Catalytic Decomposition of CH4 to Hydrogen and Carbon Nanotubes Using the Pt(1)-Fe(30)/MCM-41 Catalyst
Abstract
:1. Introduction
2. Results and Discussion
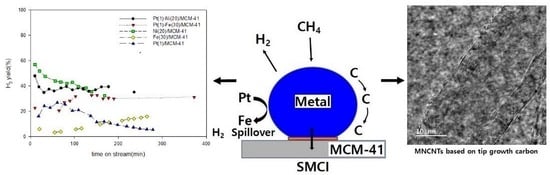
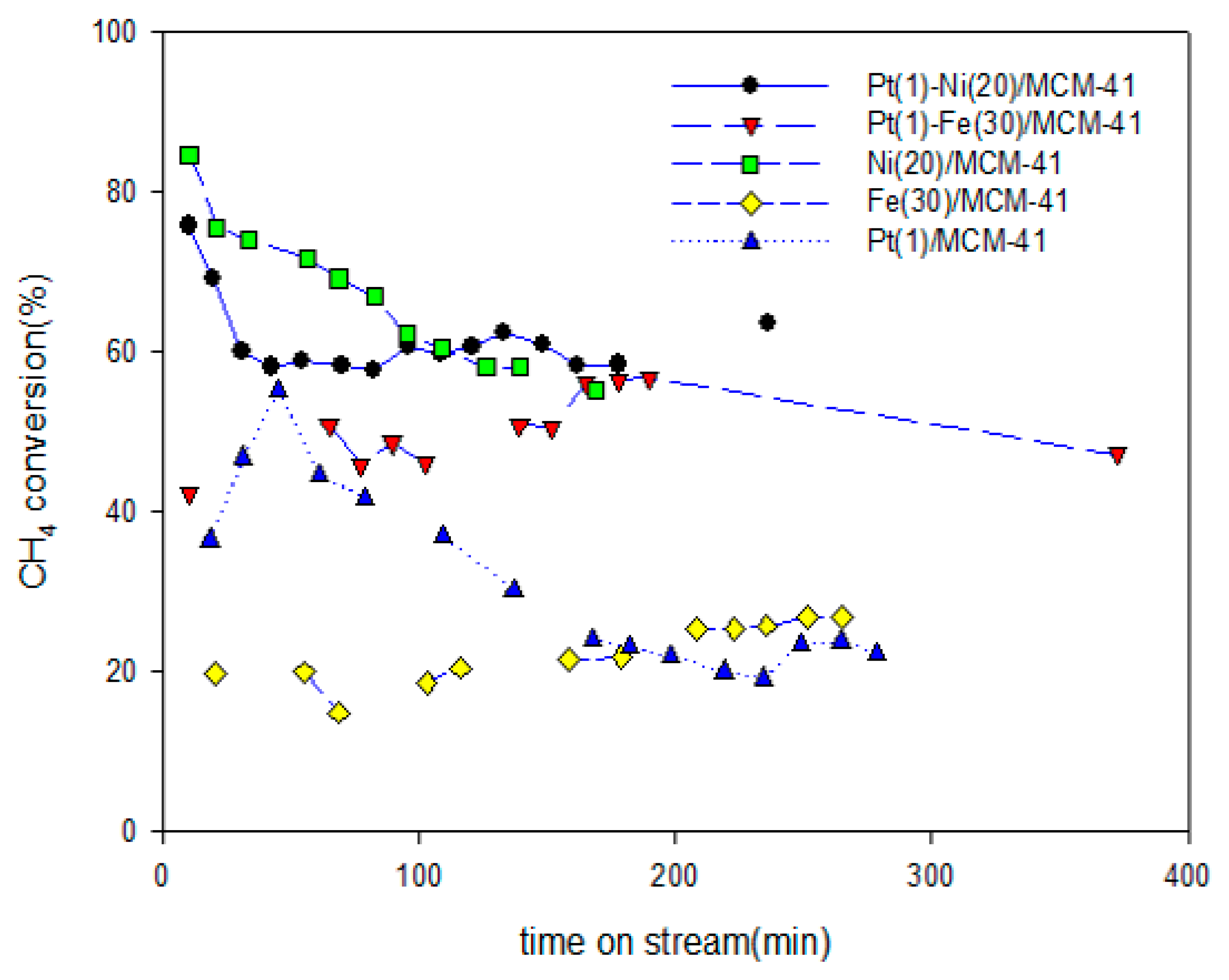
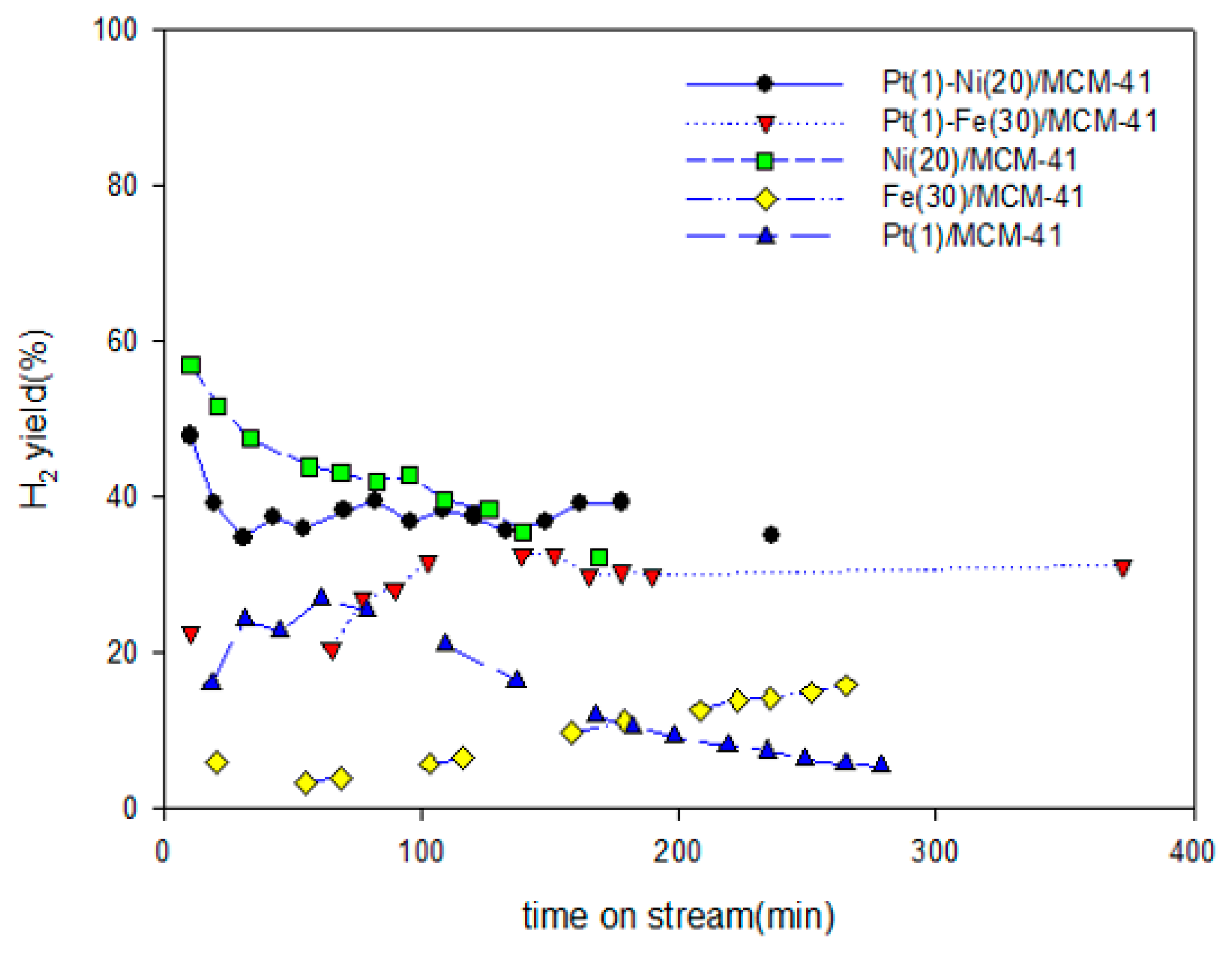
2.1. Activity of the Catalyst
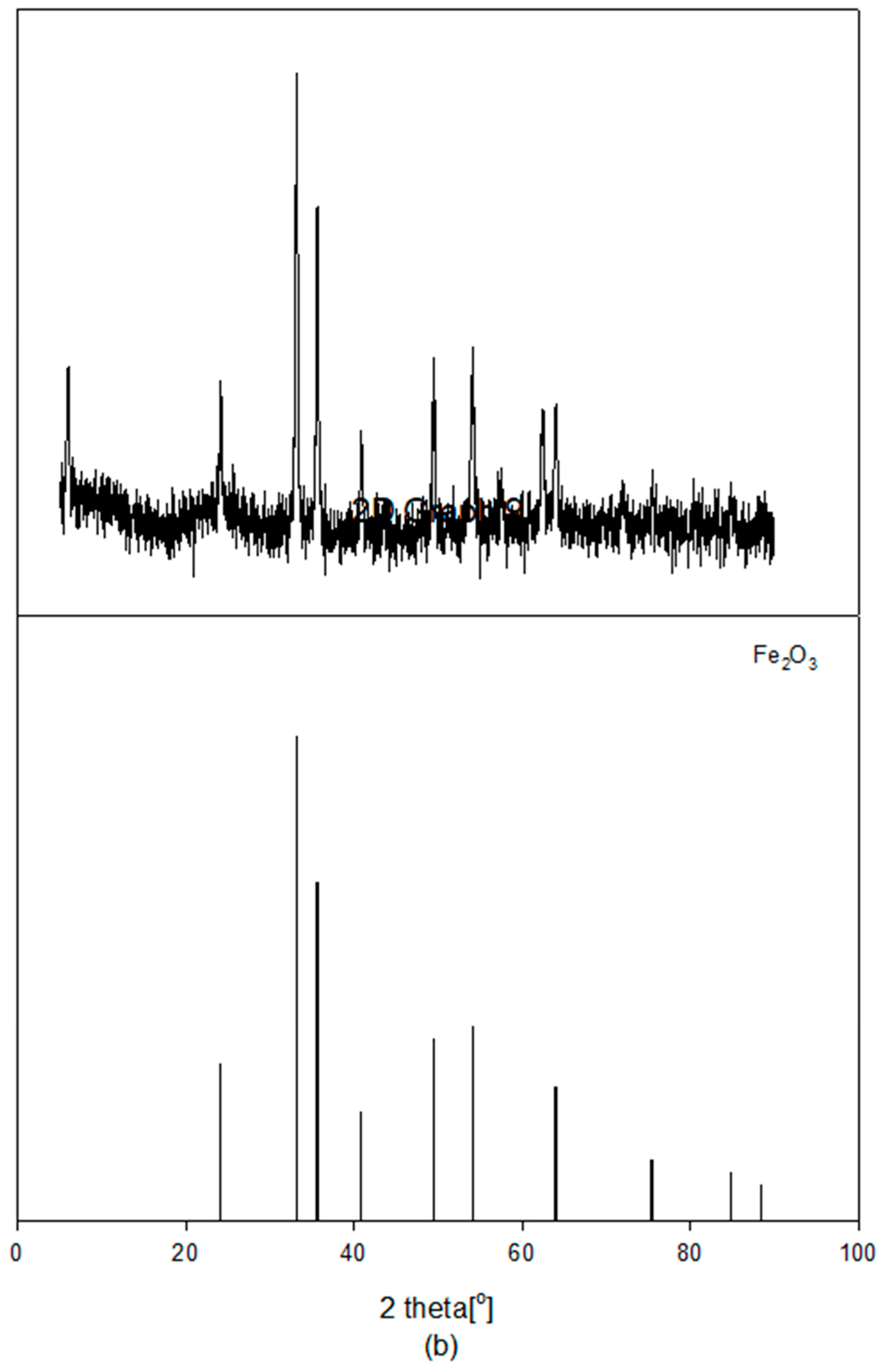
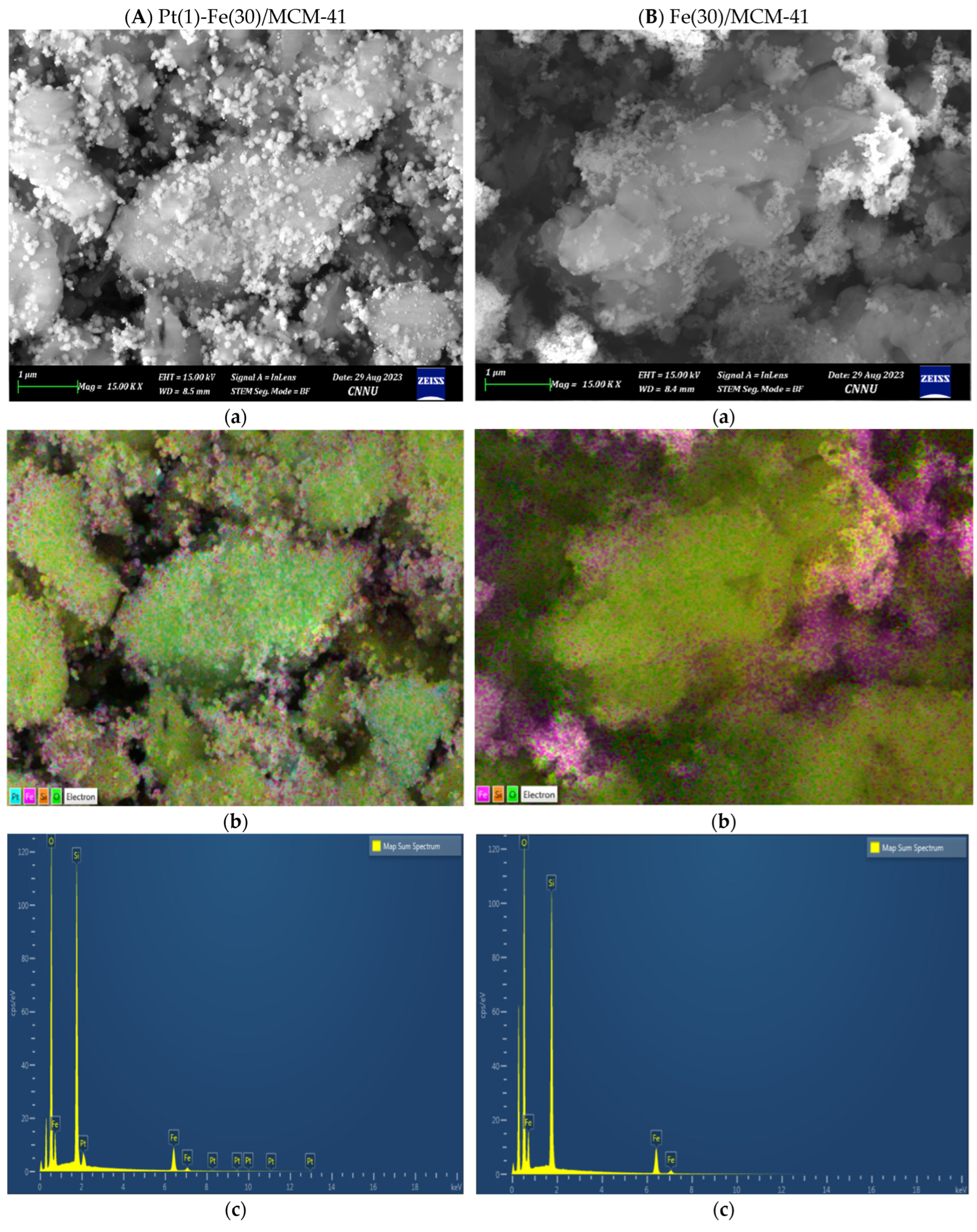
2.2. Characterization of the Fresh Catalyst
2.3. Characterization of Carbon Deposited on the Catalyst
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalyst Performance
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abbas, H.F.; Daud, W.M.A.W. Hydrogen production by methane decomposition: A review. Int. J. Hydrogen Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Wan Daud, W.M.A.; Hayashi, J.-I. A review on methane transformation to hydrogen and nanocarbon: Relevance of catalyst characteristics and experimental parameters on yield. Renew. Sustain. Energy Rev. 2017, 76, 743–767. [Google Scholar] [CrossRef]
- Surendran, S.; Pathan, M.; Walke, P.; Syam, K.R.; Babu, P.G.; Poojary, N.; Sharma, R. Green synthesis and characterization of multiwall carbon nanotubes from sugarcane bagasse as a biogenic catalyst via chemical vapour deposition method using LPG as hydrocarbon source. Mater. Lett. 2024, 359, 135904–135908. [Google Scholar] [CrossRef]
- Torres, D.; Pinilla, J.L.; Lázaro, M.J.; Moliner, R.; Suelves, I. Hydrogen and multiwall carbon nanotubes production by catalytic decomposition of methane: Thermogravimetric analysis and scaling-up of FeeMo catalysts. Int. J. Hydrogen Energy 2014, 39, 3698–3709. [Google Scholar] [CrossRef]
- Otsuka, K.; Takenaka, S.; Ohtsuki, H. Production of pure hydrogen by cyclic decomposition of methane and oxidative elimination of carbon nanofibers on supported-Ni-based catalysts. Appl. Catal. A 2004, 273, 113–124. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, X.; Hildebrandt, D.; Glasser, D. Fisher-Tropsch Synthesis Using H2/CO/CO2 Syngas Mixtures over an Iron Catalyst. Ind. Eng. Chem. Res. 2011, 50, 11002–11012. [Google Scholar] [CrossRef]
- Awadallah, A.E.; Aboul-Enein, A.A.; El-Desouki, D.S.; Aboul-Gheit, A.K. Catalytic thermal decomposition of methane to COx-free hydrogen and carbon nanotubes over MgO supported Bimetallic group VIII catalysts. Appl. Surf. Sci. 2014, 96, 100–107. [Google Scholar] [CrossRef]
- Zhao, R.; Du, X.; Cao, K.; Gong, M.; Li, Y.; Ai, J.; Ye, R.; Chen, R.; Shan, B. Highly dispersed Fe-decorated Ni nanoparticles prepared by atomic layer deposition for dry reforming of methane. Int. J. Hydrogen Energy 2023, 48, 28780–28791. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, S.; Deng, J.; Li, C.; Feng, Y.; Xie, X.; Li, N. Investigation on carbon deposition deactivation of Fe-Ni-Ca/Al2O3catalyst under volatiles reforming of biomass pyrolysis. J. Anal. Appl. Pyrolysis 2022, 168, 105778–105791. [Google Scholar] [CrossRef]
- Estephane, J.; Aouad, S.; Hany, S.; El Khoury, B.; Gennequin, C.; El Zakhem, H.; El Nakat, J.; Aboukaȉs, A.; Abi Aad, E. CO2 reforming of methane over NieCo/ZSM5 catalysts. Aging and carbon deposition study. Int. J. Hydrogen Energy 2015, 40, 9201–9208. [Google Scholar] [CrossRef]
- Ȍzdemir, H.; Ȍksȕzȍmer, M.A.F.; Gȕrkaynak, M.A. Preparation and characterization of Ni based catalysts for thecatalytic partial oxidation of methane: Effect of supportbasicity on H2/CO ratio and carbon deposition. Int. J. Hydrogen Energy 2010, 35, 12147–12160. [Google Scholar] [CrossRef]
- Wua, H.; Liua, J.; Liu, H.; Hea, D. CO2 reforming of methane to syngas at high pressure over bi-component NiCo catalyst: The anti-carbon deposition and stability of catalyst. Fuel 2019, 235, 868–877. [Google Scholar] [CrossRef]
- Han, C.; Zhu, X.; Chen, B.; Wang, X. A strategy of constructing the Ni@silicalite-1 catalyst structure with highactivity and resistance to sintering and carbon deposition for dry reformingof methane. Fuel 2024, 355, 129548–129562. [Google Scholar] [CrossRef]
- Pashchenko, D.; Makarov, I. Carbon deposition in steam methane reforming over a Ni-basedcatalyst: Experimental and thermodynamic analysis. Energy 2021, 222, 119993–120002. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arisshtirova, K.; Fierro, J.L.G.; Sener, C.; Dogu, T. MCM-41 supported PdNi catalysts for dry reforming of methane. Appl. Catal. B Environ. 2009, 92, 250–261. [Google Scholar] [CrossRef]
- Kutteri, D.A.; Wang, I.-W.; Samanta, A.; Li, L.; Hu, J. Methane decomposition to tip and base grown carbon nanotubes and COx-free H2 over mono- and bimetallic 3d transition metal catalysts. Catal. Sci. Technol. 2018, 8, 858–869. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Arshad, F.; Hassan, I.U.; Tabook, M.A.; Pedram, M.Z.; Mustaqeem, M.; Tabassum, H.; Ahmed, W.; Rezakazemi, M. Thermocatalytic Hydrogen Production Through Decomposition of Methane—A Review. Front. Chem. 2021, 9, 736801–736824. [Google Scholar] [CrossRef]
- Pudukkudy, M.; Yaakob, Z.; Jia, Q.; Takriff, M.S. Catalytic decomposition of undiluted methane into hydrogen and Carbon nanotubes over Pt promoted Ni/CeO2 catalysts. N. J. Chem. 2018, 42, 14843–14856. [Google Scholar] [CrossRef]
- Karimi, S.; Bibak, F.; Meshkani, F.; Rastegarpanah, A.; Deng, J.; Liu, Y.; Dai, H. Promotional roles of second metals in catalyzing methane decomposition over the Ni-based catalysts for hydrogen production: A critical review. Int. J. Hydrogen Energy 2021, 46, 20435–20480. [Google Scholar] [CrossRef]
- Shah, N.; Panjala, D.; Huffman, G.P. Hydrogen Production by Catalytic Decomposition of Methane. Energy Fuels 2001, 15, 1528–1534. [Google Scholar] [CrossRef]
- Takenaka, S.; Shigeta, Y.; Tanabe, E.; Otsuka, K. Methane Decomposition into Carbon Nanofibers over Supported Pd-Ni Catalysts: Characterization of the Catalysts during the Reaction. J. Phys. Chem. B 2004, 108, 7656–7664. [Google Scholar] [CrossRef]
- Wang, G.; Jin, Y.; Liu, G.; Li, Y. Production of Hydrogen and Nanocarbon from Catalytic Decomposition of Methane over a Ni-Fe/Al2O3 Catalyst. Energy Fuels 2013, 27, 4448–4456. [Google Scholar] [CrossRef]
- Shen, Y.; Lua, A.C. Synthesis of Ni and Ni-Cu supported on carbon nanotubes for hydrogen and carbon production by catalytic decomposition of methane. Appl. Catal. B Environ. 2015, 164, 61–69. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Takriff, M.S. Methane decomposition over Pd promoted Ni/MgAl2O4 catalysts for the production of COx free hydrogen and multiwalled nanotubes. Appl. Surf. Sci. 2015, 356, 1320–1326. [Google Scholar] [CrossRef]
- Tezel, E.; Figen, H.E.; Baykara, S.Z. Hydrogen production by methane decomposition using bimetallic Ni-Fe catalysts. Int. J. Hydrogen Energy 2019, 44, 9930–9940. [Google Scholar] [CrossRef]
- Mesfera, M.K.A.; Danisha, M.; Shahb, M. Synthesis and optimization of hydrotalcite derived Ni-Fe-Cu basedcatalysts for catalytic methane decomposition process using the design ofexperiment approach. J. Taiwan Inst. Chem. Eng. 2021, 128, 370–379. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhao, S.; Zhu, J.; Jin, L.; Hu, H. Preparation of bimetallic catalysts Ni-Co and Ni-Fe supported on activatedcarbon for methane decomposition. Carbon Resour. Convers. 2020, 3, 190–197. [Google Scholar] [CrossRef]
- Awadallah, A.E.; Aboul-Enein, A.A.; Deyab, M.A.; Azab, M.A.; Haggar, A.M. Impact of Cr doping on the performance of Ni/Al2O3 catalyst through methane decompositioninto COx-free hydrogen and carbon nanomaterials. Chem. Eng. Res. Des. 2022, 186, 701–712. [Google Scholar] [CrossRef]
- Sun, H.; Ren, S.; Ji, X.; Song, W.; Guo, Q.; Shen, B. Doping Fe and Zn to modulate Ni nanoparticles on IM-5 for methane decomposition to form hydrogenand CNTs. Int. J. Hydrogen Energy 2023, 48, 13081–13096. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Abdelkader, A.; Osman, A.I.; Lanre, M.S.; Fakeeha, A.H.; Alhoshan, M.; Alanazi, Y.M.; Awadallah, A.E.; Rooney, D.W. Non-supported bimetallic catalysts of Fe and Co for methane decomposition into H2 and a mixture of graphene nanosheets and carbon nanotubes. Int. J. Hydrogen Energy 2023, 48, 26506–26517. [Google Scholar] [CrossRef]
- Zhao, X.S.; Lu, G.Q.; Millar, G.J. Advances in Mesoporous Molecular Sieve MCM-41. Ind. Eng. Chem. Res. 1996, 35, 2075–2090. [Google Scholar] [CrossRef]
- Kiani, M.R.; Kamandi, R.; Nozarian, K.; Rahimpour, M.R. Introducing a novel catalyst for efficient conversion of CO2 into syngas through reverse-water-gas-shift (RWGS) reactions based on highly mesoporous structures MCM-41: Influence of the Fe incorporation. Energy Convers. Manag. 2024, 304, 118247–118263. [Google Scholar] [CrossRef]
- Lopez, L.; Montes, V.; Kušar, H.; Cabrera, S.; Boutonnet, M.; Järås, S. Syngas conversion to ethanol over a mesoporous Cu/MCM-41 catalyst: Effect of K and Fe promoters. Appl. Catal. A Gen. 2016, 526, 77–83. [Google Scholar] [CrossRef]
- Pawelec, B.; Damyanova, S.; Arishtirova, K.; Fierro, J.L.G.; Petrov, L. Structural and surface features of PtNi catalysts for reforming of methane with CO2. Appl. Catal. A Gen. 2007, 323, 188–201. [Google Scholar] [CrossRef]
- Hu, F.; Jin, C.; Lim, K.H.; Li, C.; Song, G.; Bella; Wang, T.; Ye, R.; Lu, Z.-H.; Feng, G.; et al. Promoting hydrogen spillover of NiFe/CeO2 catalyst with plasma-treatment for CO2 methanation. Fuel Process. Technol. 2023, 250, 107873–107884. [Google Scholar] [CrossRef]
- Vanoye, L.; Guicheret, B.; Rivera-Cárcamo, C.; Audevard, J.; Navarro-Ruiz, J.; del Rosal, I.; Gerber, I.C.; Campos, C.H.; Machado, B.F.; Volkman, J.; et al. Deactivation of Pd/C catalysts by irreversible loss of hydrogen spilloverability of the carbon support. J. Catal. 2023, 424, 173–188. [Google Scholar] [CrossRef]
- Li, M.; Yin, W.; Pan, J.; Zhu, Y.; Sun, N.; Zhang, X.; Wan, Y.; Luo, Z.; Yi, L.; Wang, L. Hydrogen spillover as a promising strategy for boosting heterogeneous catalysis and hydrogen storage. Chem. Eng. J. 2023, 471, 144691–144714. [Google Scholar] [CrossRef]
- Guo, J.-H.; Li, X.-D.; Cheng, X.-L.; Liu, H.-Y.; Li, S.-J.; Chen, G. The theoretical study of the bimetallic Ni/Pd, Ni/Pt and Pt/Pd catalysts for hydrogen spillover on penta-graphene. Int. J. Hydrogen Energy 2018, 43, 19121–19129. [Google Scholar] [CrossRef]
- Boudjahema, A.-G.; Bettahar, M.M. Effect of oxidative pre-treatment on hydrogen spillover for a Ni/SiO2catalyst. J. Mol. Catal. A Chem. 2017, 426, 190–197. [Google Scholar] [CrossRef]
- Ai, Q.; Yuan, Z.; Huang, R.; Yang, C.; Jiang, G.; Xiong, J.; Huang, Z.; Yuan, S. One-pot co-precipitation synthesis of Fe3O4 nanoparticles embedded in 3D carbonaceous matrix as anode for lithium ion batteries. J. Mater. Sci. 2019, 54, 4212–4224. [Google Scholar] [CrossRef]
- Chen, X.; Pang, X.; Fauteux-Lefebvre, C. The base versus tip growth mode of carbon nanotubes by catalytic hydrocarbon cracking: Review, challenges and opportunities. Carbon Trends 2023, 12, 100273–100289. [Google Scholar] [CrossRef]
- Seo, H.J. Effect of Pt as a Promoter in Decomposition of CH4 to Hydrogen over Pt(1)-Fe(30)/MCM-41 catalyst. Appl. Chem. Eng. 2023, 4, 674–678. [Google Scholar]
- Zhu, W.Z.; Miser, D.E.; Chan, W.G.; Hajaligol, M.R. Characterization of multiwalled carbon nanotubes prepared by carbon arc cathode deposit. Mater. Chem. Phys. 2003, 82, 638–647. [Google Scholar] [CrossRef]
- Serrano, D.P.; Botas, J.A.; Fierro, J.L.G.; Guil-Lŏpez, R.; Pizarro, P.; Gŏmez, G. Hydrogen production by methane decomposition: Origin of the catalytic activity of carbon materials. Fuel 2010, 89, 1241–1248. [Google Scholar] [CrossRef]
- Chen, Y.; Riu, D.-H.; Lim, Y.-S. Carbon nanotubes grown over Fe-Mo-Mg-O composite catalysts. Met. Mater. Int. 2008, 14, 385–390. [Google Scholar] [CrossRef]
- Gohier, A.; Ewels, C.P.; Minea, T.M.; Djouadi, M.A. Carbon nanotube growth mechanism switches from tip- to base-growth with decreasing catalyst particle size. Carbon 2008, 46, 1331–1338. [Google Scholar] [CrossRef]
- AL-Fatesh, A.S.; Amin, A.; Ibrahim, A.A.; Khan, W.U.; Soliman, M.A.; AL-Otaibi, R.L.; Fakeeha, A.H. Effect of Ce and Co Addition to Fe/Al2O3 for Catalytic Methane Decomposition. Catalysts 2016, 6, 40. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spetroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and non-adiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Lespade, P.; Marchand, A.; Couzi, M.; Cruege, F. Caracterisation de materiaux carbones par microspectrometrie Raman. Carbon 1984, 22, 375–385. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, X.; Luo, Z.; Liu, F.; Ye, Y.; Yin, W.; Liu, W.; Han, Y. Carbon nanotube synthesis and parametric study using CaCO3 nanocrystals as catalyst support by CVD. Mater. Chem. Phys. 2006, 95, 5–11. [Google Scholar] [CrossRef]









| Fresh Catalyst | Core Electron Levels | Atomic % |
|---|---|---|
| Pt(1)-Fe(30)/MCM-41 | Pt4f | 1.51 |
| Fe2p | 16.14 | |
| Si2p | 82.34 | |
| Fe(30)/MCM-41 | Fe2p | 13.39 |
| Si2p | 86.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.J. Catalytic Decomposition of CH4 to Hydrogen and Carbon Nanotubes Using the Pt(1)-Fe(30)/MCM-41 Catalyst. Catalysts 2024, 14, 282. https://doi.org/10.3390/catal14040282
Seo HJ. Catalytic Decomposition of CH4 to Hydrogen and Carbon Nanotubes Using the Pt(1)-Fe(30)/MCM-41 Catalyst. Catalysts. 2024; 14(4):282. https://doi.org/10.3390/catal14040282
Chicago/Turabian StyleSeo, Ho Joon. 2024. "Catalytic Decomposition of CH4 to Hydrogen and Carbon Nanotubes Using the Pt(1)-Fe(30)/MCM-41 Catalyst" Catalysts 14, no. 4: 282. https://doi.org/10.3390/catal14040282