Gadolinium-Doped Bismuth Ferrite for the Photocatalytic Oxidation of Arsenite to Arsenate under Visible Light
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photocatalysts Characterization
2.1.1. X-ray Powder Diffraction Analysis (XRD)
2.1.2. Raman Spectroscopy
2.1.3. Optical Characterization
2.1.4. Photoluminescence (PL) Spectroscopy
2.1.5. Field Emission Scanning Electron Microscopy (FE-SEM)
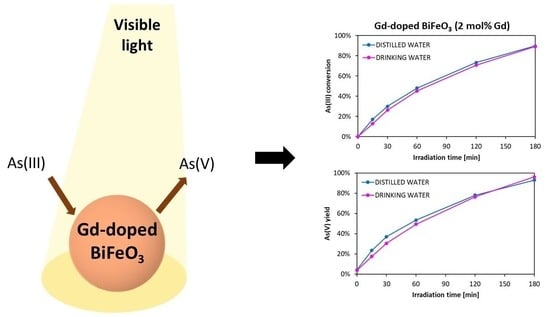
2.2. Photocatalytic Activity Results
3. Materials and Methods
3.1. Gd-BFO Preparation
3.2. Photocatalysts Characterization
3.3. Photocatalytic Activity Tests
3.4. Analytical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pezeshki, H.; Hashemi, M.; Rajabi, S.J. Removal of arsenic as a potentially toxic element from drinking water by filtration: A mini review of nanofiltration and reverse osmosis techniques. Heliyon 2023, 9, e14246. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Udensi, U.K.; Pacurari, M.; Stevens, J.J.; Patlolla, A.K.; Noubissi, F.; Kumar, S.J. State of the science review of the health effects of inorganic arsenic: Perspectives for future research. Environ. Toxicol. 2019, 34, 188–202. [Google Scholar] [CrossRef]
- Zakhar, R.; Derco, J.; Čacho, F.J. An overview of main arsenic removal technologies. Acta Chim. Slovaca 2018, 11, 107–113. [Google Scholar] [CrossRef]
- Goering, P.L.; Aposhian, H.V.; Mass, M.J.; Cebrián, M.; Beck, B.D.; Waalkes, M.P. The enigma of arsenic carcinogenesis: Role of metabolism. Toxicol. Sci. Off. J. Soc. Toxicol. 1999, 49, 5–14. [Google Scholar] [CrossRef]
- Kartinen, E.O., Jr.; Martin, C.J.J. An overview of arsenic removal processes. Desalination 1995, 103, 79–88. [Google Scholar] [CrossRef]
- Liu, R.; Qu, J. Review on heterogeneous oxidation and adsorption for arsenic removal from drinking water. J. Environ. Sci. 2021, 110, 178–188. [Google Scholar] [CrossRef]
- Navarrete-Magaña, M.; Estrella-González, A.; May-Ix, L.; Cipagauta-Díaz, S.; Gómez, R. Improved photocatalytic oxidation of arsenic (III) with WO3/TiO2 nanomaterials synthesized by the sol-gel method. J. Environ. Manag. 2021, 282, 111602. [Google Scholar] [CrossRef]
- Masuda, H. Arsenic cycling in the Earth’s crust and hydrosphere: Interaction between naturally occurring arsenic and human activities. Prog. Earth Planet. Sci. 2018, 5, 1–11. [Google Scholar] [CrossRef]
- Villaescusa, I.; Bollinger, J.-C. Arsenic in drinking water: Sources, occurrence and health effects (a review). Rev. Environ. Sci. Bio/Technol. 2008, 7, 307–323. [Google Scholar] [CrossRef]
- Olson, K.R.; Cihacek, L. The fate of Agent Blue, the arsenic based herbicide, used in South Vietnam during the Vietnam War. Open J. Soil Sci. 2020, 10, 518–577. [Google Scholar] [CrossRef]
- Chianese, L.; Murcia, J.; Hidalgo, M.; Vaiano, V.; Iervolino, G. Bismuth ferrite as innovative and efficient photocatalyst for the oxidation of As (III) to As (V) under visible light. Mater. Sci. Semicond. Process. 2023, 167, 107801. [Google Scholar] [CrossRef]
- ALSamman, M.T.; Sotelo, S.; Sánchez, J.; Rivas, B.L. Arsenic oxidation and its subsequent removal from water: An overview. Sep. Purif. Technol. 2023, 309, 123055. [Google Scholar] [CrossRef]
- Ng, K.-S.; Ujang, Z.; Le-Clech, P. Arsenic removal technologies for drinking water treatment. Rev. Environ. Sci. Bio/Technol. 2004, 3, 43–53. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Abd Manan, F. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, W.; Sun, Z. Removal of arsenic from flue gas using NaClO/NaClO2 complex absorbent. Chem. Eng. Res. Des. 2019, 144, 505–511. [Google Scholar] [CrossRef]
- Li, N.; Fan, M.; Van Leeuwen, J.; Saha, B.; Yang, H.; Huang, C.P. Oxidation of As(III) by potassium permanganate. J. Environ. Sci. 2007, 19, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Sorlini, S.; Gialdini, F. Conventional oxidation treatments for the removal of arsenic with chlorine dioxide, hypochlorite, potassium permanganate and monochloramine. Water Res. 2010, 44, 5653–5659. [Google Scholar] [CrossRef] [PubMed]
- Gude, J.; Rietveld, L.; Van Halem, D.J. As (III) oxidation by MnO2 during groundwater treatment. Water Res. 2017, 111, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Pettine, M.; Campanella, L.; Millero, F.J. Arsenite oxidation by H2O2 in aqueous solutions. Geochim. Cosmochim. Acta 1999, 63, 2727–2735. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W.J. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Wang, G. Photocatalytic Advanced Oxidation Processes for Water Treatment: Recent Advances and Perspective. Chem. Asian J. 2020, 15, 3239–3253. [Google Scholar] [CrossRef] [PubMed]
- Gowland, D.C.A.; Robertson, N.; Chatzisymeon, E. Photocatalytic Oxidation of Natural Organic Matter in Water. Water 2021, 13, 288. [Google Scholar] [CrossRef]
- Loddo, V.; Bellardita, M.; Camera-Roda, G.; Parrino, F.; Palmisano, L. Chapter 1—Heterogeneous Photocatalysis: A Promising Advanced Oxidation Process. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Mozia, S., Molinari, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–43. [Google Scholar]
- Tammaro, O.; Paparo, R.; Chianese, M.; Ritacco, I.; Caporaso, L.; Camellone, M.F.; Masenelli, B.; Lamirand, A.D.; Bluet, J.-M.; Fontana, M.; et al. Reverse micelle strategy for effective substitutional Fe-doping in small-sized CeO2 nanocrystals: Assessment of adsorption and photodegradation efficiency of ibuprofen under visible light. Chem. Eng. J. 2024, 479, 147909. [Google Scholar] [CrossRef]
- Tammaro, O.; Morante, N.; Marocco, A.; Fontana, M.; Castellino, M.; Barrera, G.; Allia, P.; Tiberto, P.; Arletti, R.; Fantini, R.; et al. The beneficial role of nano-sized Fe3O4 entrapped in ultra-stable Y zeolite for the complete mineralization of phenol by heterogeneous photo-Fenton under solar light. Chemosphere 2023, 345, 140400. [Google Scholar] [CrossRef] [PubMed]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Choi, W.; Yeo, J.; Ryu, J.; Tachikawa, T.; Majima, T. Photocatalytic oxidation mechanism of As (III) on TiO2: Unique role of As (III) as a charge recombinant species. Environ. Sci. Technol. 2010, 44, 9099–9104. [Google Scholar] [CrossRef]
- Garza-Arévalo, J.; García-Montes, I.; Reyes, M.H.; Guzmán-Mar, J.; Rodríguez-González, V.; Reyes, L.H. Fe doped TiO2 photocatalyst for the removal of As (III) under visible radiation and its potential application on the treatment of As-contaminated groundwater. Mater. Res. Bull. 2016, 73, 145–152. [Google Scholar] [CrossRef]
- Imparato, C.; Bonifazzi, M.M.; D’Errico, G.; Bifulco, A.; Tammaro, O.; Esposito, S.; Aronne, A.; Pirozzi, D. Dark and sunlight-driven dye degradation over a TiO2–dibenzoylmethane hybrid xerogel. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133148. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G. Photocatalytic removal of methyl orange azo dye with simultaneous hydrogen production using ru-modified zno photocatalyst. Catalysts 2019, 9, 964. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, Q.; Wu, A.; Jiang, M.; Liang, Z.; Jiang, B.; Fu, H. Cost-effective large-scale synthesis of ZnO photocatalyst with excellent performance for dye photodegradation. Chem. Commun. 2012, 48, 2858–2860. [Google Scholar] [CrossRef]
- Irfan, S.; Zhuanghao, Z.; Li, F.; Chen, Y.-X.; Liang, G.-X.; Luo, J.-T.; Ping, F. Critical review: Bismuth ferrite as an emerging visible light active nanostructured photocatalyst. J. Mater. Res. Technol. 2019, 8, 6375–6389. [Google Scholar] [CrossRef]
- Soltani, T.; Entezari, M.H. Photolysis and photocatalysis of methylene blue by ferrite bismuth nanoparticles under sunlight irradiation. J. Mol. Catal. A Chem. 2013, 377, 197–203. [Google Scholar] [CrossRef]
- Soltani, T.; Entezari, M. Solar photocatalytic degradation of RB5 by ferrite bismuth nanoparticles synthesized via ultrasound. Ultrason. Sonochemistry 2013, 20, 1245–1253. [Google Scholar] [CrossRef]
- Wang, F.; Chen, D.; Zhang, N.; Wang, S.; Qin, L.; Sun, X.; Huang, Y. Oxygen vacancies induced by zirconium doping in bismuth ferrite nanoparticles for enhanced photocatalytic performance. J. Colloid Interface Sci. 2017, 508, 237–247. [Google Scholar] [CrossRef]
- Brahma, S.S.; Nanda, J.; Sahoo, N.K.; Naik, B.; Das, A.A. Phase transition, electronic transitions and visible light driven enhanced photocatalytic activity of Eu–Ni co-doped bismuth ferrite nanoparticles. J. Phys. Chem. Solids 2021, 153, 110018. [Google Scholar] [CrossRef]
- Irfan, S.; Shen, Y.; Rizwan, S.; Wang, H.C.; Khan, S.B.; Nan, C.W. Band-gap engineering and enhanced photocatalytic activity of Sm and Mn doped BiFeO3 nanoparticles. J. Am. Ceram. Soc. 2017, 100, 31–40. [Google Scholar] [CrossRef]
- Guo, R.; Fang, L.; Dong, W.; Zheng, F.; Shen, M. Enhanced photocatalytic activity and ferromagnetism in Gd doped BiFeO3 nanoparticles. J. Phys. Chem. C 2010, 114, 21390–21396. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Seethya, B.N.H.; Ganganagappa, N.; Eshwaraswamy, K.B.; Kakarla, R.R. Enhanced photocatalytic activity and biosensing of gadolinium substituted BiFeO3 nanoparticles. ChemistrySelect 2018, 3, 9025–9033. [Google Scholar] [CrossRef]
- Irfan, S.; Rizwan, S.; Shen, Y.; Li, L.; Asfandiyar; Butt, S.; Nan, C.-W. The gadolinium (Gd3+) and Tin (Sn4+) Co-doped BiFeO3 nanoparticles as new solar light active photocatalyst. Sci. Rep. 2017, 7, 42493. [Google Scholar] [CrossRef]
- Anthoniappen, J.; Chang, W.S.; Soh, A.K.; Tu, C.-S.; Vashan, P.; Lim, F.S. Electric field induced nanoscale polarization switching and piezoresponse in Sm and Mn co-doped BiFeO3 multiferroic ceramics by using piezoresponse force microscopy. Acta Mater. 2017, 132, 174–181. [Google Scholar] [CrossRef]
- Kumar, M.; Chauhan, S.; Pandey, H. Effect of Gd3+ substitution on structural, morphological, and magnetic properties of BiFeO3 nanoparticles. J. Sol-Gel Sci. Technol. 2024, 109, 272–282. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, D.; Niu, F.; Wang, S.; Qin, L.; Huang, Y. Enhanced visible light photocatalytic activity of Gd-doped BiFeO3 nanoparticles and mechanism insight. Sci. Rep. 2016, 6, 26467. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, A.K.; Tripathi, P.; Srivastava, A.; Sinha, A.; Srivastava, O. Band gap engineering of Gd and Co doped BiFeO3 and their application in hydrogen production through photoelectrochemical route. Int. J. Hydrog. Energy 2017, 42, 22677–22686. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.K.; Modi, A.; Pandey, P.; Gaur, N. Variable excitation wavelength photoluminescence response and optical absorption in BiFeO3 nanostructures. J. Mater. Sci. Mater. Electron. 2017, 28, 17245–17253. [Google Scholar] [CrossRef]
- Anshul, A.; Kumar, A.; Gupta, B.K.; Kotnala, R.; Scott, J.; Katiyar, R. Photoluminescence and time-resolved spectroscopy in multiferroic BiFeO3: Effects of electric fields and sample aging. Appl. Phys. Lett. 2013, 102, 222901. [Google Scholar] [CrossRef]
- Alam, U.; Shah, T.A.; Khan, A.; Muneer, M. One-pot ultrasonic assisted sol-gel synthesis of spindle-like Nd and V codoped ZnO for efficient photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2019, 212, 427–437. [Google Scholar] [CrossRef]
- Gekko, H.; Hashimoto, K.; Kominami, H. Photocatalytic reduction of nitrite to dinitrogen in aqueous suspensions of metal-loaded titanium (IV) oxide in the presence of a hole scavenger: An ensemble effect of silver and palladium co-catalysts. Phys. Chem. Chem. Phys. 2012, 14, 7965–7970. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Kamat, P.V.; O’Shea, K.E. Mechanistic evaluation of arsenite oxidation in TiO2 assisted photocatalysis. J. Phys. Chem. A 2005, 109, 9070–9075. [Google Scholar] [CrossRef]
- Lenoble, V.; Deluchat, V.; Serpaud, B.; Bollinger, J.-C. Arsenite oxidation and arsenate determination by the molybdene blue method. Talanta 2003, 61, 267–276. [Google Scholar] [CrossRef]









| Sample | 2θ | FWHM | Crystallite Size (nm) |
|---|---|---|---|
| BFO | 32.0233 | 0.120 | 68.9 |
| 32.3455 | 0.119 | 69.5 | |
| 0.5Gd-BFO | 31.9512 | 0.133 | 62.3 |
| 32.2727 | 0.124 | 66.7 | |
| 1Gd-BFO | 32.0781 | 0.143 | 57.8 |
| 32.3955 | 0.1290 | 64.2 | |
| 2Gd-BFO | 32.0478 | 0.152 | 54.7 |
| 32.3648 | 0.136 | 60.8 | |
| 5Gd-BFO | - | - | - |
| 10Gd-BFO | - | - | - |
| Sample | Band-Gap [eV] |
|---|---|
| BFO | 2.21 |
| 0.5Gd-BFO | 2.21 |
| 1Gd-BFO | 2.18 |
| 2Gd-BFO | 2.17 |
| 5Gd-BFO | 2.07 |
| 10Gd-BFO | 2.07 |
| Radical Scavenger | Subtracted Species | Concentration |
|---|---|---|
| Isopropyl alcohol | OH∙quencher | 10 mM |
| Benzoquinone | ∙O2− quencher | 1 mM |
| Methanol | h+ quencher | 300 mM |
| Copper Sulfate | e− quencher | 1 mM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chianese, L.; Guastella, S.; Tammaro, O.; Vaiano, V.; Esposito, S.; Iervolino, G. Gadolinium-Doped Bismuth Ferrite for the Photocatalytic Oxidation of Arsenite to Arsenate under Visible Light. Catalysts 2024, 14, 258. https://doi.org/10.3390/catal14040258
Chianese L, Guastella S, Tammaro O, Vaiano V, Esposito S, Iervolino G. Gadolinium-Doped Bismuth Ferrite for the Photocatalytic Oxidation of Arsenite to Arsenate under Visible Light. Catalysts. 2024; 14(4):258. https://doi.org/10.3390/catal14040258
Chicago/Turabian StyleChianese, Laura, Salvatore Guastella, Olimpia Tammaro, Vincenzo Vaiano, Serena Esposito, and Giuseppina Iervolino. 2024. "Gadolinium-Doped Bismuth Ferrite for the Photocatalytic Oxidation of Arsenite to Arsenate under Visible Light" Catalysts 14, no. 4: 258. https://doi.org/10.3390/catal14040258
APA StyleChianese, L., Guastella, S., Tammaro, O., Vaiano, V., Esposito, S., & Iervolino, G. (2024). Gadolinium-Doped Bismuth Ferrite for the Photocatalytic Oxidation of Arsenite to Arsenate under Visible Light. Catalysts, 14(4), 258. https://doi.org/10.3390/catal14040258