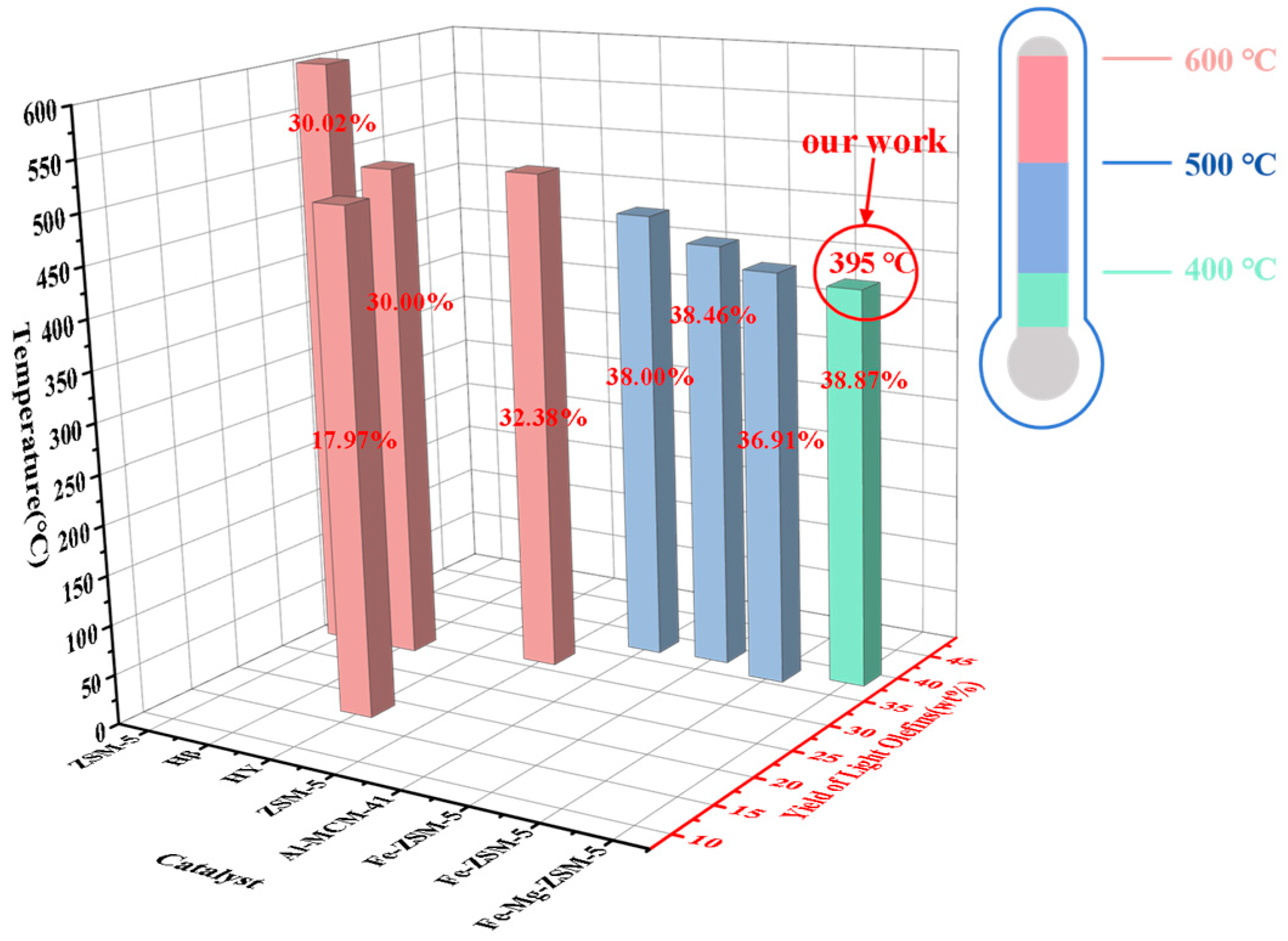
2.2.1. Catalysis Performance Comparison of Different Catalysts
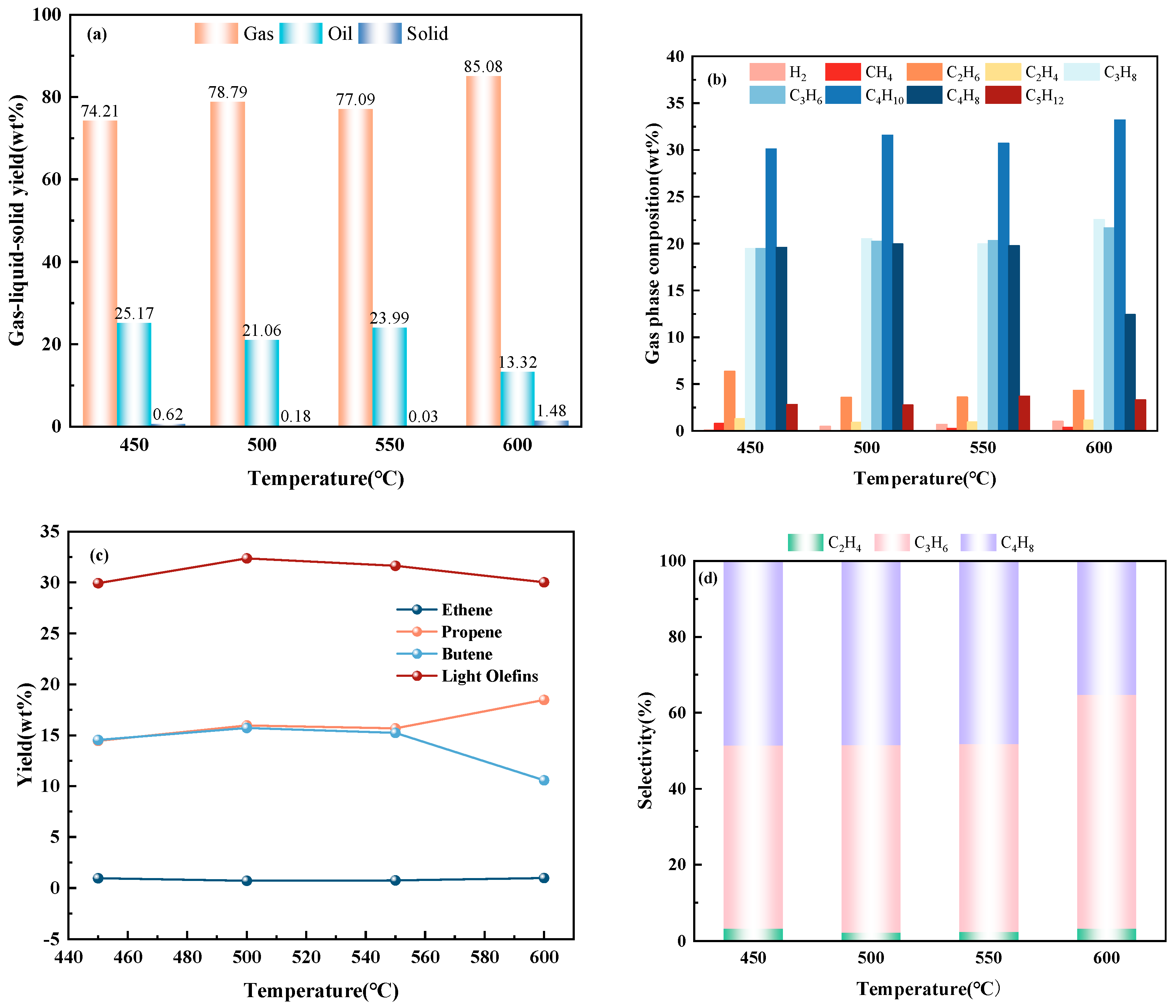
2.2.2. Catalytic Performance of ZSM-5
2.2.3. Catalytic Performance of Fe-ZSM-5
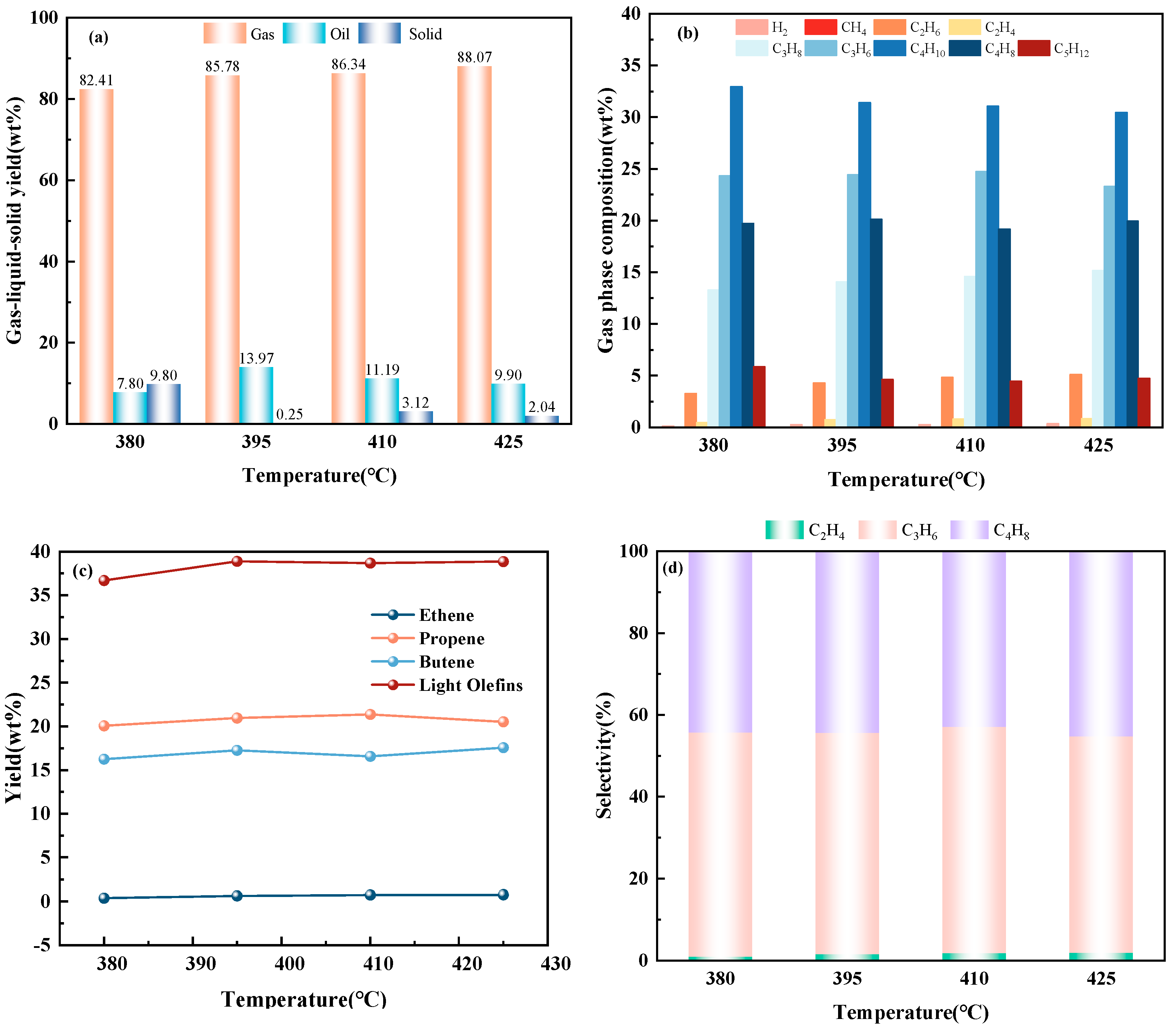
2.2.4. Catalytic Performance of Fe-Mg-ZSM-5
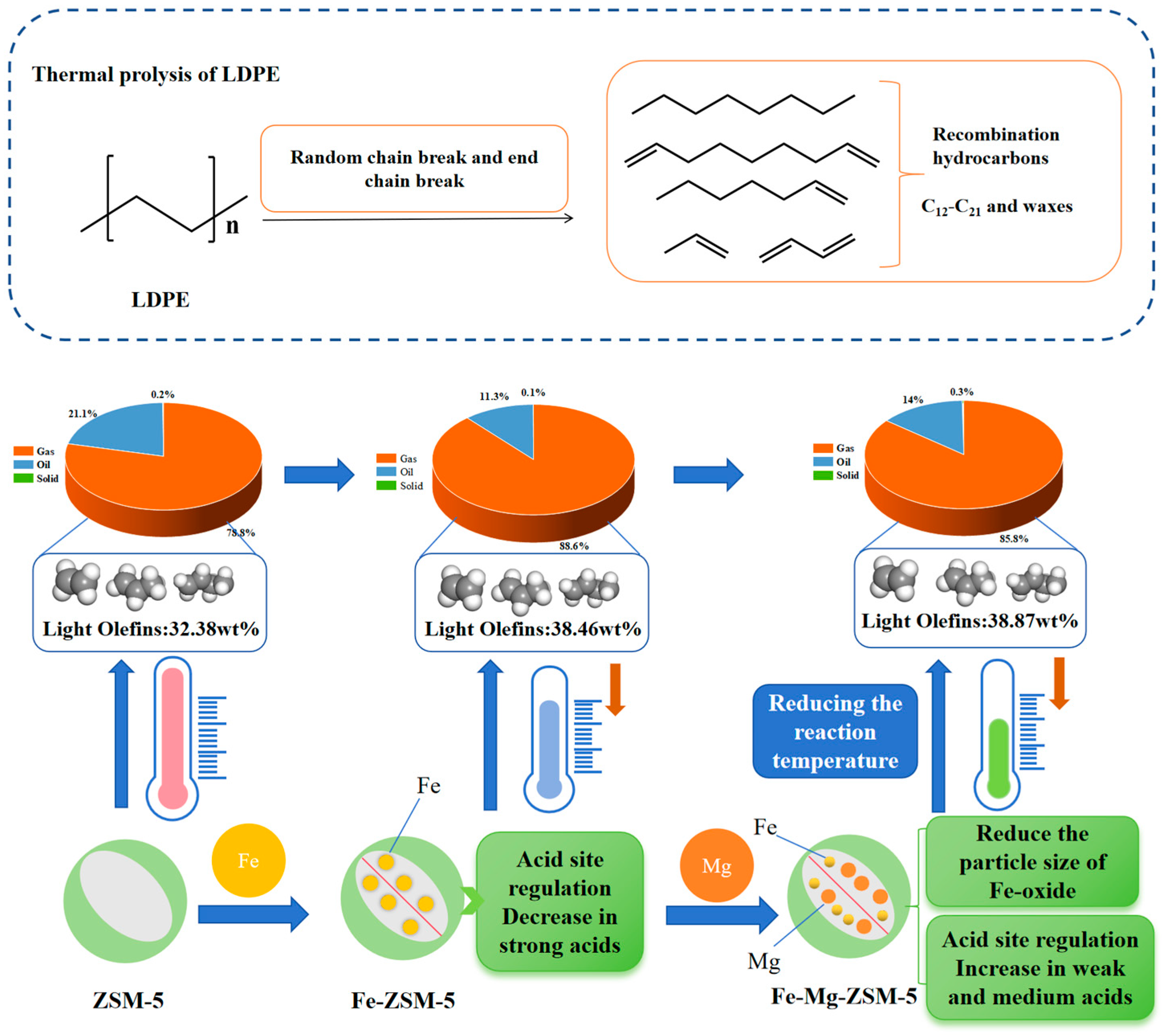
2.3. Analysis of Catalytic Performance
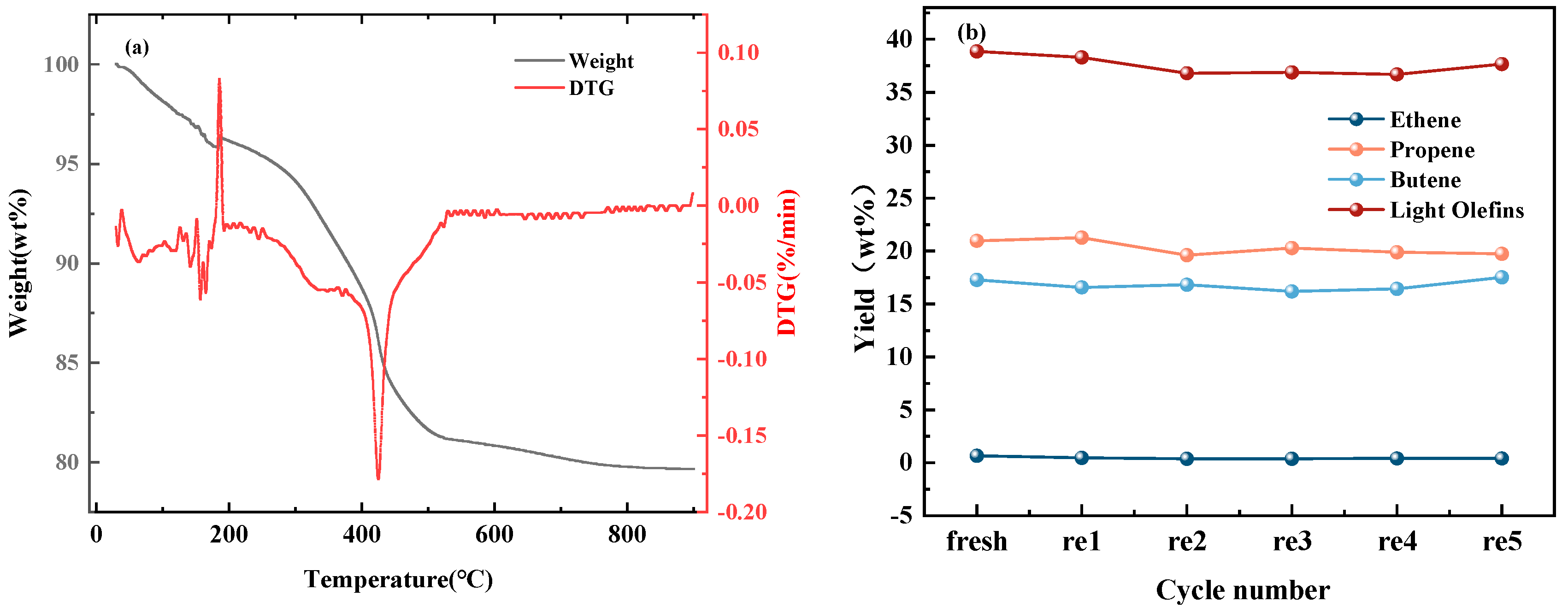
2.4. Regeneration of Spent Catalysts
3. Materials and Methods
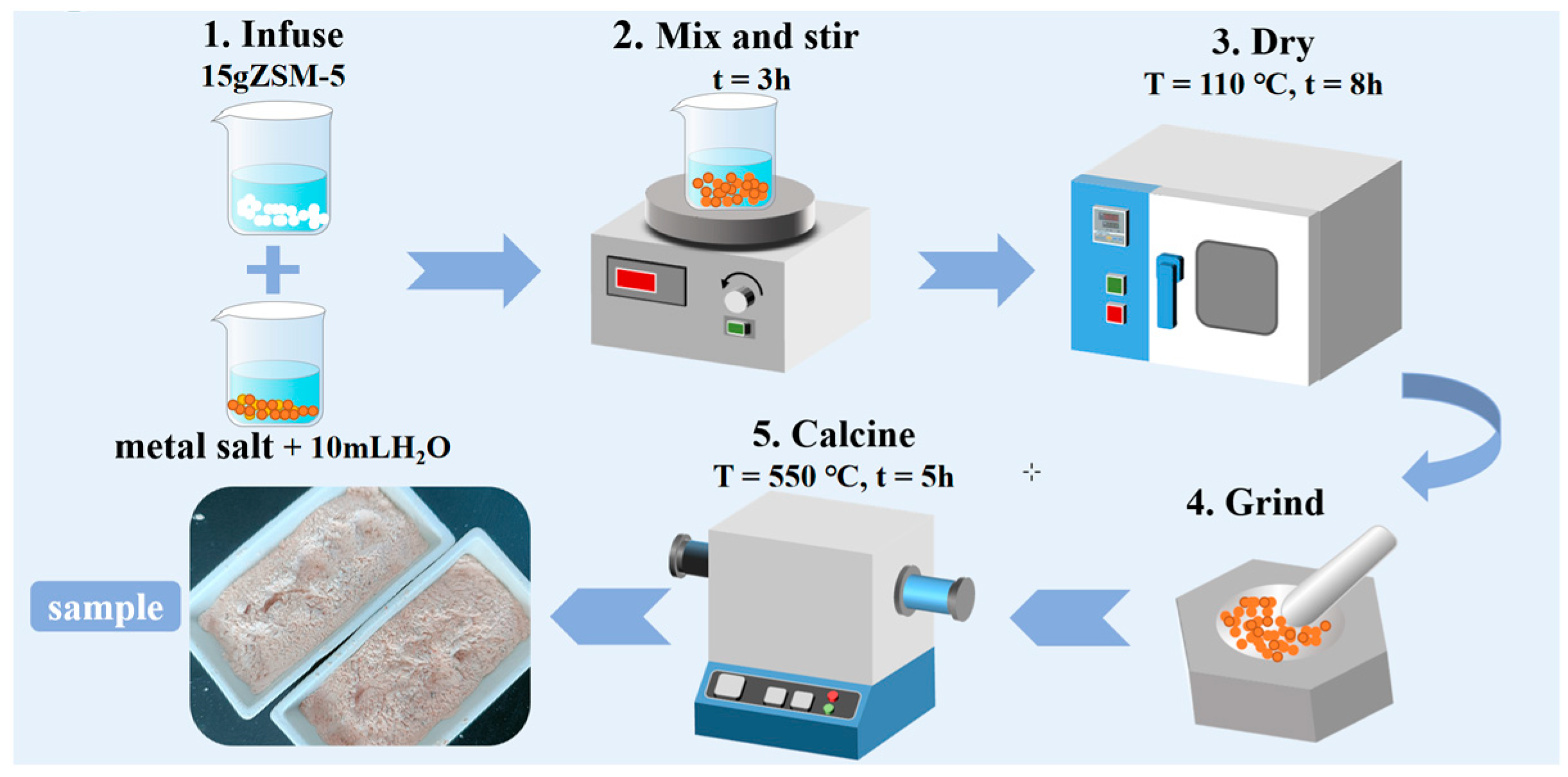
3.1. Sample Preparation
3.2. Experimental Setup
3.3. Characterization of the Samples
3.4. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, K.; Jing, Y.; Wang, Y.; Yan, N. A unified view on catalytic conversion of biomass and waste plastics. Nat. Rev. Chem. 2022, 6, 635–652. [Google Scholar] [CrossRef]
- Ellis, L.D.; Rorrer, N.A.; Sullivan, K.P.; Otto, M.; McGeehan, J.E.; Román-Leshkov, Y.; Wierckx, N.; Beckham, G.T. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 2021, 4, 539–556. [Google Scholar] [CrossRef]
- Tao, L.; Ma, X.; Ye, L.; Jia, J.; Wang, L.; Ma, P.; Liu, J. Interactions of lignin and LDPE during catalytic co-pyrolysis: Thermal behavior and kinetics study by TG-FTIR. J. Anal. Appl. Pyrolysis 2021, 158, 105267. [Google Scholar] [CrossRef]
- Danso, D.; Chow, J.; Streit, W.R. Plastics: Environmental and Biotechnological Perspectives on Microbial Degradation. Appl. Environ. Microbiol. 2019, 85, e01095-19. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Ke, L.; Dai, L.; Wu, Q.; Cobb, K.; Zeng, Y.; Zou, R.; Liu, Y.; Ruan, R. A review on catalytic pyrolysis of plastic wastes to high-value products. Energy Convers. Manag. 2022, 254, 115243. [Google Scholar] [CrossRef]
- Akgun, H.; Yapici, E.; Gunkaya, Z.; Ozkan, A.; Banar, M. Utilization of liquid product through pyrolysis of LDPE and C/LDPE as commercial wax. Environ. Sci. Pollut. Res. Int. 2021, 28, 45971–45984. [Google Scholar] [CrossRef] [PubMed]
- Mohadjer Beromi, M.; Kennedy, C.R.; Younker, J.M.; Carpenter, A.E.; Mattler, S.J.; Throckmorton, J.A.; Chirik, P.J. Iron-catalysed synthesis and chemical recycling of telechelic 1,3-enchained oligocyclobutanes. Nat. Chem. 2021, 13, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Enein, A.A.; Awadallah, A.E. Production of nanostructured carbon materials using Fe–Mo/MgO catalysts via mild catalytic pyrolysis of polyethylene waste. Chem. Eng. J. 2018, 354, 802–816. [Google Scholar] [CrossRef]
- Ding, K.; Liu, S.; Huang, Y.; Liu, S.; Zhou, N.; Peng, P.; Wang, Y.; Chen, P.; Ruan, R. Catalytic microwave-assisted pyrolysis of plastic waste over NiO and HY for gasoline-range hydrocarbons production. Energy Convers. Manag. 2019, 196, 1316–1325. [Google Scholar] [CrossRef]
- Song, J.; Sima, J.; Pan, Y.; Lou, F.; Du, X.; Zhu, C.; Huang, Q. Dielectric Barrier Discharge Plasma Synergistic Catalytic Pyrolysis of Waste Polyethylene into Aromatics-Enriched Oil. ACS Sustain. Chem. Eng. 2021, 9, 11448–11457. [Google Scholar] [CrossRef]
- Kumar, S.; Panda, A.K.; Singh, R.K. A review on tertiary recycling of high-density polyethylene to fuel. Resour. Conserv. Recycl. 2011, 55, 893–910. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, M.; Shao, J.; Yang, H.; Zeng, K.; Chen, Y.; Luo, J.; Agblevor, F.A.; Chen, H. The conversion of biomass to light olefins on Fe-modified ZSM-5 catalyst: Effect of pyrolysis parameters. Sci. Total Environ. 2018, 628–629, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, Z. Light alkane dehydrogenation to light olefin technologies: A comprehensive review. Rev. Chem. Eng. 2015, 31, 413–436. [Google Scholar] [CrossRef]
- Panda, A.K. Thermo-catalytic degradation of different plastics to drop in liquid fuel using calcium bentonite catalyst. Int. J. Ind. Chem. 2018, 9, 167–176. [Google Scholar] [CrossRef]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud WM, A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Yu, D.; Hui, H.; Li, S. Two-step catalytic co-pyrolysis of walnut shell and LDPE for aromatic-rich oil. Energy Convers. Manag. 2019, 198, 111816. [Google Scholar] [CrossRef]
- Artetxe, M.; Lopez, G.; Elordi, G.; Amutio, M.; Bilbao, J.; Olazar, M. Production of Light Olefins from Polyethylene in a Two-Step Process: Pyrolysis in a Conical Spouted Bed and Downstream High-Temperature Thermal Cracking. Ind. Eng. Chem. Res. 2012, 51, 13915–13923. [Google Scholar] [CrossRef]
- Nishu; Li, Y.; Liu, R. Catalytic pyrolysis of lignin over ZSM-5, alkali, and metal modified ZSM-5 at different temperatures to produce hydrocarbons. J. Energy Inst. 2022, 101, 111–121. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail IM, I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Zhou, Y.; Deng, S.; Zhang, H. Efficient Pyrolysis of Low-Density Polyethylene for Regulatable Oil and Gas Products by ZSM-5, HY and MCM-41 Catalysts. Catalysts 2023, 13, 382. [Google Scholar] [CrossRef]
- Li, Z.; Li, F.; Zhao, T.; Yu, H.; Ding, S.; He, W.; Song, C.; Zhang, Y.; Lin, H. The effect of steam on maximizing light olefin production by cracking of ethanol and oleic acid over mesoporous ZSM-5 catalysts. Catal. Sci. Technol. 2020, 10, 6618–6627. [Google Scholar] [CrossRef]
- Wang, J.; Shan, J.; Tian, Y.; Zhu, T.; Duan, H.; He, X.; Qiao, C.; Liu, G. Catalytic cracking of n-heptane over Fe modified HZSM-5 nanosheet to produce light olefins. Fuel 2021, 306, 121725. [Google Scholar] [CrossRef]
- Li, F.; Ding, S.; Wang, Z.; Li, Z.; Li, L.; Gao, C.; Zhong, Z.; Lin, H.; Chen, C. Production of Light Olefins from Catalytic Cracking Bio-oil Model Compounds over La2O3-Modified ZSM-5 Zeolite. Energy Fuels 2018, 32, 5910–5922. [Google Scholar] [CrossRef]
- Lima, D.S.; Perez-Lopez, O.W. Catalytic conversion of glycerol to olefins over Fe, Mo, and Nb catalysts supported on zeolite ZSM-5. Renew. Energy 2019, 136, 828–836. [Google Scholar] [CrossRef]
- Xia, S.; Zhu, Y.; Tian, Y.; He, X.; Guo, L.; Qiao, C.; Liu, G. Fe–Al isomorphous and self-pillared ZSM-5 nanosheet catalyst for cracking of n-heptane with enhanced selectivity of light olefins. J. Energy Inst. 2022, 102, 196–205. [Google Scholar] [CrossRef]
- Shao, J.A.; Jiang, H.; Yang, M.; Xiao, J.; Yang, H.; Chen, Y.; Zhang, S.; Chen, H. Catalytic fast pyrolysis of cellulose over different metal-modified ZSM-5 zeolites for light olefins. J. Anal. Appl. Pyrolysis 2022, 166, 105628. [Google Scholar] [CrossRef]
- Yang, M.; Shao, J.; Yang, Z.; Yang, H.; Wang, X.; Wu, Z.; Chen, H. Conversion of lignin into light olefins and aromatics over Fe/ZSM-5 catalytic fast pyrolysis: Significance of Fe contents and temperature. J. Anal. Appl. Pyrolysis 2019, 137, 259–265. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, F.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z.; Gu, J. Study on aromatics production via the catalytic pyrolysis vapor upgrading of biomass using metal-loaded modified H-ZSM-5. J. Anal. Appl. Pyrolysis 2017, 126, 169–179. [Google Scholar] [CrossRef]
- Schultz, E.L.; Mullen, C.A.; Boateng, A.A. Aromatic Hydrocarbon Production fromEucalyptus urophyllaPyrolysis over Several Metal-Modified ZSM-5 Catalysts. Energy Technol. 2017, 5, 196–204. [Google Scholar] [CrossRef]
- Kostyniuk, A.; Key, D.; Mdleleni, M. Effect of Fe-Mo promoters on HZSM-5 zeolite catalyst for 1-hexene aromatization. J. Saudi Chem. Soc. 2019, 23, 612–626. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Q.; Cui, M.; Qiao, X.; Fei, Z. Mg/Ca modified hierarchical porous ZSM-5 zeolite for light-olefins production from chloromethane. J. Porous Mater. 2022, 30, 521–529. [Google Scholar] [CrossRef]
- Abdalla, A.; Arudra, P.; Al-Khattaf, S.S. Catalytic cracking of 1-butene to propylene using modified H-ZSM-5 catalyst: A comparative study of surface modification and core-shell synthesis. Appl. Catal. A Gen. 2017, 533, 109–120. [Google Scholar] [CrossRef]
- Gurdeep Singh, H.K.; Yusup, S.; Quitain, A.T.; Abdullah, B.; Ameen, M.; Sasaki, M.; Kida, T.; Cheah, K.W. Biogasoline production from linoleic acid via catalytic cracking over nickel and copper-doped ZSM-5 catalysts. Environ. Res. 2020, 186, 109616. [Google Scholar] [CrossRef] [PubMed]
- Grandprix, T.M.; Kadja, T.R.S.; Ilmi, M.M.; Khalil, M.; Mukti, R.R. Subagjo Sequential mechanochemical and recrystallization methods for synthesizing hierarchically porous ZSM-5 zeolites. Microporous Mesoporous Mater. 2020, 308, 110550. [Google Scholar] [CrossRef]
- Rahimi, S.; Rostamizadeh, M. Novel Fe/B-ZSM-5 nanocatalyst development for catalytic cracking of plastic to valuable products. J. Taiwan Inst. Chem. Eng. 2021, 118, 131–139. [Google Scholar] [CrossRef]
- Chai, M.; Liu, R.; He, Y. Effects of SiO2/Al2O3 ratio and Fe loading rate of Fe-modified ZSM-5 on selection of aromatics and kinetics of corn stalk catalytic pyrolysis. Fuel Process. Technol. 2020, 206, 106458. [Google Scholar] [CrossRef]
- Kostyniuk, A.; Key, D.; Mdleleni, M. 1-hexene isomerization over bimetallic M-Mo-ZSM-5 (M: Fe, Co, Ni) zeolite catalysts: Effects of transition metals addition on the catalytic performance. J. Energy Inst. 2020, 93, 552–564. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Zhang, Z.; Cao, S.; Liu, H.; Shen, S.; Wang, W. Ultrathin Cu-Fe oxide nanosheets boosting persulfate activation to remove organic pollutants with coupling and transformation between radical and nonradical mechanism. Sep. Purif. Technol. 2022, 281, 119978. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, D.; Sun, H.; Wu, J.; Liang, P.; Zhang, H. Fe–Ce Mixed Oxides Supported on Carbon Nanotubes for Simultaneous Removal of NO and Hg0 in Flue Gas. Ind. Eng. Chem. Res. 2018, 57, 3187–3194. [Google Scholar] [CrossRef]
- Aksoy, S.; Caglar, Y.; Ilican, S.; Caglar, M. Sol–gel derived Li–Mg co-doped ZnO films: Preparation and characterization via XRD, XPS, FESEM. J. Alloys Compd. 2012, 512, 171–178. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, Z.; Xu, C.; Zhang, P.; Duan, A. FeHZSM-5 molecular sieves—Highly active catalysts for catalytic crackig of isobutane to produce ethylene and propylene. Catal. Commun. 2006, 7, 199–203. [Google Scholar] [CrossRef]
- Ji, X.; Liu, B.; Ma, W.; Chen, G.; Yan, B.; Cheng, Z. Effect of MgO promoter on Ni-Mg/ZSM-5 catalysts for catalytic pyrolysis of lipid-extracted residue of Tribonema minus. J. Anal. Appl. Pyrolysis 2017, 123, 278–283. [Google Scholar] [CrossRef]
- Wang, S.; Cao, B.; Liu, X.; Xu, L.; Hu, Y.; Afonaa-Mensah, S.; Abomohra, A.E.F.; He, Z.; Wang, Q.; Xu, S. A comparative study on the quality of bio-oil derived from green macroalga Enteromorpha clathrata over metal modified ZSM-5 catalysts. Bioresour. Technol. 2018, 256, 446–455. [Google Scholar] [CrossRef]
- Ryu, H.W.; Lim, Y.H.; Jung, W.; Nam, K.; Hwang, Y.; Roh, J.E.; Kim, D.H. Enhanced stability of CO2-assisted shale gas aromatization through the introduction of Mg promoter on Mo_ZSM-5. Chem. Eng. J. 2023, 467, 143404. [Google Scholar] [CrossRef]
- Elordi, G.; Olazar, M.; Lopez, G.; Amutio, M.; Artetxe, M.; Aguado, R.; Bilbao, J. Catalytic pyrolysis of HDPE in continuous mode over zeolite catalysts in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis 2009, 85, 345–351. [Google Scholar] [CrossRef]
- Elordi, G.; Olazar, M.; Aguado, R.; Lopez, G.; Arabiourrutia, M.; Bilbao, J. Catalytic pyrolysis of high density polyethylene in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis 2007, 79, 450–455. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; San Miguel, G.; Castro, M.C.; Madrid, S. Feedstock recycling of polyethylene in a two-step thermo-catalytic reaction system. J. Anal. Appl. Pyrolysis 2007, 79, 415–423. [Google Scholar] [CrossRef]
- Wong, S.L.; Ngadi, N.; Abdullah TA, T.; Inuwa, I.M. Conversion of low density polyethylene (LDPE) over ZSM-5 zeolite to liquid fuel. Fuel 2017, 192, 71–82. [Google Scholar] [CrossRef]
- Ji, Y.; Shi, B.; Yang, H.; Yan, W. Synthesis of isomorphous MFI nanosheet zeolites for supercritical catalytic cracking of n-dodecane. Appl. Catal. A Gen. 2017, 533, 90–98. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, M.; Chen, Z.; Zhang, X.; Yang, H.; Wang, X.; Feng, H.; Yu, J.; Gao, S. Production of monocyclic aromatics and light olefins through ex-situ catalytic pyrolysis of low-density polyethylene over Ga/P/ZSM-5 catalyst. J. Energy Inst. 2023, 108, 101235. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, G.; Liu, D.; Zhang, Y.; Zhao, L.; Gao, J.; Xu, C.; Meng, Q.; Gao, X. The advance in catalytic pyrolysis of naphtha technology using ZSM-5 as catalyst. Appl. Catal. A Gen. 2021, 628, 118399. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, H.; Yan, W. Effect of alkali metal cations modification on the acid/basic properties and catalytic activity of ZSM-5 in cracking of supercritical n-dodecane. Fuel 2019, 243, 155–161. [Google Scholar] [CrossRef]
- Inayat, A.; Inayat, A.; Schwieger, W.; Sokolova, B.; Lestinsky, P. Enhancing aromatics and olefins yields in thermo-catalytic pyrolysis of LDPE over zeolites: Role of staged catalysis and acid site density of HZSM-5. Fuel 2022, 314, 123071. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Bi, C.; Zhang, Z.; Han, D.; Wang, C.; Zhang, J.; Sun, M.; Hao, Q.; Chen, H.; Ma, X. Effective regulation of Ga active species in mesoporous ZSM-5 for catalytic upgrading of coal pyrolysis volatiles. Fuel 2022, 321, 124105. [Google Scholar] [CrossRef]
- Lopez, A.; de Marco, I.; Caballero, B.M.; Adrados, A.; Laresgoiti, M.F. Deactivation and regeneration of ZSM-5 zeolite in catalytic pyrolysis of plastic wastes. Waste Manag. 2011, 31, 1852–1858. [Google Scholar] [CrossRef]
- Carlson, T.R.; Cheng, Y.-T.; Jae, J.; Huber, G.W. Production of green aromatics and olefins by catalytic fast pyrolysis of wood sawdust. Energy Environ. Sci. 2011, 4, 145–161. [Google Scholar] [CrossRef]





| Catalysts | Acid Content (μmol/g) | |||
|---|---|---|---|---|
| Weak Acidity | Medium Acidity | Strong Acidity | Total Acidity | |
| ZSM-5 | 206.71 | 642.58 | 470.71 | 1320 |
| Fe-ZSM-5 | 182.52 | 432.77 | 473.72 | 1089 |
| Fe-Mg-ZSM-5 | 214.75 | 484.31 | 394.93 | 1094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, T.; Deng, S.; Liu, X.; Meng, Q.; Tang, M.; Wu, X.; Zhang, H. Surface Modification of Fe-ZSM-5 Using Mg for a Reduced Catalytic Pyrolysis Temperature of Low-Density Polyethylene to Produce Light Olefin. Catalysts 2024, 14, 78. https://doi.org/10.3390/catal14010078
Li Y, Liu T, Deng S, Liu X, Meng Q, Tang M, Wu X, Zhang H. Surface Modification of Fe-ZSM-5 Using Mg for a Reduced Catalytic Pyrolysis Temperature of Low-Density Polyethylene to Produce Light Olefin. Catalysts. 2024; 14(1):78. https://doi.org/10.3390/catal14010078
Chicago/Turabian StyleLi, Yincui, Ting Liu, Shengnan Deng, Xiao Liu, Qian Meng, Mengxue Tang, Xueying Wu, and Huawei Zhang. 2024. "Surface Modification of Fe-ZSM-5 Using Mg for a Reduced Catalytic Pyrolysis Temperature of Low-Density Polyethylene to Produce Light Olefin" Catalysts 14, no. 1: 78. https://doi.org/10.3390/catal14010078