Study on the Inhibition of Hydrogen Evolution Reaction by Electrocatalytic Reduction of Carbon Dioxide Using Elsholtzia Harchowensis Biochar
Abstract
:1. Introduction
2. Results and Discussion
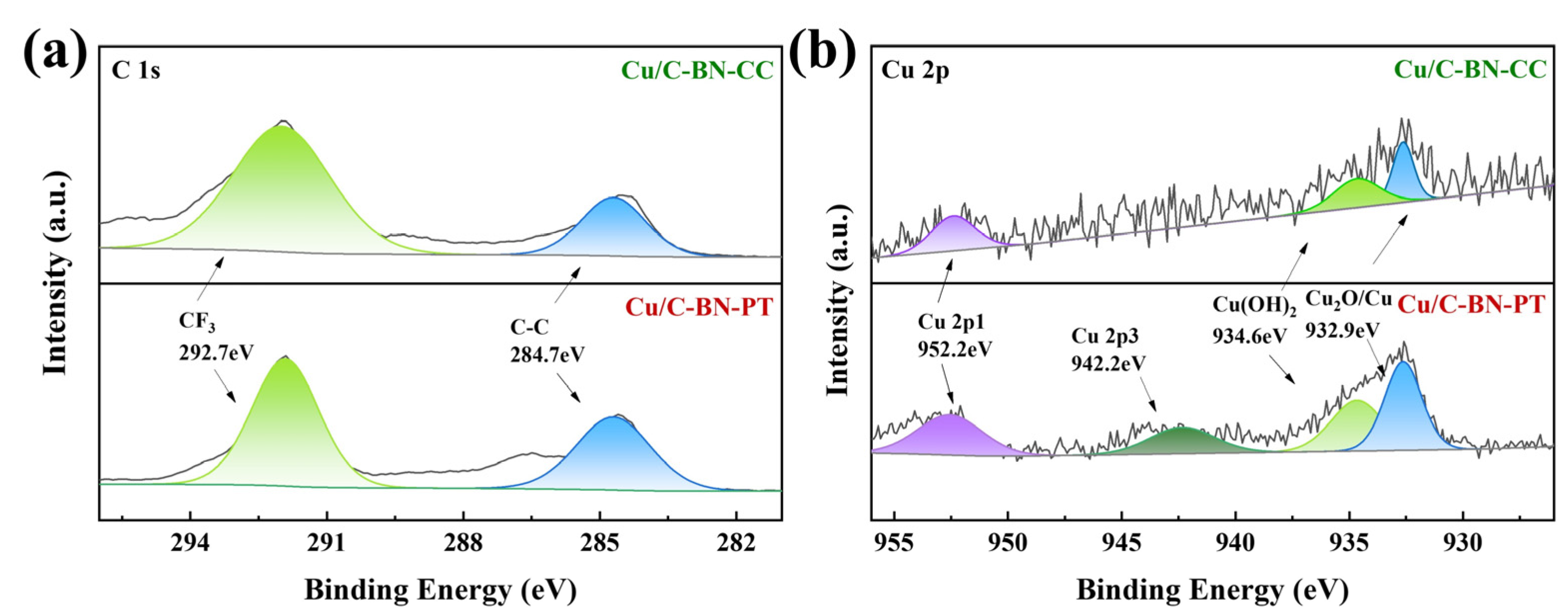
2.1. Catalyst Characterization
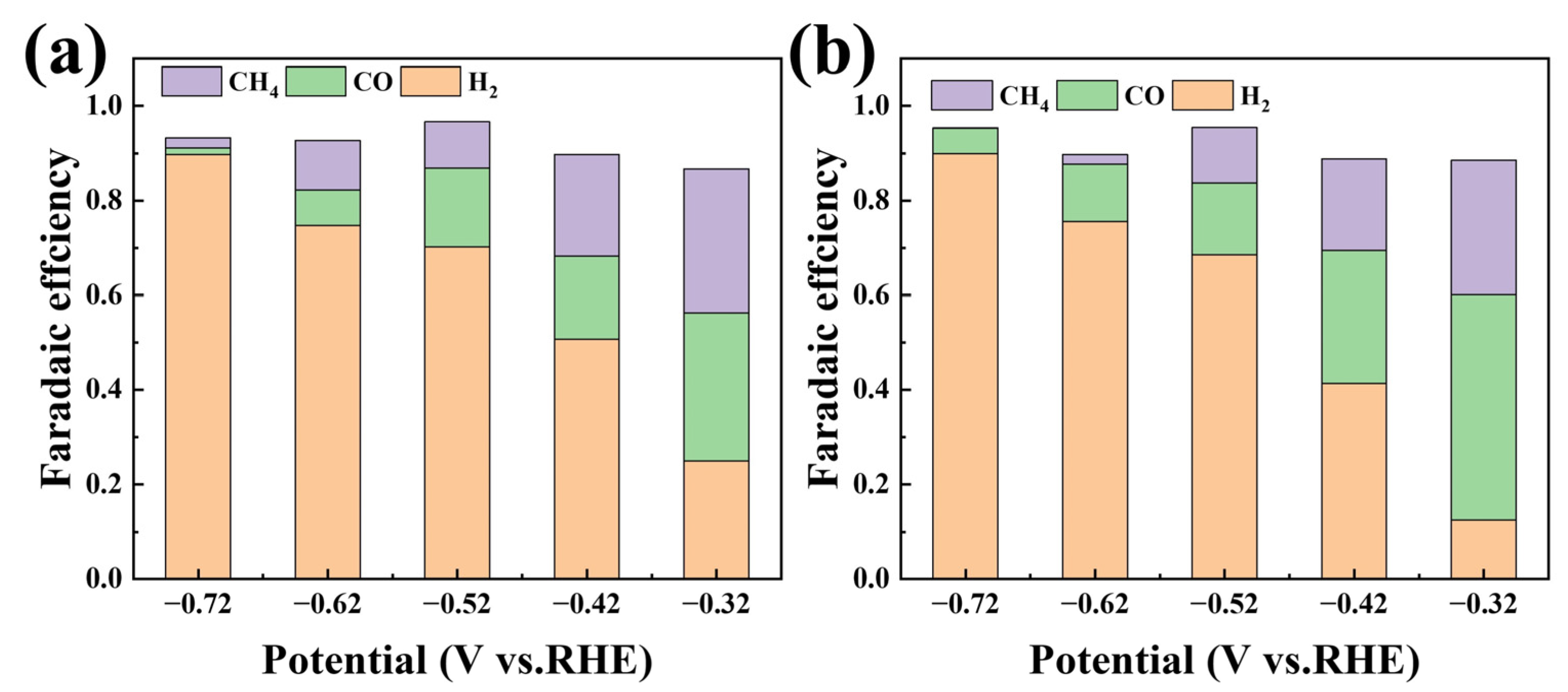
2.2. Impact of Hydrophobicity Enhancement on Electrochemical Performance
2.3. The Influence of Organic Solvent Electrolytes on Electrochemical Performance
3. Materials and Methods
3.1. Experimental Reagents and Materials
3.2. Preparation of Catalyst
3.2.1. Boron- and Nitrogen-Doped Copper Modified Biochar
3.2.2. Hydrophobic Materials
3.2.3. Electrodeposited Materials
3.2.4. Working Electrodes
3.3. Catalyst Characterization and Testing
3.3.1. Material Characterization
3.3.2. Electrochemical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, L.; Yang, X.; He, Z. Growth response and phytoextraction of copper at different levels in soils by Elsholtzia splendens. Chemosphere 2004, 55, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhao, F.-J.; Luo, Y.-M.; McGrath, S.P.; Zhang, H. Copper uptake by Elsholtzia splendens and Silene vulgaris and assessment of copper phytoavailability in contaminated soils. Environ. Pollut. 2004, 128, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wilke, B.-M.; Huang, C. The uptake of copper by plants dominantly growing on copper mining spoils along the Yangtze River, the People’s Republic of China. Plant Soil 1999, 209, 225–232. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, Z.T. Differences in accumulation and physiological response to copper stress in three populations of Elsholtzia haichowensis S. Water Air Soil Pollut. 2005, 168, 5–16. [Google Scholar] [CrossRef]
- Li, J.-T.; Gurajala, H.K.; Wu, L.-H.; van der Ent, A.; Qiu, R.-L.; Baker, A.J.M.; Tang, Y.-T.; Yang, X.-E.; Shu, W.-S. Hyperaccumulator Plants from China: A Synthesis of the Current State of Knowledge. Environ. Sci. Technol. 2018, 52, 11980–11994. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tran, K.-Q. A review on disposal and utilization of phytoremediation plants containing heavy metals. Ecotoxicol. Environ. Saf. 2021, 226, 112821. [Google Scholar] [CrossRef]
- Sas-Nowosielska, A.; Kucharski, R.; Małkowski, E.; Pogrzeba, M.; Kuperberg, J.; Kryński, K. Phytoextraction crop disposal—An unsolved problem. Environ. Pollut. 2004, 128, 373–379. [Google Scholar] [CrossRef]
- Cui, P.; Liu, C.; Su, X.; Yang, Q.; Ge, L.; Huang, M.; Dang, F.; Wu, T.; Wang, Y. Atomically Dispersed Manganese on Biochar Derived from a Hyperaccumulator for Photocatalysis in Organic Pollution Remediation. Environ. Sci. Technol. 2022, 56, 8034–8042. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.; Ma, Y.; Zhou, J.; Wang, Y.; Lu, P.; Yin, J.; Ye, R.; Zhu, Z.; Fan, Z. Recent Progresses in Electrochemical Carbon Dioxide Reduction on Copper-Based Catalysts toward Multicarbon Products. Adv. Funct. Mater. 2021, 31, 2102151. [Google Scholar] [CrossRef]
- Liotta, L.F.; Wu, H. CO2 Capture, Utilization and Storage: Catalysts Design. Catalysts 2024, 14, 80. [Google Scholar] [CrossRef]
- Lin, R.; Guo, J.; Li, X.; Patel, P.; Seifitokaldani, A. Electrochemical Reactors for CO2 Conversion. Catalysts 2020, 10, 473. [Google Scholar] [CrossRef]
- Yang, B.; Chen, L.; Xue, S.; Sun, H.; Feng, K.; Chen, Y.; Zhang, X.; Xiao, L.; Qin, Y.; Zhong, J.; et al. Electrocatalytic CO2 reduction to alcohols by modulating the molecular geometry and Cu coordination in bicentric copper complexes. Nat. Commun. 2022, 13, 5122. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Qian, K.; Yu, J.; Sun, M.; Cao, S.; Gao, J.; Yu, R.; Fang, L.; Yao, Y.; Lu, X.; et al. MOF-Transformed In2O3-x@C Nanocorn Electrocatalyst for Efficient CO2 Reduction to HCOOH. Nano-Micro Lett. 2022, 14, 167. [Google Scholar] [CrossRef]
- Wang, H.; Jia, J.; Song, P.; Wang, Q.; Li, D.; Min, S.; Qian, C.; Wang, L.; Li, Y.F.; Ma, C.; et al. Efficient Electrocatalytic Reduction of CO2 by Nitrogen-Doped Nanoporous Carbon/Carbon Nanotube Membranes: A Step Towards the Electrochemical CO2 Refinery. Angew. Chem. Int. Ed. 2017, 56, 7847–7852. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry; Vayenas, C.G., White, R.E., Gamboa-Aldeco, M.E., Eds.; Springer: New York, NY, USA, 2008; pp. 89–189. [Google Scholar]
- Gu, Z.; Shen, H.; Shang, L.; Lv, X.; Qian, L.; Zheng, G. Nanostructured Copper-Based Electrocatalysts for CO2 Reduction. Small Methods 2018, 2, 1800121. [Google Scholar] [CrossRef]
- Wu, B.; Chen, J.; Qian, L. Recent Advances in Heterogeneous Electroreduction of CO2 on Copper-Based Catalysts. Catalysts 2022, 12, 860. [Google Scholar] [CrossRef]
- Hong, X.; Zhu, H.; Du, D.; Zhang, Q.; Li, Y. Research Progress of Copper-Based Bimetallic Electrocatalytic Reduction of CO2. Catalysts 2023, 13, 376. [Google Scholar] [CrossRef]
- Chen, S.; Liu, W.; Mei, Z.; Li, H.; Zhao, W.; Zhao, J.; Tao, H. The synthesis of copper-modified biochar from Elsholtzia Harchowensis and its electrochemical activity towards the reduction of carbon dioxide. Front. Chem. 2023, 11, 1238424. [Google Scholar] [CrossRef]
- Liu, W.; Chen, S.; Mei, Z.; Li, L.; Li, H.; Zhao, W.; Tao, H. Boron and nitrogen doping modulating the coordination environment of copper in biochar for reformative electrocatalytic CO2 reduction. Surf. Interfaces 2024, 44, 103608. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Savéant, J.-M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2012, 42, 2423–2436. [Google Scholar] [CrossRef]
- Kumar, B.; Llorente, M.; Froehlich, J.; Dang, T.; Sathrum, A.; Kubiak, C.P. Photochemical and Photoelectrochemical Reduction of CO2. Annu. Rev. Phys. Chem. 2012, 63, 541–569. [Google Scholar] [CrossRef] [PubMed]
- Burgers, I.; Pérez-Gallent, E.; Goetheer, E.; Kortlever, R. Electrochemical CO2 Reduction on Copper in Propylene Carbonate: Influence of Water Content and Temperature on the Product Distribution. Energy Technol. 2023, 11, 2201465. [Google Scholar] [CrossRef]
- Fang, M.; Wang, M.; Wang, Z.; Zhang, Z.; Zhou, H.; Dai, L.; Jiang, L. Hydrophobic, Ultrastable Cuδ+ for Robust CO2 Electroreduction to C2 Products at Ampere-Current Levels. J. Am. Chem. Soc. 2023, 145, 11323–11332. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Wei, L.; Li, H.; Yang, B.; Liu, K.; Fu, J.; Li, H.; Liu, H.; Hu, J.; Lu, Y.-R.; et al. Enhancing CO2 reduction by suppressing hydrogen evolution with polytetrafluoroethylene protected copper nanoneedles. J. Mater. Chem. A 2020, 8, 15936–15941. [Google Scholar] [CrossRef]
- Blom, M.J.W.; van Swaaij, W.P.M.; Mul, G.; Kersten, S.R.A. Increased hydrogen partial pressure suppresses and reverses hydrogen evolution during Pd catalysed electrolysis of CO2. Sustain. Energy Fuels 2020, 4, 4459–4463. [Google Scholar] [CrossRef]
- Valenti, M.; Prasad, N.P.; Kas, R.; Bohra, D.; Ma, M.; Balasubramanian, V.; Chu, L.; Gimenez, S.; Bisquert, J.; Dam, B.; et al. Suppressing H2 Evolution and Promoting Selective CO2 Electroreduction to CO at Low Overpotentials by Alloying Au with Pd. ACS Catal. 2019, 9, 3527–3536. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, F.; Li, J.; Zeng, G.; Ye, Y.; Larson, D.M.; Yano, J.; Crumlin, E.J.; Ager, J.W.; Wang, L.-W.; et al. Investigation and mitigation of degradation mechanisms in Cu2O photoelectrodes for CO2 reduction to ethylene. Nat. Energy 2021, 6, 1124–1132. [Google Scholar] [CrossRef]
- Li, X.; Hu, Y.; Zhang, C.; Xiao, C.; Cheng, J.; Chen, Y. Electro-activating of peroxymonosulfate via boron and sulfur co-doped macroporous carbon nanofibers cathode for high-efficient degradation of levofloxacin. J. Hazard. Mater. 2023, 442, 130016. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Chen, S.; Mei, Z.; Li, L.; Tao, H. Study on the Inhibition of Hydrogen Evolution Reaction by Electrocatalytic Reduction of Carbon Dioxide Using Elsholtzia Harchowensis Biochar. Catalysts 2024, 14, 172. https://doi.org/10.3390/catal14030172
Liu W, Chen S, Mei Z, Li L, Tao H. Study on the Inhibition of Hydrogen Evolution Reaction by Electrocatalytic Reduction of Carbon Dioxide Using Elsholtzia Harchowensis Biochar. Catalysts. 2024; 14(3):172. https://doi.org/10.3390/catal14030172
Chicago/Turabian StyleLiu, Wei, Shiqi Chen, Ziwei Mei, Liang Li, and Hong Tao. 2024. "Study on the Inhibition of Hydrogen Evolution Reaction by Electrocatalytic Reduction of Carbon Dioxide Using Elsholtzia Harchowensis Biochar" Catalysts 14, no. 3: 172. https://doi.org/10.3390/catal14030172




