Extended Hydrogen-Bonded Molybdenum Arrays Derived from Carboxylic Acids and Dianilines: ROP Capability of the Complexes and Parent Acids and Dianilines
Abstract
:1. Introduction
2. Results and Discussion
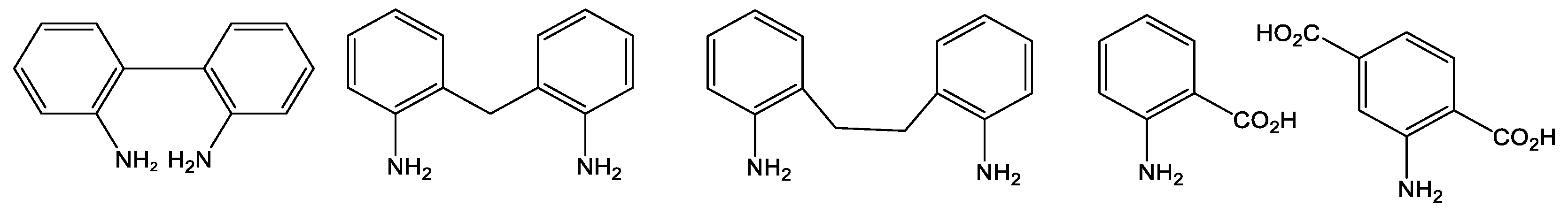
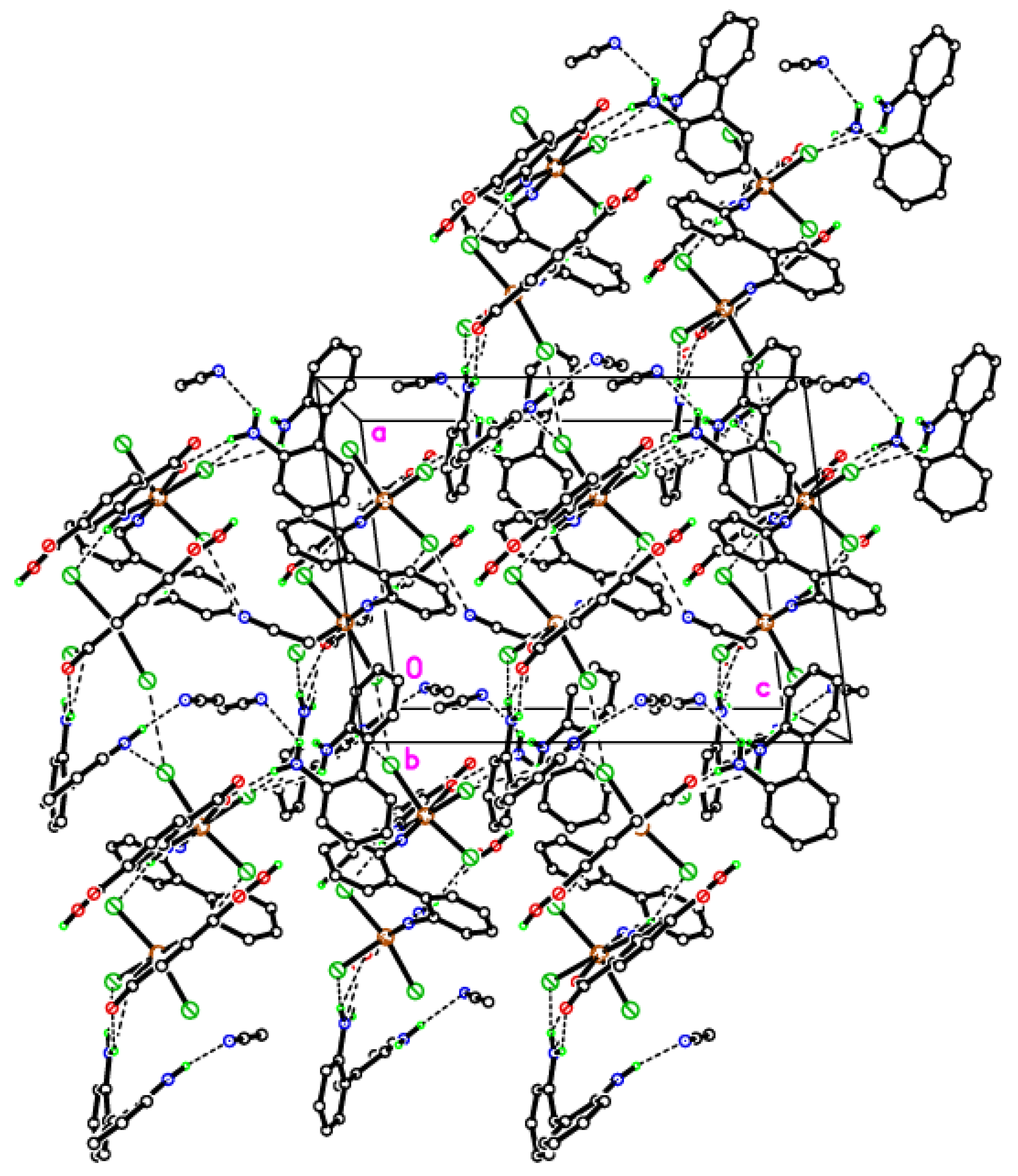
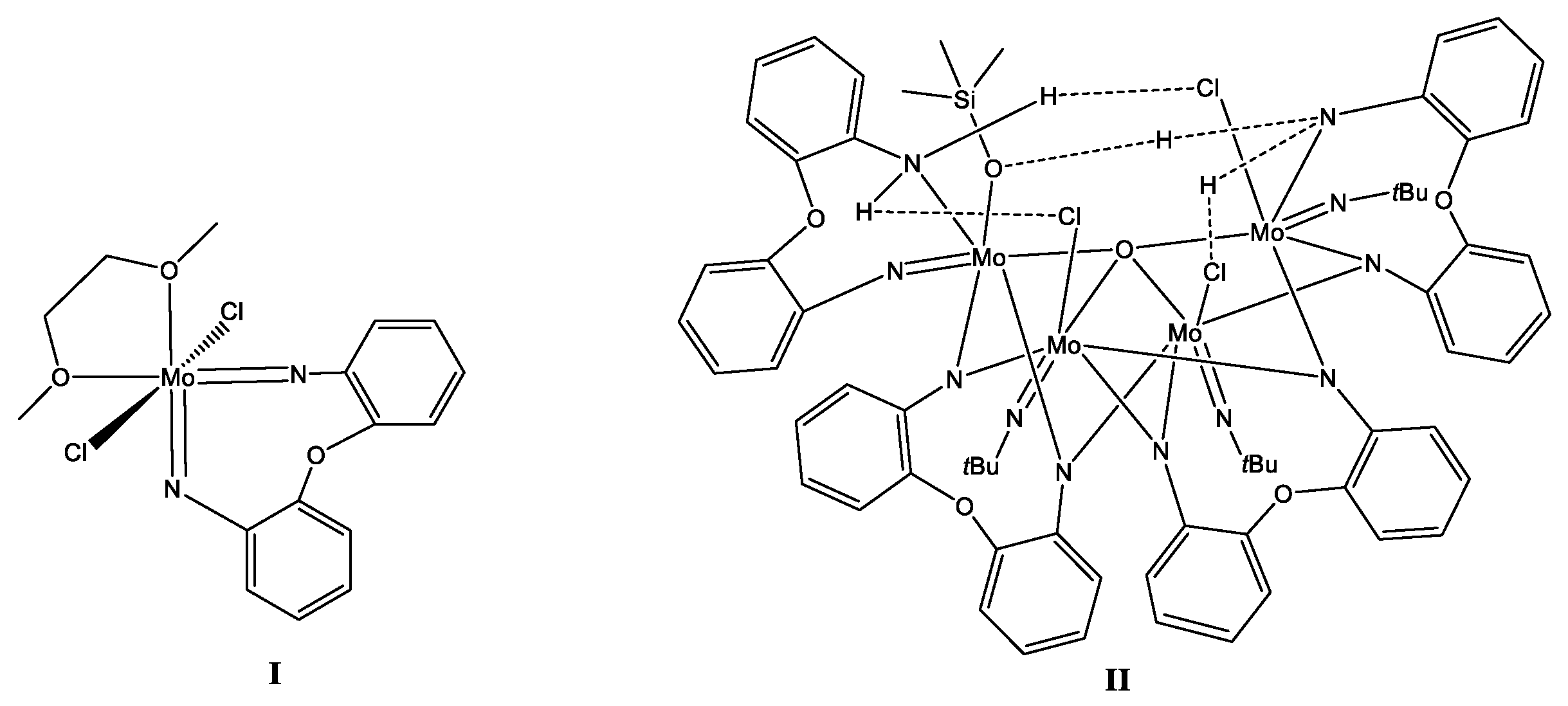
2.1. Synthesis and Characterization of Hydrogen-Bonded 2,2′-Diaminobiphenyl-Derived Molybdenum Complex
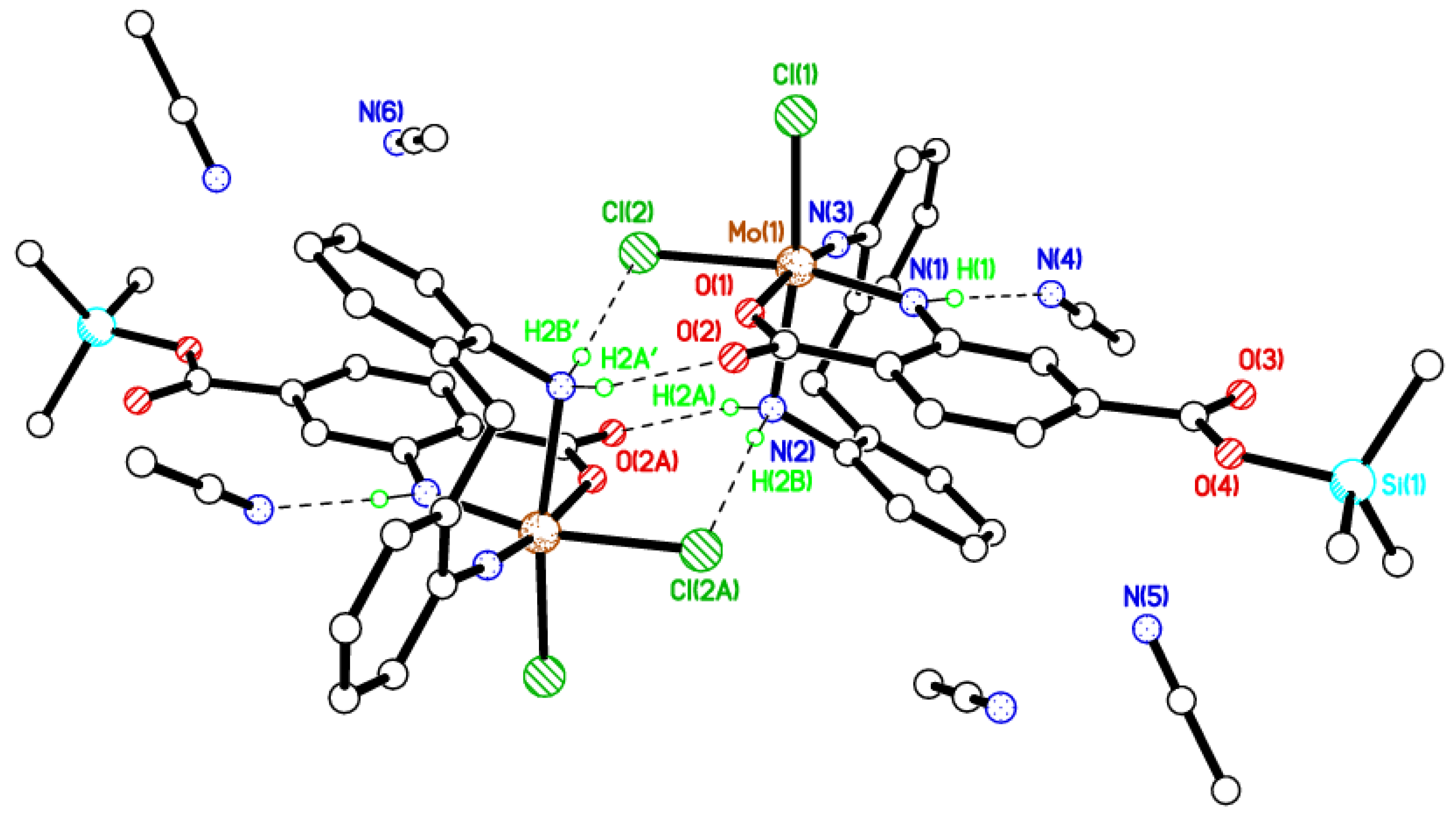
2.2. Synthesis and Characterization of the Methylene-Bridged Dianiline-Derived Molybdenum Complex
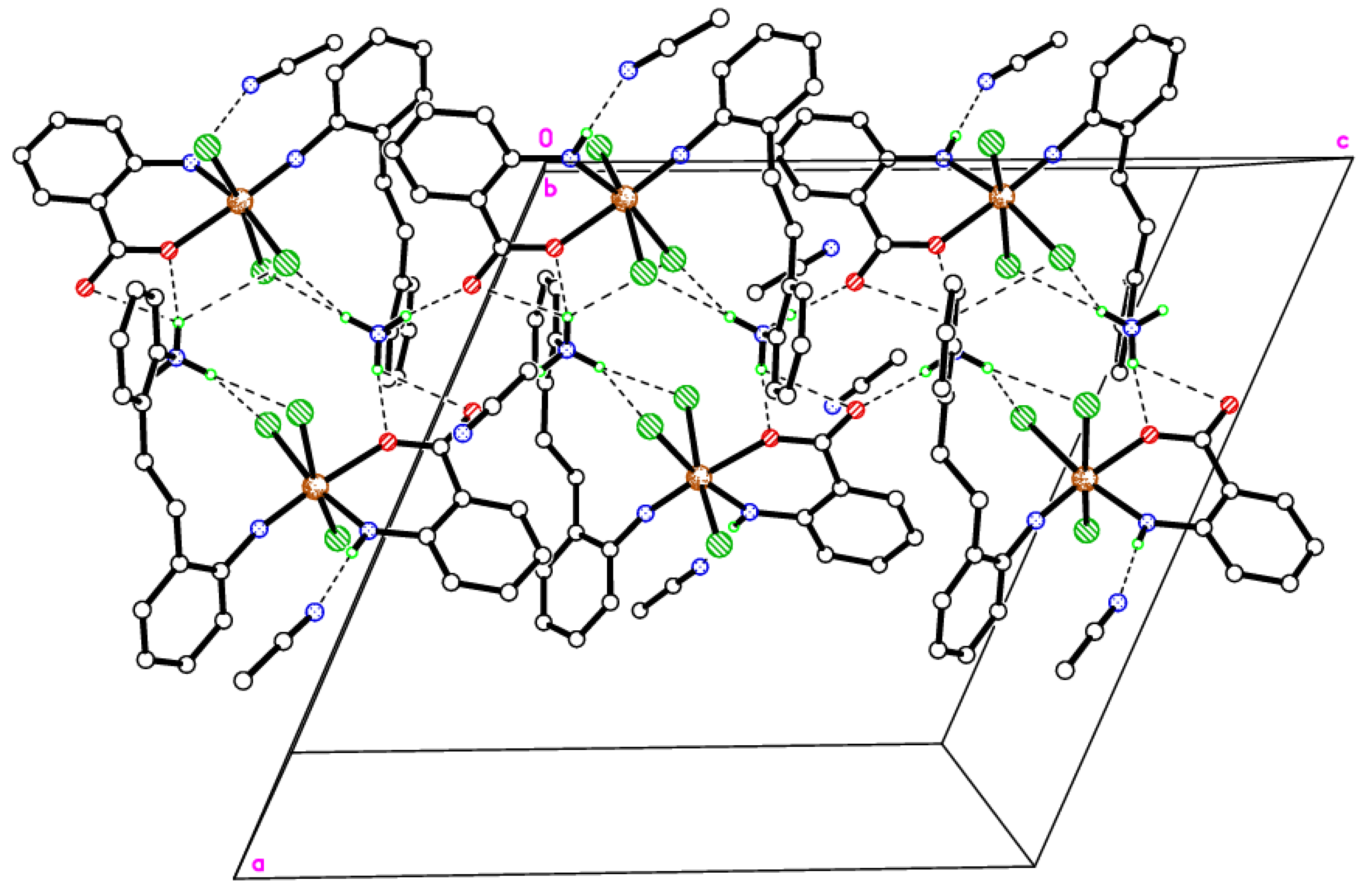
2.3. Synthesis and Characterization of the Ethylene-Bridged Dianiline-Derived Molybdenum Complex
3. Ring Opening Polymerization (ROP)
3.1. Ring Opening Polymerization of ε-Caprolactone (ε-CL)
3.2. Ring Opening Polymerization of δ-Valerolactone (δ-VL)
4. Materials and Methods
4.1. Synthesis of [(MoCl3[2,2′-N(C6H4)]2}{HNC6H3-1-(CO2),4-(CO2H)]·2[2,2′-NH2(C6H4)]2·3.5MeCN (1·3.5MeCN)
4.2. Synthesis of [MoCl2(O2CC6H3NHCO2SiMe3)(NC6H4CH2C6H4NH2)]·3(C2H3N) (2·3MeCN)
4.3. Synthesis of [MoCl3{1,2-(NH)(CO2)C6H4}{NC6H4CH2CH2C6H4NH3}]·MeCN (3·MeCN)
4.4. ROP of ε-Caprolactone (ε-CL)
4.5. X-ray Crystallography
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schrock, R.R.; Hoveyda, A.H. Molybdenum and Tungsten Imido Alkylidene Complexes as Efficient Olefin-Metathesis Catalysts. Angew. Chem. Int. Ed. 2003, 42, 4592–4633. [Google Scholar] [CrossRef] [PubMed]
- Gibson, V.C.; Redshaw, C.; Clegg, W.; Elsegood, M.R.J. Synthesis and characterisation of molybdenum complexes bearing highly functionalised imido substituents. Dalton Trans. 1997, 3207–3212. [Google Scholar] [CrossRef]
- Redshaw, C.; Gibson, V.C.; Clegg, W.; Edwards, A.J.; Miles, B. Pentamethylcyclopentadienyl tungsten complexes containing imido, hydrazido and amino acid derived N-O chelate ligands. Dalton Trans. 1997, 3343–3347. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Clegg, W.; Elsegood, M.R.J. Carboxylate versus imidobenzoate bonding for anthranilic acid derivatives. Crystals structures of [W(η-Cp*)Cl3(η2-O2CC6H4NH2-2)] and [ReCl(OEt)(PPh3)2(NC6H4CO2)]. Inorg. Chem. Commun. 2001, 4, 95–99. [Google Scholar] [CrossRef]
- Humphrey, S.M.; Redshaw, C.; Holmes, K.E.; Elsegood, M.R.J. Acid/amide bonding for anthranilic acid derivatives: Crystal structures of W(X)Cl3(HO2CC6H4NH-2) (X = O, NPh). Inorg. Chim. Acta 2005, 358, 222–226. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Clegg, W.; Elsegood, M.R.J.; Siemeling, U.; Turk, T. Synthesis and X-ray crystal structures of hydridotris(3,5-dimethylpyrazolyl)borate (Tp’) molybdenum(VI) bis(imido) complexes. Polyhedron 2004, 23, 189–194. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Clegg, W.; Elsegood, M.R.J. Supramolecular metallocalixarene chemistry: Linking metallocalixarenes through imido bridges. Chem. Commun. 1998, 1969–1970. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Clegg, W.; Elsegood, M.R.J.; Siemeling, U.; Türk, T. Bridged bis(imido)molybdenum complexes: Isolobal analogues of ansa-zirconocenes and bizirconocenes. J. Chem. Soc. Dalton Trans. 1996, 4513–4515. [Google Scholar] [CrossRef]
- Siemeling, U.; Tűrk, T.; Schoeller, W.W.; Redshaw, C.; Gibson, V.C. Benefits of the chelate effect: Preparation of an unsymmetrical ansa-bis(imido)molybdenum complex containing a seven-membered chelate ring. Inorg. Chem. 1998, 37, 4738–4739. [Google Scholar] [CrossRef]
- Redshaw, C.; Gibson, V.C.; Elsegood, M.R.J.; Clegg, W. New coordination modes at molybdenum for 2-diphenylphosphinoaniline derived ligands. Chem. Commun. 2007, 1951–1953. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, Q.-K.; Redshaw, C.; Elsegood, M.R.J. Molybdenum complexes derived from the oxydianiline [(2-NH2C6H4)2O]: Synthesis, characterization and ε-caprolactone ROP capability. Dalton Trans. 2015, 44, 13133–13140. [Google Scholar] [CrossRef]
- Kretzschmar, E.A.; Kipke, J.; Sundermeyer, J. The first chiral diimido chelate complexes of molybdenum and tungsten: Transition metal diimido complexes on the way to asymmetric catalysis. Chem. Commun. 1999, 2381–2382. [Google Scholar] [CrossRef]
- Singh, N.; Ogunseitan, O.A.; Wong, M.H.; Tang, Y. Sustainable materials alternative to petrochemical plastics pollution: A review analysis. Sustain. Horiz. 2022, 2, 100016. [Google Scholar] [CrossRef]
- Keefe, B.J.; Hillmeyer, M.A.; Tolman, W.B. Polymerization of lactide and related cyclic esters by discrete metal complexes. J. Chem. Soc. Dalton Trans. 2001, 2215–2224. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef] [PubMed]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M. Stereocontrolled ring-opening polymerization of cyclic esters: Synthesis of new polyester microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Arbaoui, A.; Redshaw, C. Metal catalysts for ε-caprolactone polymerisation. Polym. Chem. 2010, 1, 801–826. [Google Scholar] [CrossRef]
- Dove, A.P. Organic Catalysis for Ring Opening Polymerization. ACS Macro Lett. 2012, 1, 1409–1412. [Google Scholar] [CrossRef]
- Sarazin, Y.; Carpentier, J.-F. Discrete Cationic Complexes for Ring-Opening Polymerization Catalysis of Cyclic Esters and Epoxides. Chem. Rev. 2015, 115, 3564–3614. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, D.; Zhang, W.; Solan, G.A.; Ma, Y.; Sun, W.-H. Recent progress in the application of group 1, 2 & 13 metal complexes as catalysts for the ring opening polymerization of cyclic esters. Inorg. Chem. Front. 2019, 6, 2619–2652. [Google Scholar]
- Nifant’ev, I.; Ivchenko, P. Coordination Ring-Opening Polymerization of Cyclic Esters: A Critical Overview of DFT Modeling and Visualization of the Reaction Mechanisms. Molecules 2019, 24, 4117. [Google Scholar] [CrossRef]
- Tazekas, E.; Lowy, P.A.; Rahman, M.A.; Lykkeberg, A.; Zhou, Y.; Chambenahalli, R.; Garden, J.A. Main group metal polymerisation catalysts. Chem. Soc. Rev 2022, 51, 8793–8814. [Google Scholar] [CrossRef]
- Naz, F.; Abdur, R.M.; Mumtaz, F.; Elkadi, M.; Verpoort, F. Advances in cyclic ester ring-opening polymerization using heterogeneous catalysts. Appl. Organomet. Chem. 2023, 37, e7296. [Google Scholar] [CrossRef]
- Wang, Y.; Wang. X.; Zhang, W.; Sun, W.-H. Progress of Ring Opening Polymerization of Cyclic Esters Catalyzed by Iron Compounds. Organometallics 2023, 42, 1680–1692. [Google Scholar] [CrossRef]
- Báez, J.E.; Martῐnez-Richa, A. Synthesis and characterization of poly(ε-caprolactone) and copolyesters by catalysis with molybdenum compounds: Polymers with acid-functional asymmetric telechelic architecture. Polymer 2005, 46, 12118. [Google Scholar] [CrossRef]
- Maruta, Y.; Abiko, A. Bis(salicylaldehydato)dioxomolybdenum complexes: Catalysis for ring-opening polymerization. Polym. Bull. 2014, 71, 1433. [Google Scholar] [CrossRef]
- Al-Khafaji, Y.; Prior, T.J.; Elsegood, M.R.J.; Redshaw, C. Molybdenum(VI) imido complexes derived from chelating phenols: Synthesis, characterization and ɛ-caprolactone ROP capability. Catalysts 2015, 5, 1928–1947. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Y.; Prior, T.J.; Elsegood, M.R.J.; Wang, K.; Xing, T.; Redshaw, C. Mono-oxo molybdenum(VI) and tungsten(VI) complexes bearing chelating aryloxides: Synthesis, structure and ring opening polymerization of cyclic esters. Dalton Trans. 2019, 48, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Elsegood, M.R.J.; Dale, S.H.; Redshaw, C. Pentamethylcyclopentadienyl molybdenum(V) complexes derived from iodoanilines: Synthesis, structure, and ROP of ε-caprolactone. Catalysts 2021, 11, 1554. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, H.; Li, Z.; Wei, F.; Gao, Y.; Xu, J.; Wang, H.; Liu, J.; Guo, T.; Guo, K. Tuning the H-bond donicity boosts carboxylic acid efficiency in ring-opening polymerization. Eur. Polym. J. 2019, 112, 799–808. [Google Scholar] [CrossRef]
- Jehanno, C.; Mezzasalma, L.; Sardon, H.; Ruipérez, F.; Coulembier, O.; Taton, D. Benzoic Acid as an Efficient Organocatalyst for the Statistical Ring Opening Copolymerization of ε-Caprolactone and L-Lactide: A Computational Investigation. Macromolecules 2019, 52, 9238–9247. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C.; Li, Z.; Wu, W.; Zhi, X.; Zhang, Q.; Wu, H.; Wang, X.; Cui, S.; Guo, K. A squaramide and tertiary amine: An excellent hydrogen-bonding pair organocatalyst for living polymerization. Polym. Chem. 2015, 6, 3754–3757. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J.; Li, Z.; Xu, S.; Wang, X.; Wang, H.; Guo, T.; Gao, Y.; Zhang, L.; Guo, K. Squaramide and amine binary H-bond organocatalysis in polymerizations of cyclic carbonates, lactones, and lactides. Polym. Chem. 2017, 8, 7054–7068. [Google Scholar] [CrossRef]
- Du, F.; Zheng, Y.; Yuan, W.; Shan, G.; Bao, Y.; Jie, S.; Pan, P. Solvent-Free Ring-Opening Polymerization of Lactones with Hydrogen Bonding Bisurea Catalyst. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 90–100. [Google Scholar] [CrossRef]
- Jain, I.; Malik, P. Advances in urea and thiourea catalyzed ring opening polymerization: A brief overview. Eur. Polym. J. 2020, 133, 109791. [Google Scholar] [CrossRef]
- Printz, G.; Ryzhakov, D.; Gourlaouen, C.; Jacques, B.; Messaoudi, S.; Dumas, F.; Le Bideau, F.; Dagorne, S. First Use of Thiosquaramides as Polymerization Catalysts: Controlled ROP of Lactide Implicating Key Secondary Interactions for Optimal Performance. ChemCatChem 2024, 16, e202301207. [Google Scholar] [CrossRef]
- Kazakov, O.I.; Datta, P.P.; Isajani, M.; Kiesewetter, E.T.; Kiesewetter, M.K. Cooperative Hydrogen-Bond Pairing in Organocatalytic Ring Opening Polymerization. Macromolecules 2014, 47, 7463–7468. [Google Scholar] [CrossRef] [PubMed]
- Spink, S.S.; Kazakov, O.I.; Kiesewetter, E.T.; Kiesewetter, M.K. Rate Accelerated Organocatalytic Ring-Opening Polymerization of L-Lactide via the Application of a Bis(thiourea) H-bond Donating Cocatalyst. Macromolecules 2015, 48, 6127–6131. [Google Scholar] [CrossRef] [PubMed]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Cryst. 1990, B46, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Basenko, S.V.; Soldatenko, A.S.; Vashchenko, A.V.; Smimov, V.I. Silacyclophanones 3*. Cyclic organosilicon esters of ortho-phthalic acids. Chem. Heterocycl. Compd. 2019, 55, 1139. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Dorn, H.; Gindl, J.; Noltemeye, M.; Schmidt, H.-G.; Roesky, H.W. Synthesis and structural characterization of sulfonates, phosphinates and carboxylates of organometallic Group 4 metal fluorides. J. Organomet. Chem. 1998, 550, 1–6. [Google Scholar] [CrossRef]
- Basenko, S.V.; Soldatenko, A.S.; Vashchenko, O.V.; Smirnov, V.I. Silacyclophanones 2*. Cyclic organosilicon esters of terephthalic acid. Chem. Heterocycl. Compd. 2018, 54, 826–828. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wang, F.-C.; Ko, B.-T.; Yu, T.-L.; Lin, C.-C. Ring-Opening Polymerization of ε-Caprolactone and L-Lactide Using Aluminum Thiolates as Initiator. Macromolecules 2001, 34, 356–361. [Google Scholar] [CrossRef]
- Save, M.; Schappacher, M.; Soum, A. Controlled Ring-Opening Polymerization of Lactones and Lactides Initiated by Lanthanum Isopropoxide, 1. General Aspects and Kinetics. Macromol. Chem. Phys. 2002, 203, 889–899. [Google Scholar] [CrossRef]
- Al-Khafaji, Y.F.; Elsegood, M.R.J.; Frese, J.W.A.; Redshaw, C. Ring opening polymerization of lactides and lactones by multimetallic alkyl zinc complexes derived from the acids Ph2C(X)CO2H (X = OH, NH2). RSC Adv. 2017, 7, 4510–4517. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, K.-Q.; Al-Khafaji, Y.; Mo, S.; Prior, T.J.; Elsegood, M.R.J.; Redshaw, C. Organoaluminium complexes derived from anilines or Schiff bases for the ring-opening polymerization of ε-caprolactone, δ-valerolactone and rac-lactide. Eur. J. Inorg. Chem. 2017, 2017, 1951–1965. [Google Scholar] [CrossRef]
- Handbook of Ring Opening Polymerization; Dubois, P.; Coulembier, O.; Raquez, J.-M. (Eds.) Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar] [CrossRef]
- Aikawa, K.; Mikami, K. Dual chirality control of palladium(II) complexes bearing Tropos biphenyl diamine ligands. Chem. Commun. 2005, 5799–5801. [Google Scholar] [CrossRef] [PubMed]
- APEX 2 & SAINT Software for CCD Diffractometers; Bruker AXS Inc.: Madison, WI, USA, 2006.
- CrysAlisPRO Software, Oxford Rigaku Diffraction; Rigaku Corporation: Wrocław, Poland, 2021 & 2023.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]






| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| N3—H3A···Cl6 | 0.88 | 2.42 | 3.278 (8) | 164 |
| O3—H3···O2 i | 0.84 | 1.73 | 2.564 (9) | 170 |
| N4—H4···Cl3 | 0.88 | 2.32 | 3.190 (8) | 172 |
| O7—H7···O6 ii | 0.84 | 1.89 | 2.707 (10) | 165 |
| N5—H5A···N10 | 0.88 | 2.53 | 3.37 (2) | 159 |
| N5—H5B···Cl4 iii | 0.88 | 2.74 | 3.413 (11) | 135 |
| N6—H6A···O2 | 0.88 | 2.56 | 3.319 (12) | 145 |
| N6—H6A···N5 | 0.88 | 2.28 | 2.736 (13) | 112 |
| N6—H6B···Cl2 | 0.88 | 2.44 | 3.307 (9) | 170 |
| N7—H7A···O5 | 0.88 | 2.13 | 2.990 (10) | 165 |
| N7—H7B···N11 | 0.88 | 2.34 | 3.02 (2) | 135 |
| N8—H8A···Cl5 | 0.88 | 2.95 | 3.432 (11) | 116 |
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| N1—H1···N4 | 0.82 (1) | 2.13 (1) | 2.9518 (17) | 176 (2) |
| N2—H2A···O1 i | 0.81 (1) | 2.47 (2) | 2.9215 (14) | 116 (2) |
| N2—H2A···O2 i | 0.81 (1) | 2.28 (1) | 3.0736 (15) | 168 (2) |
| N2—H2B···Cl2 i | 0.84 (1) | 2.68 (1) | 3.5139 (11) | 174 (2) |
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| N1—H1···N7 | 0.81 (2) | 2.19 (2) | 2.995 (4) | 172 (3) |
| N3—H3A···O2 i | 0.87 (2) | 1.96 (2) | 2.828 (3) | 172 (3) |
| N3—H3B···Cl1 | 0.87 (2) | 2.54 (2) | 3.323 (3) | 151 (3) |
| N3—H3B···Cl2 | 0.87 (2) | 2.85 (3) | 3.502 (3) | 133 (3) |
| N3—H3C···O3 | 0.88 (2) | 1.93 (2) | 2.803 (3) | 174 (3) |
| N3—H3C···O4 | 0.88 (2) | 2.55 (3) | 3.054 (3) | 117 (2) |
| N4—H4···N8 | 0.81 (2) | 2.21 (2) | 3.010 (4) | 170 (3) |
| N6—H6A···O4 ii | 0.88 (2) | 1.98 (2) | 2.847 (3) | 171 (3) |
| N6—H6B···Cl1 | 0.87 (2) | 2.99 (3) | 3.446 (3) | 115 (2) |
| N6—H6B···O1 | 0.87 (2) | 2.00 (2) | 2.858 (3) | 171 (3) |
| N6—H6B···O2 | 0.87 (2) | 2.59 (3) | 3.059 (3) | 115 (2) |
| N6—H6C···Cl5 | 0.87 (2) | 2.70 (2) | 3.416 (3) | 141 (3) |
| N6—H6C···Cl6 | 0.87 (2) | 2.59 (2) | 3.326 (3) | 143 (3) |
| Entry | Catalyst | [CL]:[Cat]:BnOH | Conversion a (%) b | Mn (obsd)b | Mn Corrected c | Mncalcd | Ð b |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 500:1:2 | 100 | 14,820 | 8300 | 57,180 | 2.78 |
| 2 e | 1 | 500:1:2 | 100 | 16,590 | 9290 | 57,180 | 3.00 3.00 |
| 3 | 2 | 500:1:1 | 100 | 10,430 | 5840 | 57,180 | 1.12 |
| 4 e | 2 | 500:1:1 | 99 | 22,880 | 12,810 | 56,610 | 1.85 |
| 5 | 3 | 500:1:1 | 86 | 56,520 | 31,650 | 49,190 | 3.66 |
| 6 e | 3 | 500:1:1 | 99 | 34,200 | 19,150 | 56,610 | 2.84 |
| 7 | I | 500:1:1 | 100 | 64,160 | 35,930 | 57,180 | 3.83 |
| 8 e | I | 500:1:1 | 100 | 34,110 | 19,100 | 57,180 | 3.61 |
| 9 | II | 500:1:1 | 100 | 21,040 | 11,780 | 57,180 | 2.26 |
| 10 e | II | 500:1:1 | 97 | 15,620 | 8750 | 55,460 | 3.69 |
| 11 e | [2,2′-NH2(C6H4)]2 | 500:1 | - | - | - | - | |
| 12 e | [2,2′-(NH2)C6H4]2CH2 | 500:1 | - | - | - | - | |
| 13 e | [2,2′-(NH2)C6H4]2CH2CH2 | 500:1 | - | - | - | - | |
| 14 e | 1,2-(NH2)(CO2H)C6H4 | 500:1 | 98 | 14,090 | 7890 | 56,070 | 1.37 |
| 15 e | H2NC6H3-1,4-(CO2H)2 | 500:1 | 28 | - | - | - |
| Entry | Catalyst | [VL]:[Cat]:BnOH | Conversion a (%) a | Mn (obsd) b | Mn Corrected c | Mn calc d | Ð b |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 500:1:2 | 93 | 22,160 | 12,630 | 46,570 | 2.38 |
| 2 e | 1 | 500:1:2 | 98 | 29,320 | 16,710 | 49,080 | 1.38 |
| 3 | 2 | 500:1:1 | 83 | 24,390/10,910 | 13,900/6220 | 41,570 | 1.24/1.06 |
| 4 e | 2 | 500:1:1 | 96 | 27,470 | 15,660 | 48,080 | 2.71 |
| 5 | 3 | 500:1:1 | 76 | 23,460 | 13,370 | 38,060 | 2.75 |
| 6 e | 3 | 500:1:1 | 86 | 16,770 | 9560 | 43,070 | 1.90 |
| 7 | I | 500:1:1 | 94 | 10,560 | 6020 | 47,070 | 1.69 |
| 8e | I | 500:1:1 | 94 | 24,460 | 13,940 | 47,070 | 2.06 |
| 9 | II | 500:1:1 | 98 | 29,400/5930 | 16,760/3380 | 49,080 | 1.69/1.47 |
| 10 e | II | 500:1:1 | 97 | 20,440/4720 | 11,650/2690 | 48,580 | 1.39/1.23 |
| 11 e | [2,2′-NH2(C6H4)]2 | 500:1 | 95 | 15,910 | 9070 | 47,580 | 2.29 |
| 12 e | [2,2′-(NH2)C6H4]2CH2 | 500:1 | 94 | 29,490 | 16,810 | 47,070 | 1.21 |
| 13 e | [2,2′-(NH2)C6H4]2CH2CH2 | 500:1 | 94 | 29,860 | 17,020 | 47,070 | 1.32 |
| 14 e | 1,2-(NH2)(CO2H)C6H4 | 500:1 | 99 | 8140 | 4640 | 49,580 | 2.69 |
| 15 e | H2NC6H3-1,4-(CO2H)2 | 500:1 | 13 | - | - | - |
| Compound | 1·2C12H12N2·3.5MeCN | 2·3MeCN | 3·MeCN |
|---|---|---|---|
| CCDC No. | 2,286,024 | 2,286,025 | 2,286,026 |
| Formula | C28H18Cl6Mo2N4O8·2(C12H12N2)·3.5(C2H3N) | C24H25Cl2MoN3O4Si·3(C2H3N) | C21H20Cl3MoN3O2·(C2H3N) |
| Formula weight | 1455.20 | 737.56 | 589.74 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | Cc | P21/n | P21/c |
| Unit cell dimensions | |||
| a (Å) | 12.2500(2) | 15.57139(19) | 17.9901(13) |
| b (Å) | 31.4061(7) | 13.33765(14) | 16.5331(12) |
| c (Å) | 16.2619(2) | 17.8684(2) | 18.5902(13) |
| β (º) | 97.3910(13) | 110.7234(14) | 114.058(2) |
| V (Å3) | 6204.37(19) | 3470.91(7) | 5049.0(6) |
| Z | 4 | 4 | 8 |
| Temperature (K) | 100(2) | 100(2) | 160(2) |
| Wavelength (Å) | 1.54178 | 0.71073 | 0.71073 |
| Calculated density (g.cm−3) | 1.558 | 1.411 | 1.552 |
| Absorption coefficient (mm−1) | 6.22 | 0.61 | 0.87 |
| Transmission factors (min./max.) | 0.045 and 0.143 | 0.761 and 1.000 | 0.674 and 0.862 |
| Crystal size (mm3) | 0.04 × 0.04 × 0.02 | 0.20 × 0.14 × 0.01 | 0.20 × 0.20 × 0.14 |
| θ(max) (°) | 75.7 | 33.6 | 28.5 |
| Reflections measured | 20,721 | 143,190 | 30,725 |
| Unique reflections | 8359 | 12,893 | 11,549 |
| Rint | 0.052 | 0.035 | 0.046 |
| Reflections with F2 > 2σ(F2) | 7358 | 11,128 | 7877 |
| Number of parameters | 798 | 412 | 621 |
| R1 [F2 > 2σ(F2)] | 0.058 | 0.031 | 0.041 |
| wR2 (all data) | 0.144 | 0.072 | 0.094 |
| GOOF, S | 1.03 | 1.05 | 0.99 |
| Largest difference peak and hole (e Å−3) | 1.67 and −0.36 | 0.87 and −0.80 | 0.51 and −0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clegg, W.; Elsegood, M.R.J.; Redshaw, C. Extended Hydrogen-Bonded Molybdenum Arrays Derived from Carboxylic Acids and Dianilines: ROP Capability of the Complexes and Parent Acids and Dianilines. Catalysts 2024, 14, 214. https://doi.org/10.3390/catal14030214
Clegg W, Elsegood MRJ, Redshaw C. Extended Hydrogen-Bonded Molybdenum Arrays Derived from Carboxylic Acids and Dianilines: ROP Capability of the Complexes and Parent Acids and Dianilines. Catalysts. 2024; 14(3):214. https://doi.org/10.3390/catal14030214
Chicago/Turabian StyleClegg, William, Mark R. J. Elsegood, and Carl Redshaw. 2024. "Extended Hydrogen-Bonded Molybdenum Arrays Derived from Carboxylic Acids and Dianilines: ROP Capability of the Complexes and Parent Acids and Dianilines" Catalysts 14, no. 3: 214. https://doi.org/10.3390/catal14030214







