3D-Printed Monoliths Based on Cu-Exchanged SSZ-13 as Catalyst for SCR of NOx
Abstract
:1. Introduction
2. Results and Discussion
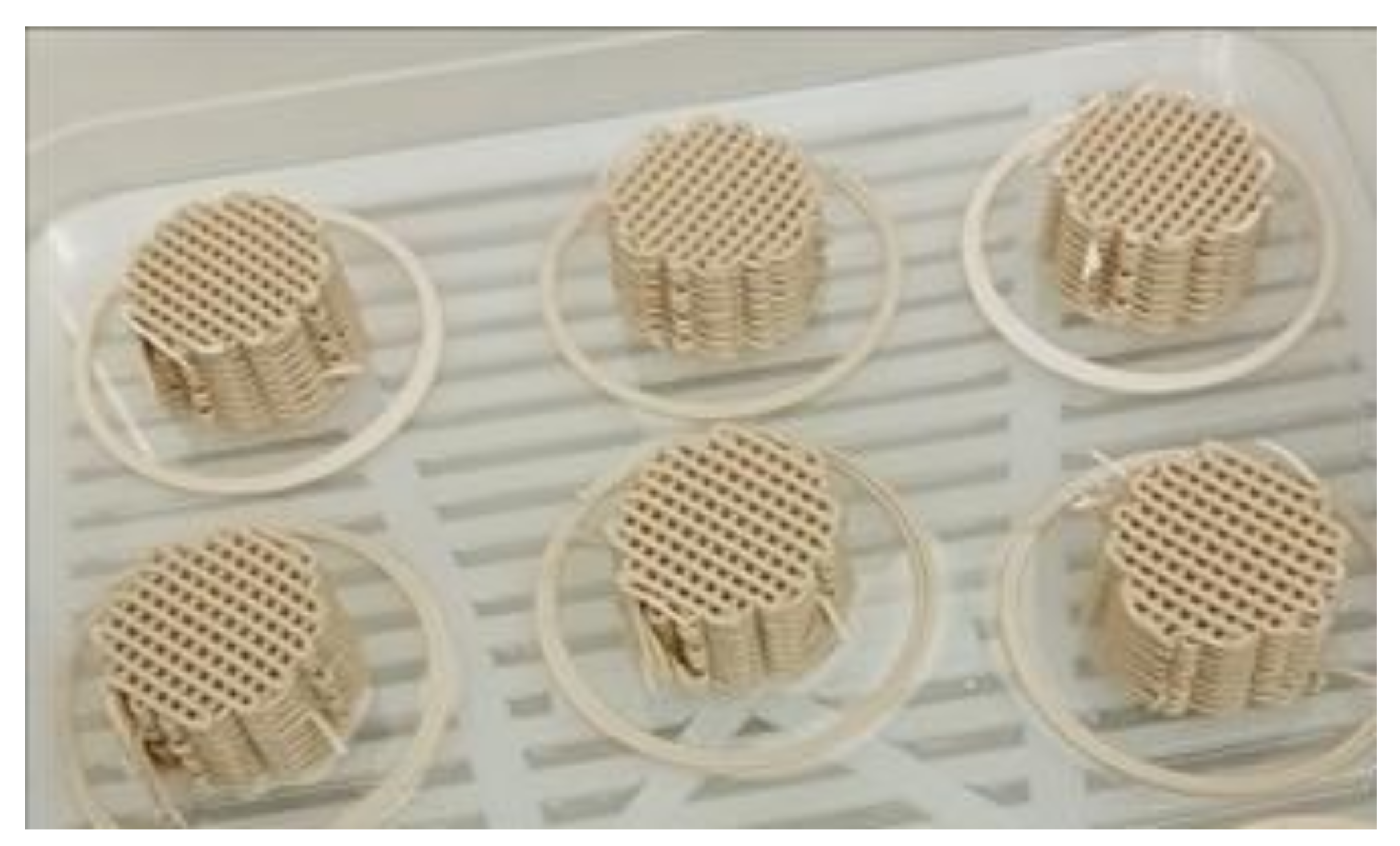
2.1. Catalysts Preparation
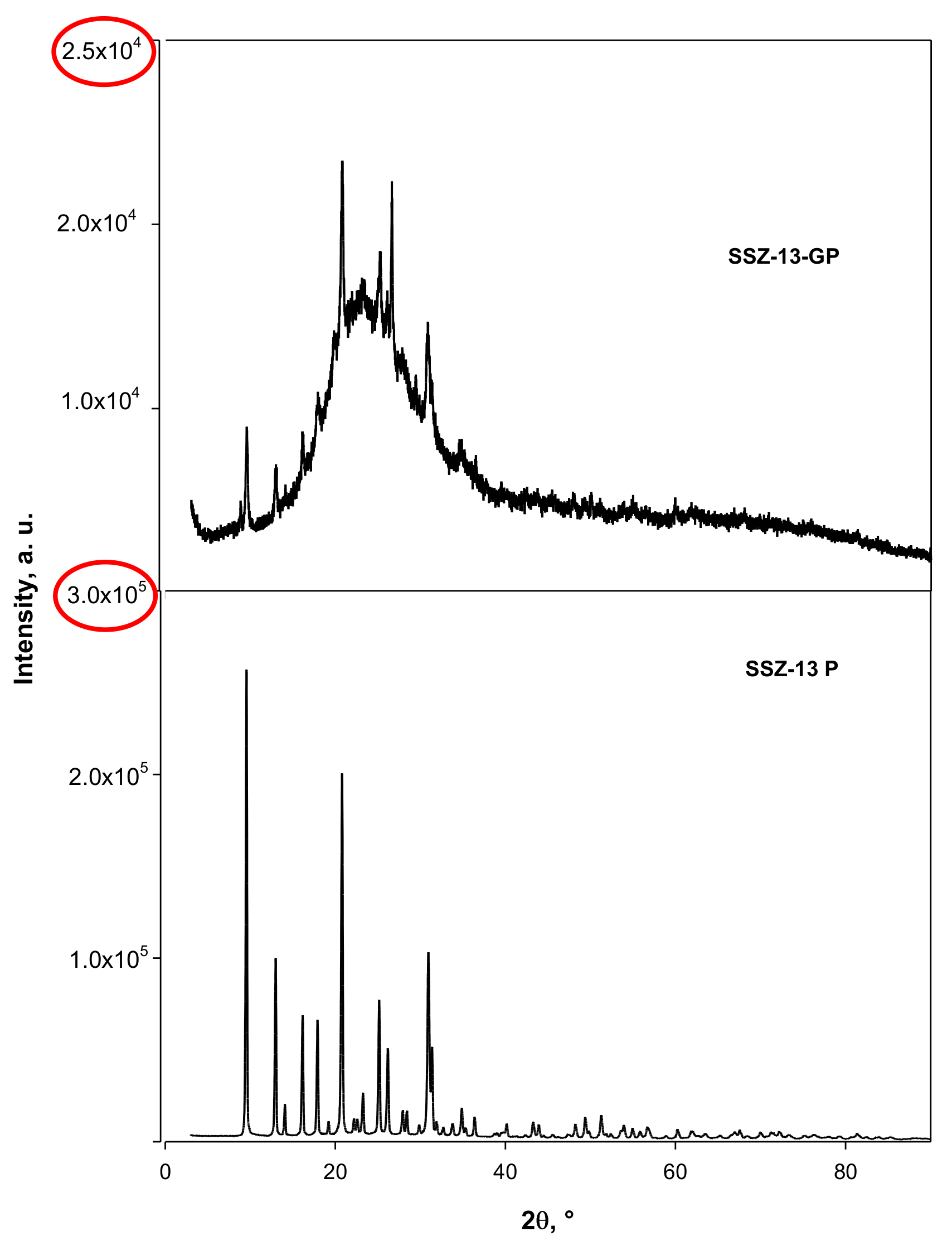
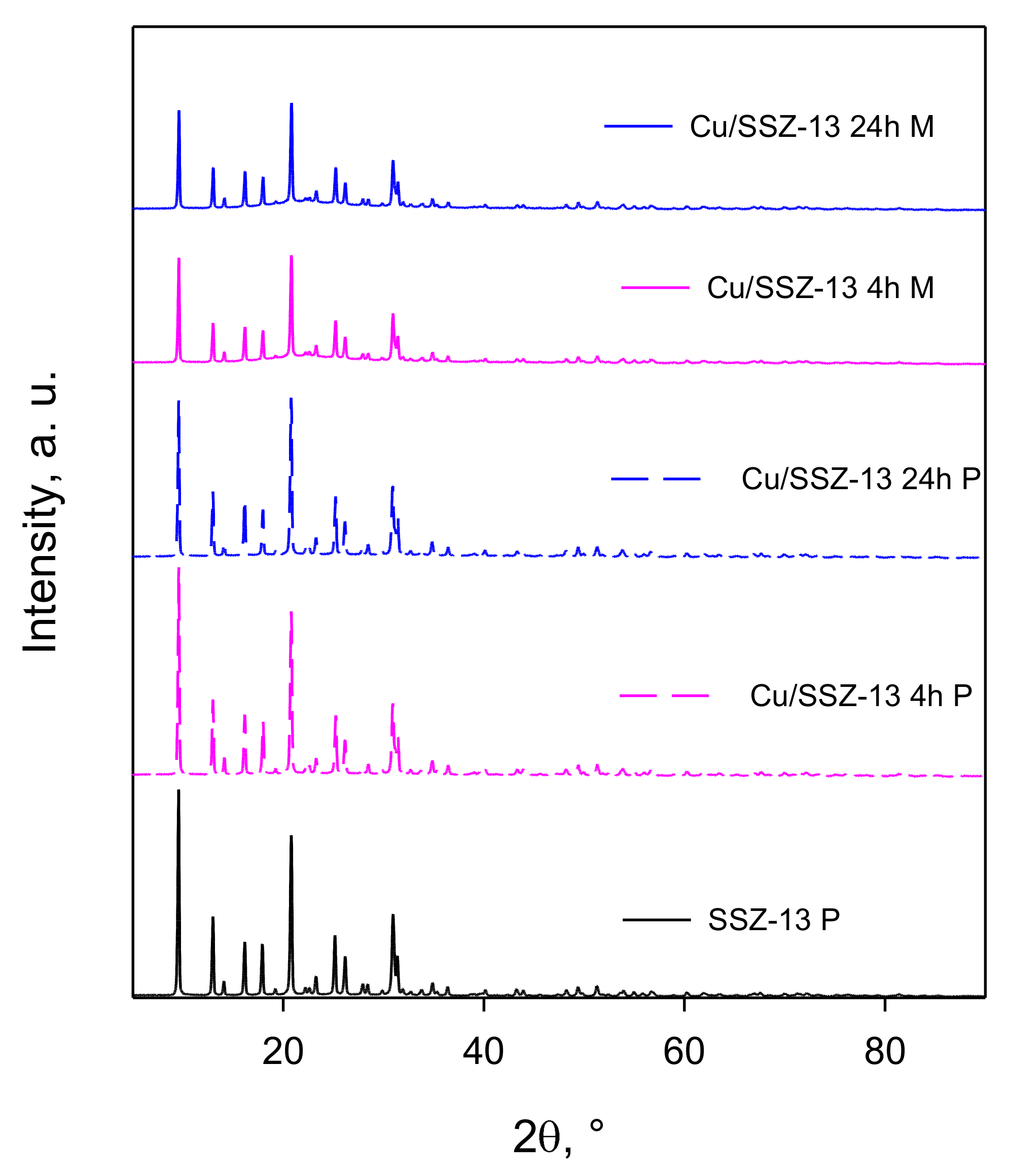
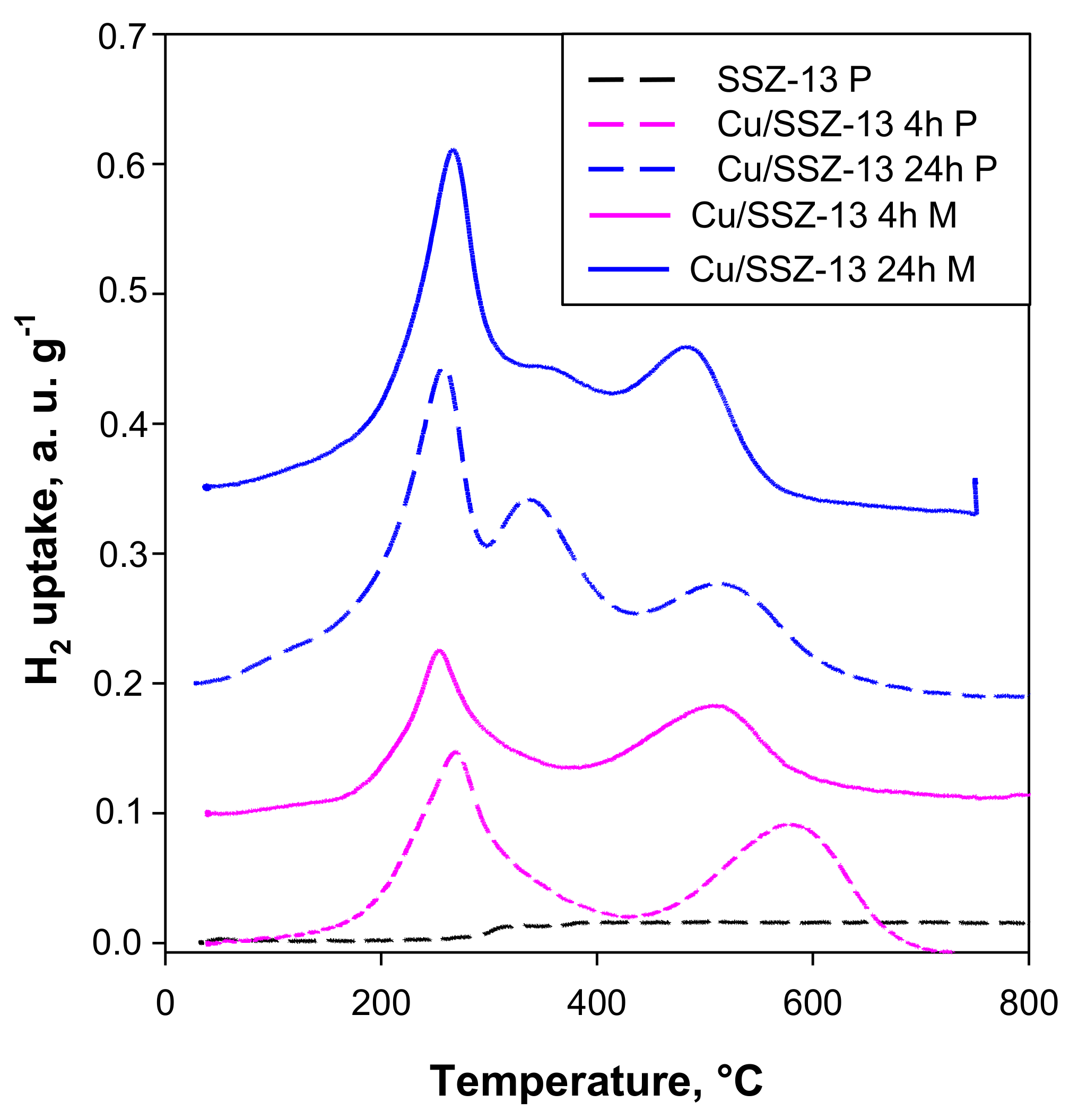
2.2. Catalysts Characterization
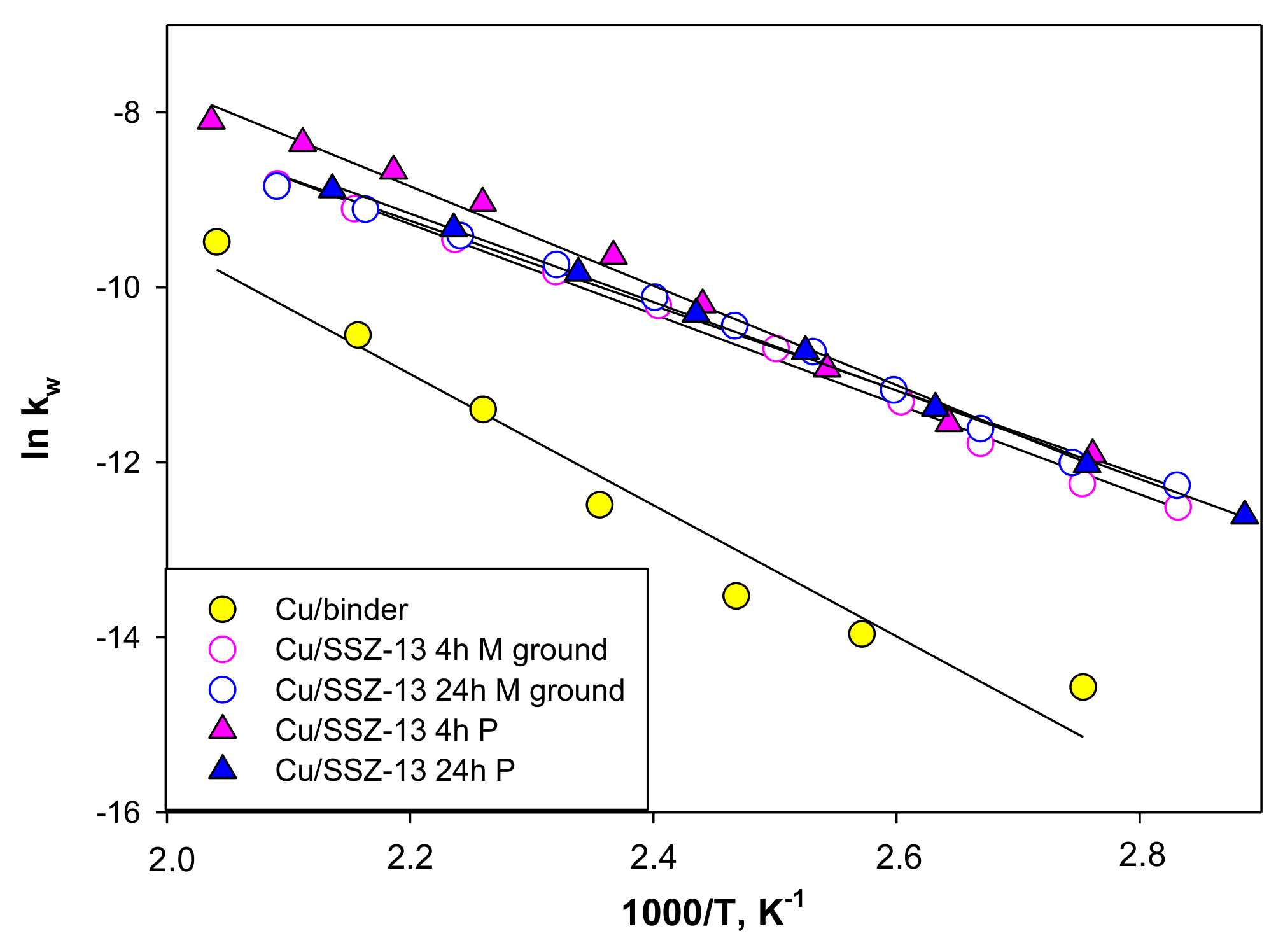
2.3. NH3-SCR Tests
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kwak, J.A.; Zhu, H.; Lee, J.H.; Peden, C.H.F.; Szanyi, J. Two different cationic positions in Cu-SSZ-13? Chem. Commun. 2012, 48, 4758–4760. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Chen, Z.; Pang, L.; Ming, S.; Dong, C.; Albert, K.B.; Liu, P.; Wang, J.; Zhu, D.; Chen, H.; et al. Steam and alkali resistant Cu-SSZ-13 catalyst for the selective catalytic reduction of NOx in diesel exhaust. Chem. Eng. J. 2018, 334, 344–354. [Google Scholar] [CrossRef]
- Shan, Y.; Du, J.; Zhang, Y.; Shan, W.; Shi, X.; Yu, Y.; Zhang, R.; Meng, X.; Xiao, F.-S.; He, H. Selective catalytic reduction of NOx with NH3: Opportunities and challenges of Cu-based small-pore zeolites. Natl. Sci. Rev. 2021, 8, nwab010. [Google Scholar] [CrossRef] [PubMed]
- Putluru, S.S.R.; Riisager, A.; Fehrmann, R. Alkali resistant Cu/zeolite deNOx catalysts for flue gas cleaning in biomass fired applications. Appl. Catal. B Environ. 2011, 101, 183–188. [Google Scholar] [CrossRef]
- Chen, B.; Xu, R.; Zhang, R. Economical way to synthesize SSZ-13 with abundant ion-exchanged Cu+ for an extraordinary performance in selective catalytic reduction (SCR) of NOx by ammonia. Environ. Sci. Technol. 2014, 48, 13909–13916. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.L.; Kamp, C.J.; Gao, F.; Wang, Y.; Engelhard, M.H. Coating Distribution in a Commercial SCR Filter. Emiss. Control Sci. Technol. 2018, 4, 260–270. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, Y.; Cavataio, G.; McCabe, R.W.; Fu, L.; Li, J. Characterization of commercial Cu-SSZ-13 and Cu-SAPO-34 catalysts with hydrothermal treatment for NH3-SCR of NOx in diesel exhaust. Chem. Eng. J. 2013, 225, 323–330. [Google Scholar] [CrossRef]
- Paolucci, C.; Di Iorio, J.R.; Ribeiro, F.H.; Gounder, R.; Schneider, W.F. Chapter One—Catalysis Science of NOx Selective Catalytic Reduction with Ammonia Over Cu-SSZ-13 and Cu-SAPO-34. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 2016; Volume 59, pp. 1–107. [Google Scholar]
- Song, J.; Wang, Y.; Walter, E.D.; Washton, N.M.; Mei, D.; Kovarik, L.; Engelhard, M.H.; Prodinger, S.; Wang, Y.; Peden, C.H.F.; et al. Toward Rational Design of Cu/SSZ-13 Selective Catalytic Reduction Catalysts: Implications from Atomic-Level Understanding of Hydrothermal Stability. ACS Catal. 2017, 7, 8214–8227. [Google Scholar] [CrossRef]
- Luo, J.; Gao, F.; Kamasamudram, K.; Currier, N.; Peden, C.H.F.; Yezerets, A. New insights into Cu/SSZ-13 SCR catalyst acidity. Part I: Nature of acidic sites probed by NH3 titration. J. Catal. 2017, 348, 291–299. [Google Scholar] [CrossRef]
- Daya, R.; Trandal, D.; Dadi, R.K.; Li, H.; Joshi, S.Y.; Luo, J.; Kumar, A.; Yezerets, A. Kinetics and thermodynamics of ammonia solvation on Z2Cu, ZCuOH and ZCu sites in Cu-SSZ-13—Implications for hydrothermal aging. Appl. Catal. B Environ. 2021, 297, 120444. [Google Scholar] [CrossRef]
- Jangjou, Y.; Do, Q.; Gu, Y.; Lim, L.-G.; Sun, H.; Wang, D.; Kumar, A.; Li, J.; Grabow, L.C.; Epling, W.S. Nature of Cu Active Centers in Cu-SSZ-13 and Their Responses to SO2 Exposure. ACS Catal. 2018, 8, 1325–1337. [Google Scholar] [CrossRef]
- Du, J.; Shan, Y.; Sun, Y.; Gao, M.; Liu, Z.; Shi, X.; Yu, Y.; He, H. Unexpected increase in low-temperature NH3-SCR catalytic activity over Cu-SSZ-39 after hydrothermal aging. Appl. Catal. B Environ. 2021, 294, 120237. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, S.; Wu, X.; Ma, T.; Liu, L.; Jin, B.; Liu, M.; Liu, J.; Ran, R.; Si, Z.; et al. Improved hydrothermal durability of Cu-SSZ-13 NH3-SCR catalyst by surface Al modification: Affinity and passivation. J. Catal. 2022, 405, 199–211. [Google Scholar] [CrossRef]
- Liu, B.; Lv, N.; Wang, C.; Zhang, H.; Yue, Y.; Xu, J.; Bi, X.; Bao, X. Redistributing Cu species in Cu-SSZ-13 zeolite as NH3-SCR catalyst via a simple ion-exchange. Chin. J. Chem. Eng. 2022, 41, 329–341. [Google Scholar] [CrossRef]
- Peng, C.; Yan, R.; Peng, H.; Mi, Y.; Liang, J.; Liu, W.; Wang, X.; Song, G.; Wu, P.; Liu, F. One-pot synthesis of layered mesoporous ZSM5 plus Cu ion-exchange: Enhanced NH3-SCR performance on Cu-ZSM5 with hierarchical pore structures. J. Hazard. Mater. 2020, 385, 121593. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ye, T.; Qu, H.; Zhu, T.; Zhong, Q. Progressive regulation of Al sites and Cu distribution to increase hydrothermal stability of hierarchical SSZ-13 for the selective catalytic reduction reaction. Appl. Catal. B Environ. 2022, 303, 120867. [Google Scholar] [CrossRef]
- Wijayanti, K.; Xie, K.; Kumar, A.; Kamasamudram, K.; Olsson, L. Effect of gas compositions on SO2 poisoning over Cu/SSZ-13 used for NH3-SCR. Appl. Catal. B Environ. 2017, 219, 142–154. [Google Scholar] [CrossRef]
- Wijayanti, K.; Leistner, K.; Chand, S.; Kumar, A.; Kamasamudram, K.; Currier, N.W.; Yezerets, A.; Olsson, L. Deactivation of Cu-SSZ-13 by SO2 exposure under SCR conditions. Catal. Sci. Technol. 2016, 6, 2565–2579. [Google Scholar] [CrossRef]
- Lisi, L.; Pirone, R.; Russo, G.; Stanzione, V. Cu-ZSM5 based monolith reactors for NO decomposition. Chem. Eng. J. 2009, 154, 341–347. [Google Scholar] [CrossRef]
- Nova, I.; Bounechada, D.; Maestri, R.; Tronconi, E.; Heibel, A.K.; Collins, T.A.; Boger, T. Influence of the substrate properties on the performances of NH3-SCR monolithic catalysts for the aftertreatment of diesel exhaust: An experimental and modeling study. Ind. Eng. Chem. Res. 2011, 50, 299–309. [Google Scholar] [CrossRef]
- Cepollaro, E.M.; Cimino, S.; Lisi, L.; Botti, R.; Colombo, P.; Franchin, G. Cu-exchanged 3D-printed Geopolymer/ZSM-5 Monolith for Selective Catalytic Reduction of NOx. Chem. Eng. Trans. 2021, 84, 67–72. [Google Scholar]
- Cepollaro, E.M.; Botti, R.; Franchin, G.; Lisi, L.; Colombo, P.; Cimino, S. Cu/ZSM5-Geopolymer 3D-Printed Monoliths for the NH3-SCR of NOx. Catalysts 2021, 11, 1212. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Zhu, Y.; He, H.; Wang, N.; Yang, X.; Liu, L. One-pot synthesis of hierarchical MnCu-SSZ-13 catalyst with excellent NH3-SCR activity at low temperatures. Microporous Mesoporous Mater. 2022, 333, 111720. [Google Scholar] [CrossRef]
- Thommes, M.; Cychosz, K. Physical adsorption characterization of nanoporous materials: Progress and challenges. Adsorption 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tran, D.; Szanyi, J.; Peden, C.H.F.; Lee, J.H. The Effect of Copper Loading on the Selective Catalytic Reduction of Nitric Oxide by Ammonia Over Cu-SSZ-13. Catal. Lett. 2012, 142, 295–301. [Google Scholar] [CrossRef]
- Clemens, A.K.S.; Shishkin, A.; Carlsson, P.-A.; Skoglundh, M.; Martínez-Casado, F.J.; Matĕj, Z.; Balmes, O.; Härelind, H. Reaction-driven Ion Exchange of Copper into Zeolite SSZ-13. ACS Catal. 2015, 5, 6209–6218. [Google Scholar] [CrossRef]
- Delavernhe, L.; Pilavtepe, M.; Emmerich, K. Cation exchange capacity of natural and synthetic hectorite. Appl. Clay Sci. 2018, 151, 175–180. [Google Scholar] [CrossRef]
- Khurana, I.; Albarracin-Caballero, J.D.; Shih, A.J. Identification and quantification of multinuclear Cu active sites derived from monomeric Cu moieties for dry NO oxidation over Cu-SSZ-13. J. Catal. 2022, 413, 111–1122. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Wang, J.; Wang, Z.; Chen, Z.; Li, X.; Shen, M.; Yan, W.; Kang, X. The Role of Impregnated Sodium Ions in Cu/SSZ-13 NH3-SCR Catalysts. Catalysts 2018, 8, 593. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, G.; Hill, A.J.; Luo, B.; Jing, G. One-step ion-exchange from Na-SSZ-13 to Cu-SSZ-13 for NH3-SCR by adjusting the pH value of Cu-exchange solution: The effect of H+ ions on activity and hydrothermal stability. Microporous Mesoporous Mater. 2021, 324, 111271. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Walter, E.D.; Szanyi, J.; Wang, Y.; Gao, F. Influences of Na+ co-cation on the structure and performance of Cu/SSZ-13 selective catalytic reduction catalysts. Catal. Today 2020, 339, 233–240. [Google Scholar] [CrossRef]
- Gargiulo, N.; Caputo, D.; Totarella, G.; Lisi, L.; Cimino, S. Me-ZSM-5 monolith foams for the NH3-SCR of NO. Catal. Today 2018, 304, 112–118. [Google Scholar] [CrossRef]




| Sample | Form | BET Area (m2 g−1) | V Micro (cm3 g−1) | V Total (cm3 g−1) |
|---|---|---|---|---|
| SSZ-13 P | Powder | 686 | 0.26 | 0.39 |
| SSZ-13 M | 3D Monolith | 516 | 0.19 | 0.35 |
| Cu-SSZ-13 P (4 h) | Powder | 557 | 0.22 | 0.34 |
| Cu-SSZ-13 M (4 h) | 3D Monolith | 512 | 0.18 | 0.37 |
| Cu-SSZ-13 P (24 h) | Powder | 542 | 0.21 | 0.30 |
| Cu-SSZ-13 M (24 h) | 3D Monolith | 463 | 0.16 | 0.36 |
| Sample | Cu from ICP-MS (wt%) | H2 Uptake (mmol g−1) | Cu Estimated from Total H2 Uptake (wt%) | Desorbed NH3 (mmol g−1) |
|---|---|---|---|---|
| SSZ-13 P | - | - | - | 3.56 |
| Cu/SSZ-13 P (4 h) | 5.09 | 0.68 | 4.3 | 2.65 |
| Cu/SSZ-13 M (4 h) | 2.91 | 0.47 | 3.0 | 1.69 |
| Cu/SSZ-13 P (24 h) | 6.23 | 0.95 | 6.0 | 2.92 |
| Cu/SSZ-13 M (24 h) | 3.33 | 0.84 | 5.3 | 1.86 |
| Catalyst | Ea (kJ mol−1) | kwcatalyst (cm3 g−1 s−1) | kwzeol (cm3 g−1 s−1) |
|---|---|---|---|
| Cu/binder | 62 | 4.96 | - |
| Cu-SSZ-13 4 h P | 49 | 58.9 | 58.9 |
| Cu-SSZ-13 24 h P | 42 | 45.2 | 45.2 |
| Cu-SSZ-13 4 h M | 46 | 44.7 | 74.5 |
| Cu-SSZ-13 24 h M | 44 | 49.4 | 82.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cepollaro, E.M.; Cimino, S.; D’Agostini, M.; Gargiulo, N.; Franchin, G.; Lisi, L. 3D-Printed Monoliths Based on Cu-Exchanged SSZ-13 as Catalyst for SCR of NOx. Catalysts 2024, 14, 85. https://doi.org/10.3390/catal14010085
Cepollaro EM, Cimino S, D’Agostini M, Gargiulo N, Franchin G, Lisi L. 3D-Printed Monoliths Based on Cu-Exchanged SSZ-13 as Catalyst for SCR of NOx. Catalysts. 2024; 14(1):85. https://doi.org/10.3390/catal14010085
Chicago/Turabian StyleCepollaro, Elisabetta M., Stefano Cimino, Marco D’Agostini, Nicola Gargiulo, Giorgia Franchin, and Luciana Lisi. 2024. "3D-Printed Monoliths Based on Cu-Exchanged SSZ-13 as Catalyst for SCR of NOx" Catalysts 14, no. 1: 85. https://doi.org/10.3390/catal14010085







