Co3O4-rGO—Synthesis, Characterization, and Evaluation of Photocatalytic Activities
Abstract
:1. Introduction
2. Results and Discussion
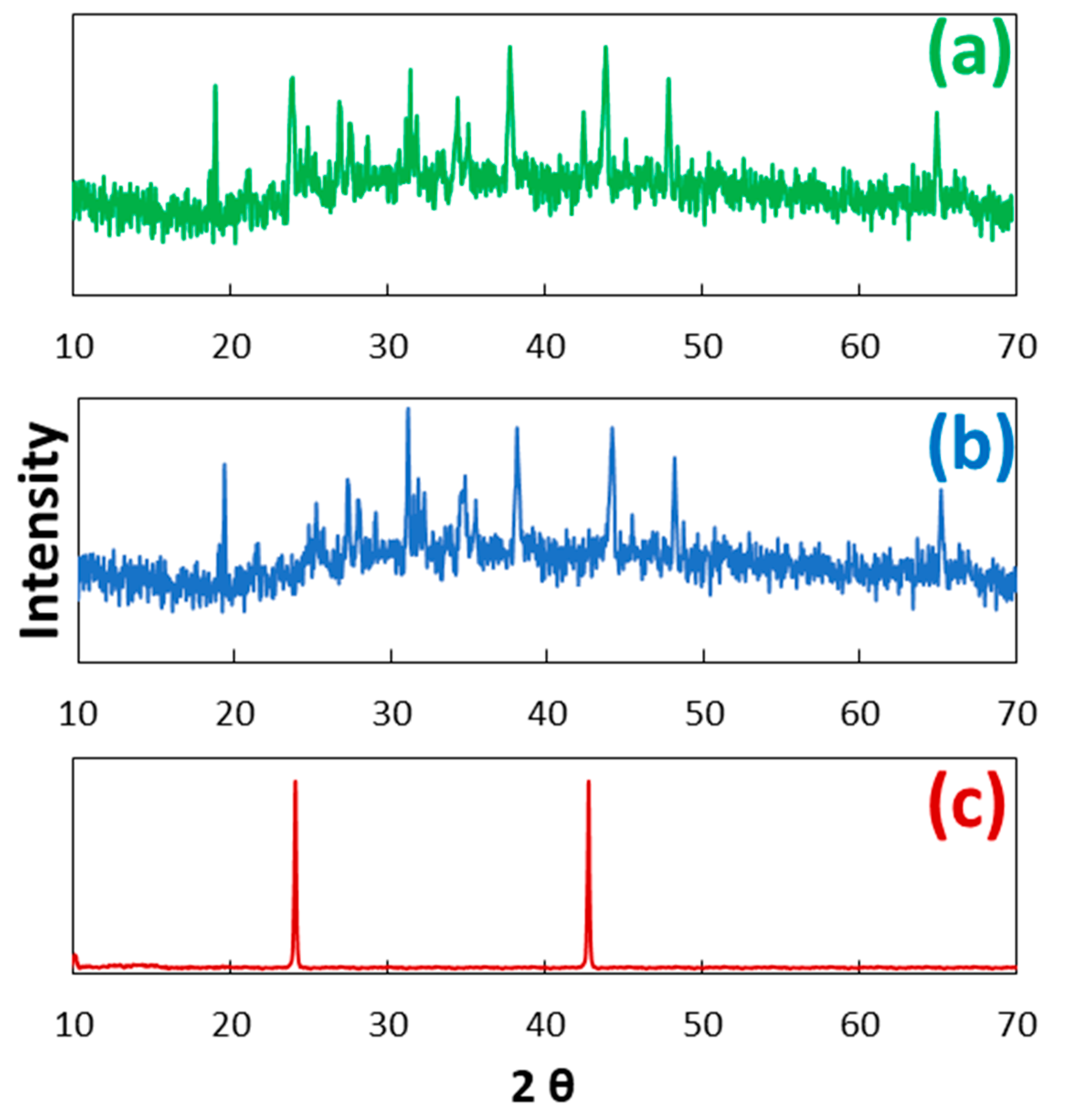
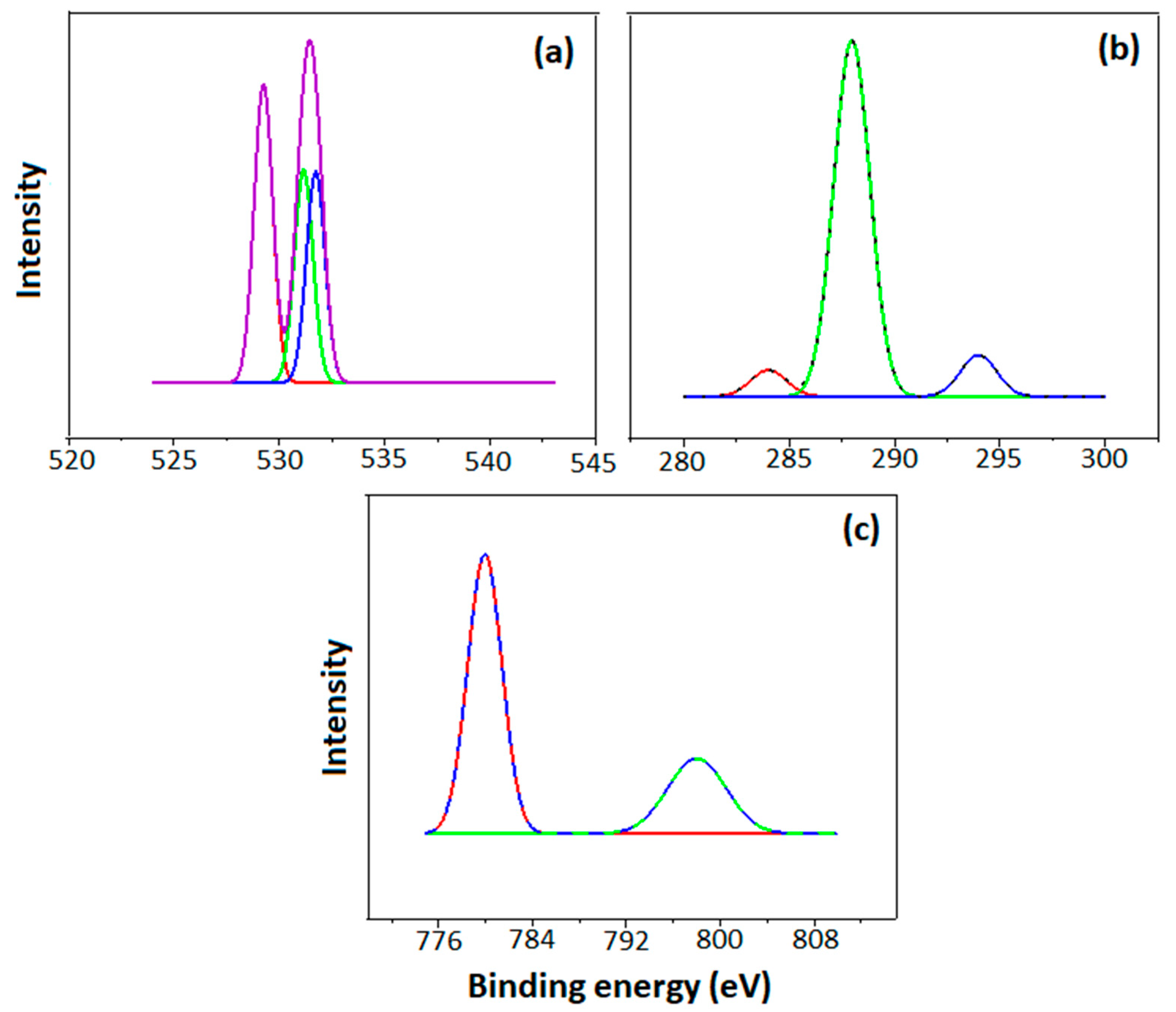
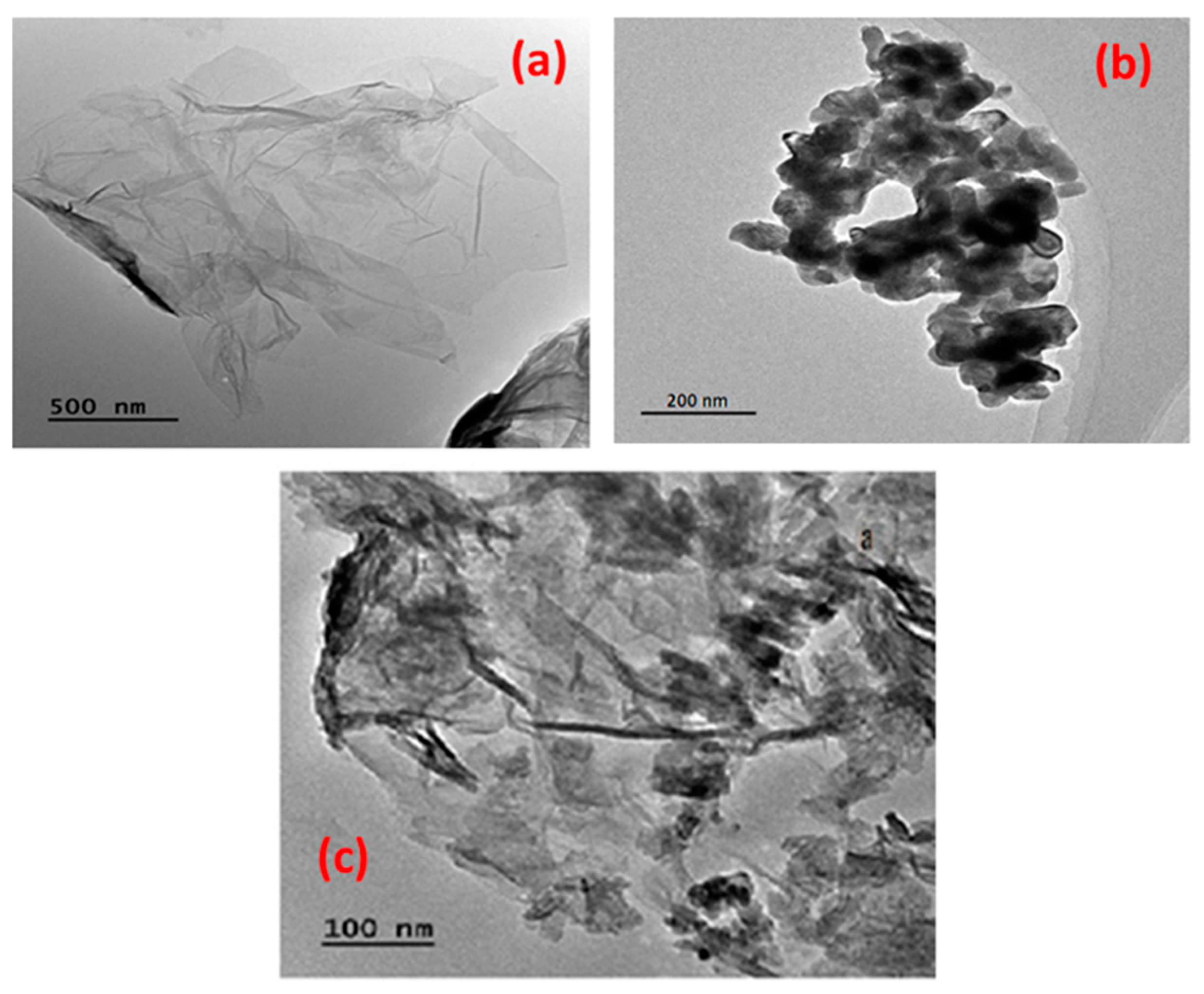
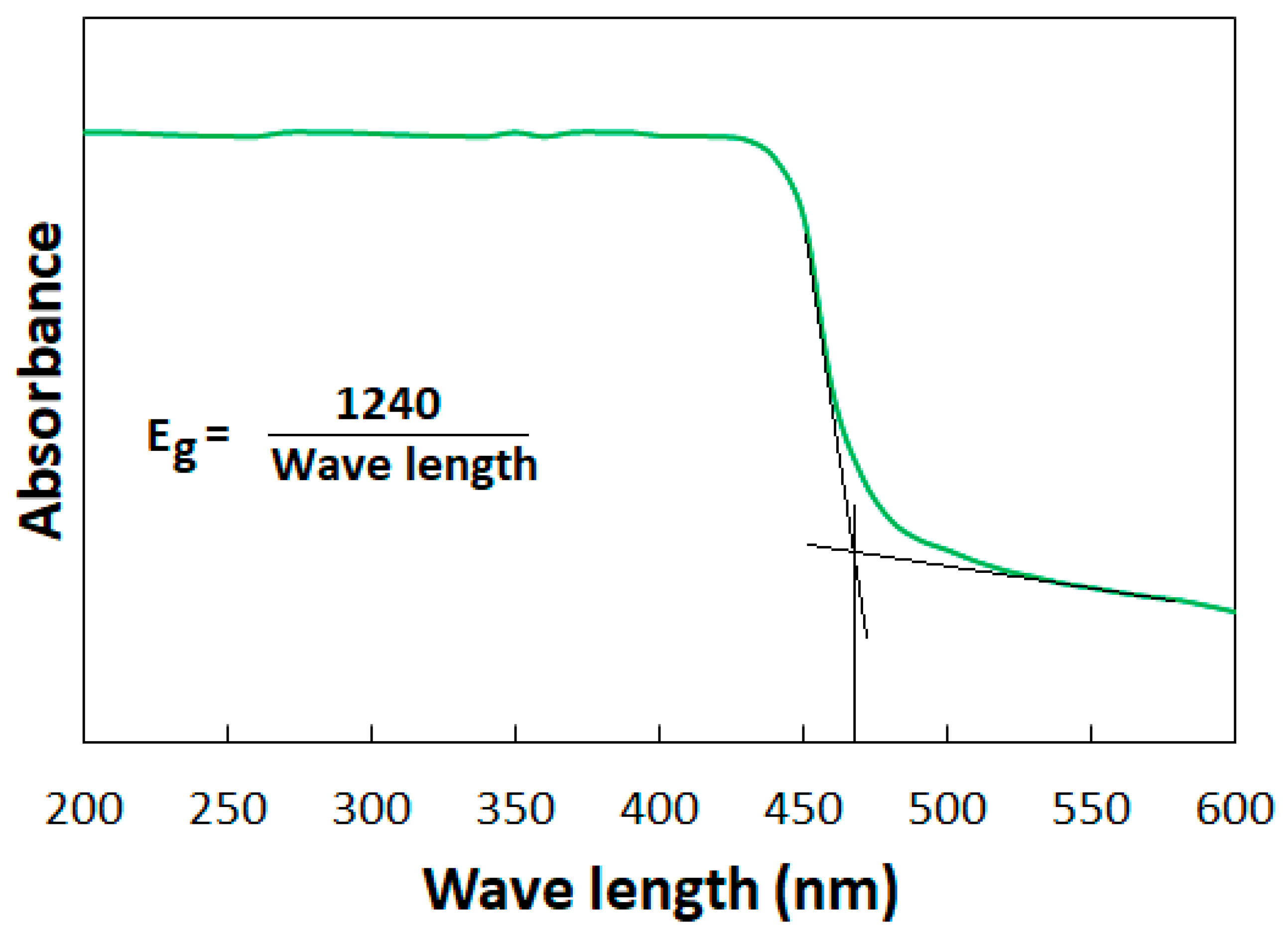
2.1. Characterization of Co3O4-rGO
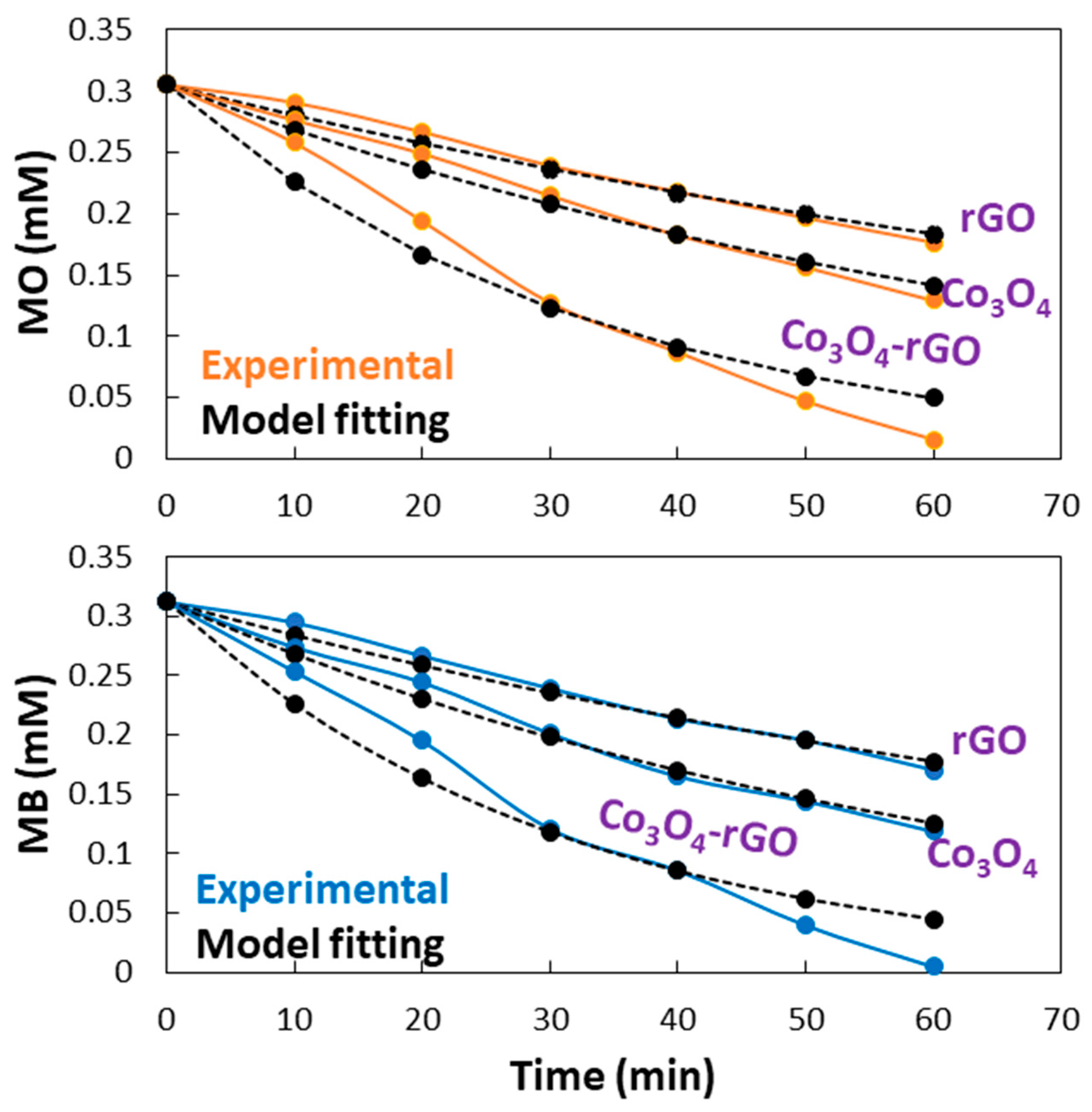
2.2. Photocatalytic Efficiency of Co3O4-rGO
2.3. Optimization of Catalyst Dose
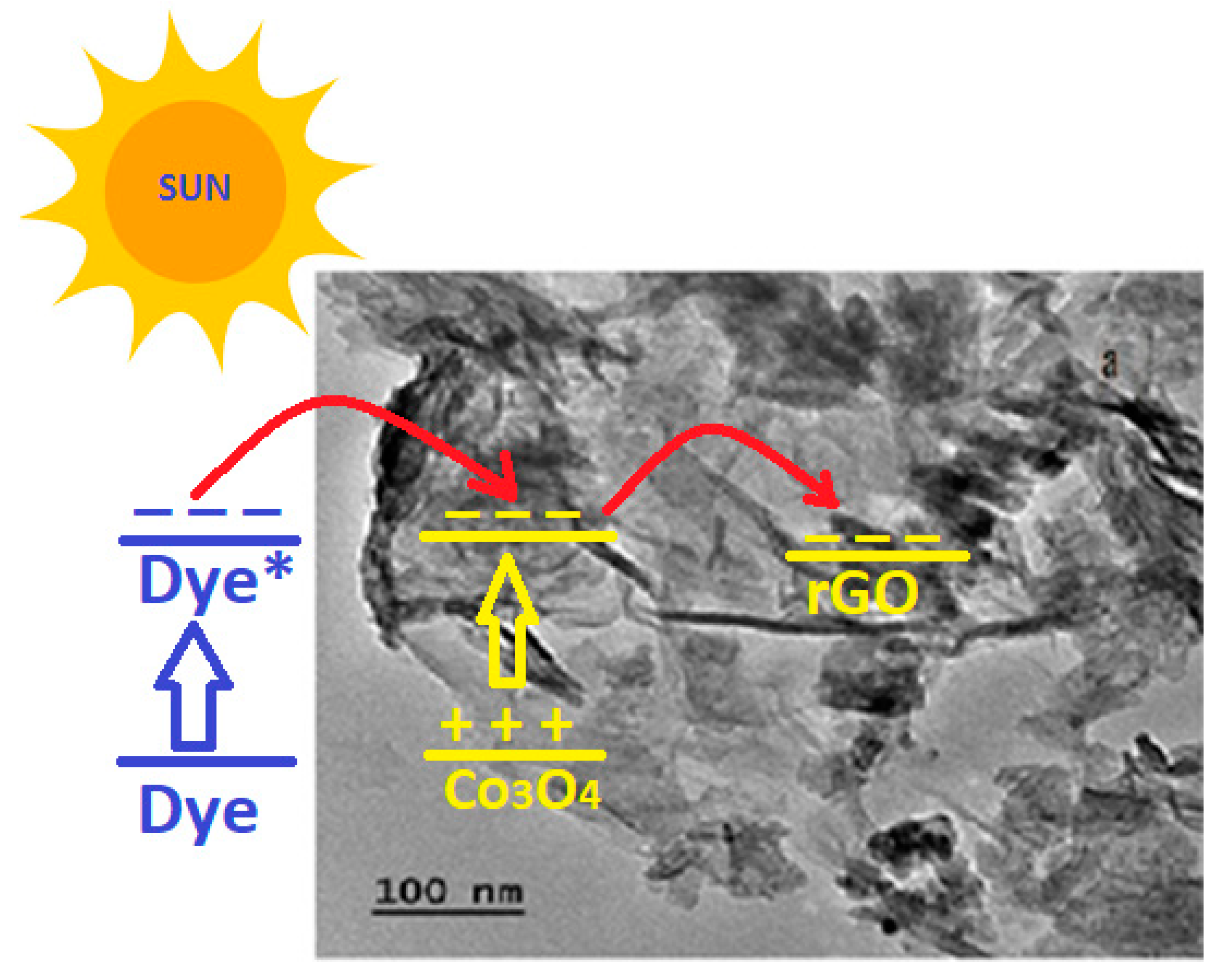
2.4. Mechanism and Kinetics of Photodegradation
| (Reaction 1) | |
| (Reaction 2) | |
| (Reaction 3) | |
| (Reaction 4) | |
| (Reaction 5) | |
| (Reaction 6) | |
| (Reaction 7) |
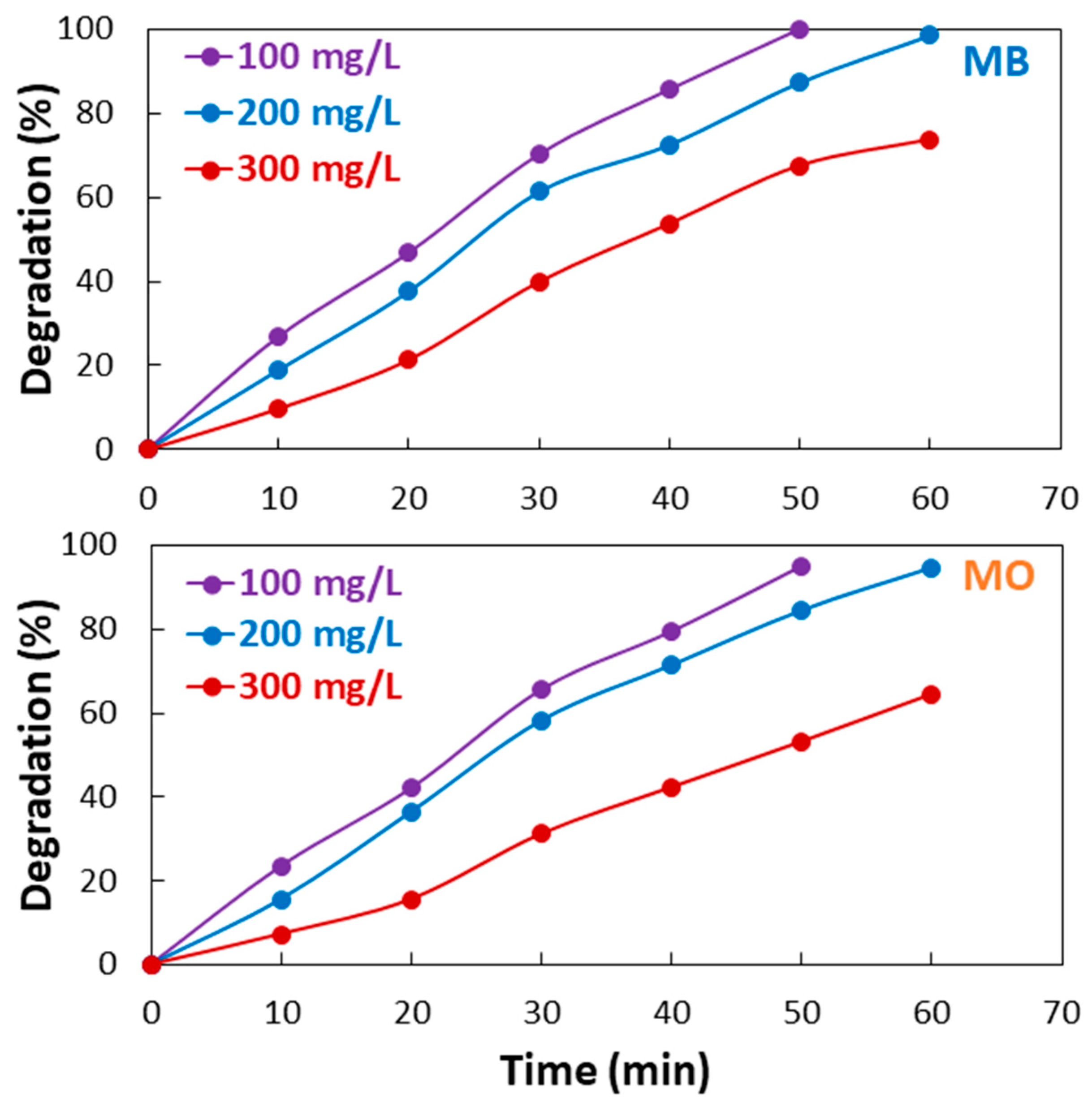
2.5. Effect of Dye Concentration
3. Experimental Process
3.1. Reagents
3.2. Synthesis of Co3O4-rGO
3.3. Characterization of Co3O4-rGO
3.4. Photocatalytic Activity of Co3O4-rGO
4. Conclusions
- The successful synthesis of Co3O4-rGO was confirmed through characterization with XRD, XPS, TEM, and FTIR analyses.
- The fabricated Co3O4-rGO composite showed efficient catalytic performance in the photodegradation of MB and MO. The photocatalytic activity of Co3O4-rGO was higher than the two-fold and three-fold catalytic activity of Co3O4 and rGO, respectively. The increase in the initial concentration of MB and MO resulted in a decrease in the photocatalytic performance.
- About 98, 62, and 45% photodegradation of methylene blue was observed in 60 min of reaction using Co3O4-rGO, Co3O4, and rGO as photocatalysts, respectively. Similarly, the photodegradation of methyl orange was 92, 57, and 42% over Co3O4-rGO, Co3O4, and rGO as photocatalysts, respectively.
- About 80% and 68% reductions in COD and BOD were detected in the 50 mL (200 mg/L) solution of MB/MO after treatment with Co3O4-rGO for 60 min. The decrease in COD and BOD of the dye solutions indicates that Co3O4-rGO can be employed as an efficient catalyst for the photodegradation of the dyes.
- The enhanced photocatalytic efficiency of Co3O4-rGO was attributed to the behavior of rGO as an electron reservoir that captures the photoexcited electron from Co3O4.
- The Co3O4-rGO was found to be an efficient and reusable photocatalyst for the degradation of methylene blue and methyl orange.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamran, U.; Bhatti, H.N.; Noreen, S.; Tahir, M.A.; Park, S.J. Chemically Modified Sugarcane Bagasse-Based Biocomposites for Efficient Removal of Acid Red 1 Dye: Kinetics, Isotherms, Thermodynamics, and Desorption Studies. Chemosphere 2022, 291, 132796. [Google Scholar] [CrossRef]
- Zafar, L.; Khan, A.; Kamran, U.; Park, S.J.; Bhatti, H.N. Eucalyptus (camaldulensis) Bark-Based Composites for Efficient Basic Blue 41 Dye Biosorption from Aqueous Stream: Kinetics, Isothermal, and Thermodynamic Studies. Surf. Interfaces 2022, 31, 101897. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, J.; Qiao, R.; Zhao, N.; Zhao, M.; Kong, L. Facile Preparation and Controllable Absorption of a Composite Based on PMo12/Ag Nanoparticles: Photodegradation Activity and Mechanism. ChemistrySelect 2022, 7, e202103668. [Google Scholar] [CrossRef]
- Pang, S.; Zhou, C.; Sun, Y.; Zhang, K.; Ye, W.; Zhao, X.; Cai, L.; Hui, B. Natural Wood-Derived Charcoal Embedded with Bimetallic Iron/Cobalt Sites to Promote Ciprofloxacin Degradation. J. Clean. Prod. 2023, 414, 137569. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Guo, X.; Chen, Z.; Zhang, W.; Wang, Y.; Tang, X.; Zhang, Y.; Zhao, Y. Sulfur-Doped g-C3N4/RGO Porous Nanosheets for Highly Efficient Photocatalytic Degradation of Refractory Contaminants. J. Mater. Sci. Technol. 2020, 41, 117–126. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.H.; Oh, C.; Zhang, K.; Park, J.H. Artificial Photosynthesis for High-Value-Added Chemicals: Old Material, New Opportunity. Carbon Energy 2022, 4, 21–44. [Google Scholar] [CrossRef]
- Bankole, O.M.; Olaseni, S.E.; Adeyemo, M.A.; Ogunlaja, A.S. Microwave-Assisted Synthesis of Cobalt Oxide/Reduced Graphene Oxide (Co3O4-RGO) Composite and Its Sulfite Enhanced Photocatalytic Degradation of Organic Dyes. Z. Fur. Phys. Chem. 2020, 234, 1681–1708. [Google Scholar] [CrossRef]
- Shah, K.W.; Li, W. A Review on Catalytic Nanomaterials for Volatile Organic Compounds VOC Removal and Their Applications for Healthy Buildings. Nanomaterials 2019, 9, 910. [Google Scholar] [CrossRef]
- Xu, C.; Ravi Anusuyadevi, P.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured Materials for Photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, F.; Shi, Q.; Li, M.; Dai, Y.; Zhang, Z.; Zhu, C. Responses of Arid Plant Species Diversity and Composition to Environmental Factors. J. For. Res. (Harbin) 2023, 34, 1723–1734. [Google Scholar] [CrossRef]
- Poudel, M.B.; Awasthi, G.P.; Kim, H.J. Novel Insight into the Adsorption of Cr(VI) and Pb(II) Ions by MOF Derived Co-Al Layered Double Hydroxide @hematite Nanorods on 3D Porous Carbon Nanofiber Network. Chem. Eng. J. 2021, 417, 129312. [Google Scholar] [CrossRef]
- Wang, Z.; Mei, Y.; Ling, J.; Yang, M.; Li, Y. Phthalimide Based New Photocatalysts Featured with Highly Selective Photodegradation of Azo-Dyes and Good Photocatalytic Activity in Both Homogeneous and Heterogeneous Systems. J. Photochem. Photobiol. A Chem. 2023, 435, 114346. [Google Scholar] [CrossRef]
- Kumar, A.; Raorane, C.J.; Syed, A.; Bahkali, A.H.; Elgorban, A.M.; Raj, V.; Kim, S.C. Synthesis of TiO2, TiO2/PAni, TiO2/PAni/GO Nanocomposites and Photodegradation of Anionic Dyes Rose Bengal and Thymol Blue in Visible Light. Environ. Res. 2023, 216, 114741. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Luo, M.; Khan, S.; Asghar, H.; Saeed, M.; Khan, S.; Khan, A.; Humayun, M.; Guo, L.; Shi, B. Green synthesis of SrO bridged LaFeO3/g-C3N4 nanocomposites for CO2 conversion and bisphenol A degradation with new insights into mechanism. Environ. Res. 2022, 207, 112650. [Google Scholar] [CrossRef] [PubMed]
- Kotp, Y.H. Fabrication of Cerium Titanate Cellulose Fiber Nanocomposite Materials for the Removal of Methyl Orange and Methylene Blue from Polluted Water by Photocatalytic Degradation. Environ. Sci. Pollut. Res. 2022, 29, 81583–81608. [Google Scholar] [CrossRef]
- Eissa, D.; Hegab, R.H.; Abou-Shady, A.; Kotp, Y.H. Green Synthesis of ZnO, MgO and SiO2 Nanoparticles and Its Effect on Irrigation Water, Soil Properties, and Origanum Majorana Productivity. Sci. Rep. 2022, 12, 5780. [Google Scholar] [CrossRef]
- Deng, J.; Lei, W.; Fu, J.; Jin, H.; Xu, Q.; Wang, S. Enhanced Selective Photooxidation of Toluene to Benzaldehyde over Co3O4-Modified BiOBr/AgBr S-Scheme Heterojunction. Sol. RRL 2022, 6, 2200279. [Google Scholar] [CrossRef]
- Xu, Q.; Xia, Z.; Zhang, J.; Wei, Z.; Guo, Q.; Jin, H.; Tang, H.; Li, S.; Pan, X.; Su, Z.; et al. Recent Advances in Solar-Driven CO2 Reduction over g-C3N4-Based Photocatalysts. Carbon Energy 2023, 5, e205. [Google Scholar]
- Mousavi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on Magnetically Separable Graphitic Carbon Nitride-Based Nanocomposites as Promising Visible-Light-Driven Photocatalysts. J. Mater. Sci. Mater. Electron. 2018, 29, 1719–1747. [Google Scholar] [CrossRef]
- Rao, G.; Zhao, H.; Chen, J.; Deng, W.; Jung, B.; Abdel-Wahab, A.; Batchelor, B.; Li, Y. FeOOH and Fe2O3 Co-Grafted TiO2 Photocatalysts for Bisphenol A Degradation in Water. Catal. Commun. 2017, 97, 125–129. [Google Scholar] [CrossRef]
- Moussa, H.; Chouchene, B.; Gries, T.; Balan, L.; Mozet, K.; Medjahdi, G.; Schneider, R. Growth of ZnO Nanorods on Graphitic Carbon Nitride GCN Sheets for the Preparation of Photocatalysts with High Visible-Light Activity. ChemCatChem 2018, 10, 4987–4997. [Google Scholar] [CrossRef]
- Saeed, M.; Usman, M.; Ibrahim, M.; ul Haq, A.; Khan, I.; Ijaz, H.; Akram, F. Enhanced Photo Catalytic Degradation of Methyl Orange Using p–n Co3O4-TiO2 Hetero-Junction as Catalyst. Int. J. Chem. React. Eng. 2020, 18, 20200004. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Khosa, M.K.K.; Akram, N.; Khalid, S.; Adeel, M.; Nisar, A.; Sherazi, S. Azadirachta indica Leaves Extract Assisted Green Synthesis of Ag-TiO2 for Degradation of Methylene Blue and Rhodamine B Dyes in Aqueous Medium. Green Process. Synth. 2019, 8, 659–666. [Google Scholar] [CrossRef]
- Tan, X.Q.; Ng, S.F.; Mohamed, A.R.; Ong, W.J. Point-to-Face Contact Heterojunctions: Interfacial Design of 0D Nanomaterials on 2D g-C3N4 towards Photocatalytic Energy Applications. Carbon Energy 2022, 4, 665–730. [Google Scholar] [CrossRef]
- Poudel, M.B.; Yu, C.; Kim, H.J. Synthesis of Conducting Bifunctional Polyaniline@mn-TiO2 Nanocomposites for Supercapacitor Electrode and Visible Light Driven Photocatalysis. Catalysts 2020, 10, 546. [Google Scholar] [CrossRef]
- Peng, W.; Li, H.; Liu, Y.; Song, S. A Review on Heavy Metal Ions Adsorption from Water by Graphene Oxide and Its Composites. J. Mol. Liq. 2017, 230, 496–504. [Google Scholar] [CrossRef]
- Hernández-Gordillo, A.; Obregón, S.; Paraguay-Delgado, F.; Rodríguez-González, V. Effective Photoreduction of a Nitroaromatic Environmental Endocrine Disruptor by AgNPs Functionalized on Nanocrystalline TiO2. RSC Adv. 2015, 5, 15194–15197. [Google Scholar] [CrossRef]
- Wang, L.; Du, Y.; Zhu, Q.; Song, J.; Ou, K.; Xie, G.; Yu, Z. Regulating the Alkyl Chain Length of Quaternary Ammonium Salt to Enhance the Inkjet Printing Performance on Cationic Cotton Fabric with Reactive Dye Ink. ACS Appl. Mater. Interfaces 2023, 15, 19750–19760. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, A.; Huang, G.; Yang, M.; Li, D.; Teng, M.; Han, D. Enhanced Efficiency with CDCA Co-Adsorption for Dye-Sensitized Solar Cells Based on Metallosalophen Complexes. Sol. Energy 2020, 209, 316–324. [Google Scholar] [CrossRef]
- Munde, A.V.; Mulik, B.B.; Dighole, R.P.; Sathe, B.R. Cobalt Oxide Nanoparticle-Decorated Reduced Graphene Oxide (Co3O4-RGO): Active and Sustainable Nanoelectrodes for Water Oxidation Reaction. New J. Chem. 2020, 44, 15776–15784. [Google Scholar] [CrossRef]
- Garakani, M.A.; Abouali, S.; Zhang, B.; Takagi, C.A.; Xu, Z.L.; Huang, J.Q.; Huang, J.; Kim, J.K. Cobalt Carbonate/ and Cobalt Oxide/Graphene Aerogel Composite Anodes for High Performance Li-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 18971–18980. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G. Development of Highly Efficient and Durable Reduced Graphene Oxide Decorated with Ag/Co3O4 Nanocomposite towards Photocatalytic C[Sbnd]H Activation. J. Photochem. Photobiol. A Chem. 2020, 394, 112494. [Google Scholar] [CrossRef]
- Shahid, M.M.; Rameshkumar, P.; Numan, A.; Shahabuddin, S.; Alizadeh, M.; Khiew, P.S.; Chiu, W.S. A Cobalt Oxide Nanocubes Interleaved Reduced Graphene Oxide Nanocomposite Modified Glassy Carbon Electrode for Amperometric Detection of Serotonin. Mater. Sci. Eng. C 2019, 100, 388–395. [Google Scholar] [CrossRef]
- Li, D.; Shi, D.; Chen, Z.; Liu, H.; Jia, D.; Guo, Z. Enhanced Rate Performance of Cobalt Oxide/Nitrogen Doped Graphene Composite for Lithium Ion Batteries. RSC Adv. 2013, 3, 5003–5008. [Google Scholar] [CrossRef]
- Nguyen, D.C.T.; Cho, K.Y.; Oh, W.C. Synthesis of Frost-like CuO Combined Graphene-TiO2 by Self-Assembly Method and Its High Photocatalytic Performance. Appl. Surf. Sci. 2017, 412, 252–261. [Google Scholar] [CrossRef]
- Tran, M.H.; Jeong, H.K. Synthesis and Characterization of Silver Nanoparticles Doped Reduced Graphene Oxide. Chem. Phys. Lett. 2015, 630, 80–85. [Google Scholar] [CrossRef]
- Verma, S.; Mungse, H.P.; Kumar, N.; Choudhary, S.; Jain, S.L.; Sain, B.; Khatri, O.P. Graphene Oxide: An Efficient and Reusable Carbocatalyst for Aza-Michael Addition of Amines to Activated Alkenes. Chem. Commun. 2011, 47, 12673–12675. [Google Scholar] [CrossRef]
- Hu, C.; Lu, T.; Chen, F.; Zhang, R. A Brief Review of Graphene–Metal Oxide Composites Synthesis and Applications in Photocatalysis. J. Chin. Adv. Mater. Soc. 2013, 1, 21–39. [Google Scholar] [CrossRef]
- Al Nafiey, A.; Addad, A.; Sieber, B.; Chastanet, G.; Barras, A.; Szunerits, S.; Boukherroub, R. Reduced Graphene Oxide Decorated with Co3O4 Nanoparticles (RGO-Co3O4) Nanocomposite: A Reusable Catalyst for Highly Efficient Reduction of 4-Nitrophenol, and Cr(VI) and Dye Removal from Aqueous Solutions. Chem. Eng. J. 2017, 322, 375–384. [Google Scholar] [CrossRef]
- Jha, A.; Mhamane, D.; Suryawanshi, A.; Joshi, S.M.; Shaikh, P.; Biradar, N.; Ogale, S.; Rode, C.V. Triple Nanocomposites of CoMn2O4, Co3O4 and Reduced Graphene Oxide for Oxidation of Aromatic Alcohols. Catal. Sci. Technol. 2014, 4, 1771–1778. [Google Scholar] [CrossRef]
- Taourati, R.; Khaddor, M.; el Kasmi, A. Stable ZnO Nanocatalysts with High Photocatalytic Activity for Textile Dye Treatment. Nano-Struct. Nano-Objects 2019, 18, 100303. [Google Scholar] [CrossRef]
- Kumbhakar, P.; Pramanik, A.; Biswas, S.; Kole, A.K.; Sarkar, R.; Kumbhakar, P. In-Situ Synthesis of RGO-ZnO Nanocomposite for Demonstration of Sunlight Driven Enhanced Photocatalytic and Self-Cleaning of Organic Dyes and Tea Stains of Cotton Fabrics. J. Hazard. Mater. 2018, 360, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Raizada, P.; Sudhaik, A.; Patial, S.; Hasija, V.; Parwaz Khan, A.A.; Singh, P.; Gautam, S.; Kaur, M.; Nguyen, V.H. Engineering Nanostructures of CuO-Based Photocatalysts for Water Treatment: Current Progress and Future Challenges. Arab. J. Chem. 2020, 13, 8424–8457. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Xi, C.; Cheng, C.; Zou, C.; Zhang, R.; Xie, Y.; Guo, Z.; Tang, C.; Dong, C.; et al. A Hydrogen-Deficient Nickel–Cobalt Double Hydroxide for Photocatalytic Overall Water Splitting. Angew. Chem. 2020, 132, 11607–11612. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Hong, Z.L.; Xu, J.F.; Khalid, N.R.; Elhissi, A.; Ahmed, W. A Facile One-Step Approach to Synthesizing ZnO/Graphene Composites for Enhanced Degradation of Methylene Blue under Visible Light. Appl. Surf. Sci. 2013, 274, 273–281. [Google Scholar] [CrossRef]
- Song, C.; Wang, L.J.; Sun, S.M.; Wu, Y.; Xu, L.J.; Gan, L. Preparation of Visible-Light Photocatalysts of Bi2O3/Bi Embedded in Porous Carbon from Bi-Based Metal Organic Frameworks for Highly Efficient Rhodamine B Removal from Water. Xinxing Tan Cailiao/New Carbon Mater. 2020, 35, 609–618. [Google Scholar] [CrossRef]
- Saeed, M.; Alwadai, N.; ben Farhat, L.; Baig, A.; Nabgan, W.; Iqbal, M. Co3O4-Bi2O3 Heterojunction: An Effective Photocatalyst for Photodegradation of Rhodamine B Dye. Arab. J. Chem. 2022, 15, 103732. [Google Scholar] [CrossRef]
- Zada, N.; Saeed, K.; Khan, I. Decolorization of Rhodamine B Dye by Using Multiwalled Carbon Nanotubes/Co–Ti Oxides Nanocomposite and Co–Ti Oxides as Photocatalysts. Appl. Water Sci. 2020, 10, 40. [Google Scholar] [CrossRef]
- Saroyan, H.; Kyzas, G.Z.; Deliyanni, E.A. Effective Dye Degradation by Graphene Oxide Supported Manganese Oxide. Process. 2019, 7, 40. [Google Scholar] [CrossRef]
- Saeed, M.; Albalawi, K.; Khan, I.; Akram, N.; Abd El-Rahim, I.H.A.; Alhag, S.K.; Ezzat Ahmed, A. Faiza Synthesis of P-n NiO-ZnO Heterojunction for Photodegradation of Crystal Violet Dye. Alex. Eng. J. 2022, 65, 561–574. [Google Scholar] [CrossRef]
- Jiao, T.; Guo, H.; Zhang, Q.; Peng, Q.; Tang, Y.; Yan, X.; Li, B. Reduced Graphene Oxide-Based Silver Nanoparticle-Containing Composite Hydrogel as Highly Efficient Dye Catalysts for Wastewater Treatment. Sci. Rep. 2015, 5, 11873. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cai, J.; Li, Z. Self-Assembly of Semiconductor Nanoparticles/Reduced Graphene Oxide (RGO) Composite Aerogels for Enhanced Photocatalytic Performance and Facile Recycling in Aqueous Photocatalysis. ACS Sustain. Chem. Eng. 2015, 3, 277–282. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, Y.; Nie, W.; Chen, P. MoS2–GO Nanocomposites Synthesized via a Hydrothermal Hydrogel Method for Solar Light Photocatalytic Degradation of Methylene Blue. Appl. Surf. Sci. 2015, 357, 1606–1612. [Google Scholar] [CrossRef]
- Choi, J.; Reddy, D.A.; Islam, M.J.; Ma, R.; Kim, T.K. Self-Assembly of CeO2 Nanostructures/Reduced Graphene Oxide Composite Aerogels for Efficient Photocatalytic Degradation of Organic Pollutants in Water. J. Alloys Compd. 2016, 688, 527–536. [Google Scholar] [CrossRef]
- Cai, R.; Wu, J.G.; Sun, L.; Liu, Y.J.; Fang, T.; Zhu, S.; Li, S.Y.; Wang, Y.; Guo, L.F.; Zhao, C.E.; et al. 3D Graphene/ZnO Composite with Enhanced Photocatalytic Activity. Mater. Des. 2016, 90, 839–844. [Google Scholar] [CrossRef]
- Cai, J.; Liu, W.; Li, Z. One-Pot Self-Assembly of Cu2O/RGO Composite Aerogel for Aqueous Photocatalysis. Appl. Surf. Sci. 2015, 358, 146–151. [Google Scholar] [CrossRef]
- Qiu, B.; Xing, M.; Zhang, J. Mesoporous TiO2 Nanocrystals Grown in Situ on Graphene Aerogels for High Photocatalysis and Lithium-Ion Batteries. J. Am. Chem. Soc. 2014, 136, 5852–5855. [Google Scholar] [CrossRef]
- Qiu, B.; Deng, Y.; Du, M.; Xing, M.; Zhang, J. Ultradispersed Cobalt Ferrite Nanoparticles Assembled in Graphene Aerogel for Continuous Photo-Fenton Reaction and Enhanced Lithium Storage Performance. Sci. Rep. 2016, 6, 29099. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, N.; Lai, J.; Liu, R.; Liu, X. Tannic Acid Functionalized Graphene Hydrogel for Entrapping Gold Nanoparticles with High Catalytic Performance toward Dye Reduction. J. Hazard. Mater. 2015, 300, 615–623. [Google Scholar] [CrossRef]



| Photocatalyst | MB | MO | ||
|---|---|---|---|---|
| k | R2 | k | R2 | |
| rGO | 0.0094 | 0.995 | 0.0085 | 0.995 |
| Co3O4 | 0.0152 | 0.992 | 0.0128 | 0.994 |
| Co3O4-rGO | 0.0323 | 0.983 | 0.0303 | 0.985 |
| Concentration (mg/L) | MB | MO | ||
|---|---|---|---|---|
| k | R2 | k | R2 | |
| 100 | 0.0432 | 0.991 | 0.0363 | 0.991 |
| 200 | 0.0323 | 0.983 | 0.0303 | 0.985 |
| 300 | 0.0206 | 0.987 | 0.0151 | 0.988 |
| No | Graphene-Catalyst | Dye | Activity |
|---|---|---|---|
| 1 | Ag [51] | MB RhB | Almost complete degradation in 70 min using 10 mg/L of dye solution |
| 2 | BiOBr [52] | RhB | Almost complete degradation in 5 h using 20 mg/L dye solution |
| 3 | MoS2 [53] | MB | A 99% degradation in 60 min using 10 mg/L dye solution |
| 4 | CeO2 [54] | RhB | An 85% degradation in 2 h using 10 mg/L dye solution |
| 5 | ZnO [55] | MO | A 92% degradation in 3 h using 5 mM dye solution |
| 6 | Cu2O [56] | MO | A 70% degradation in 5 h using 5 mg/L dye solution |
| 7 | TiO2 [57] | MO | A 90% degradation in 5 h using 10 mg/L dye solution |
| 8 | CoFe2O4 [58] | MO | A 78% degradation in 30 min using 10 mg/L dye solution |
| 9 | Au [59] | MB (10 mg/L) | Almost complete degradation in 10 min using 10 mg/L dye solution |
| 10 | Co3O4 (this work) | MB MO | Complete degradation in 60 min using 100 mg/L dye solution |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, M.; Albadran, F.H.; Zahoor, A.F.; Nisar, A.; Al-Mutairi, A.A.; Al-Hussain, S.A.; Irfan, A.; Zaki, M.E.A. Co3O4-rGO—Synthesis, Characterization, and Evaluation of Photocatalytic Activities. Catalysts 2024, 14, 96. https://doi.org/10.3390/catal14020096
Saeed M, Albadran FH, Zahoor AF, Nisar A, Al-Mutairi AA, Al-Hussain SA, Irfan A, Zaki MEA. Co3O4-rGO—Synthesis, Characterization, and Evaluation of Photocatalytic Activities. Catalysts. 2024; 14(2):96. https://doi.org/10.3390/catal14020096
Chicago/Turabian StyleSaeed, Muhammad, Firas H. Albadran, Ameer Fawad Zahoor, Asif Nisar, Aamal A. Al-Mutairi, Sami A. Al-Hussain, Ali Irfan, and Magdi E. A. Zaki. 2024. "Co3O4-rGO—Synthesis, Characterization, and Evaluation of Photocatalytic Activities" Catalysts 14, no. 2: 96. https://doi.org/10.3390/catal14020096









