Enhancing Oxygen Evolution Reaction with Two-Dimensional Nickel Oxide on Au (111)
Abstract
:1. Introduction
2. Results
2.1. Preparation and Characterization of NiOx/Au (111) Surfaces
2.2. OER Activity of NiOx/Au (111) Catalysts
2.3. Computational Studies
3. Materials and Methods
3.1. Model Catalyst Preparation and Characterization
3.2. Electrochemical Measurements
3.3. Computational Details
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, W.H.; Nong, H.N.; Choi, C.H.; Chae, K.H.; Hwang, Y.J.; Min, B.K.; Strasser, P.; Oh, H.-S. Carbon-supported IrCoOx nanoparticles as an efficient and stable OER electrocatalyst for practicable CO2 electrolysis. Appl. Catal. B 2020, 269, 118820. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Chang, K.-C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, T.; Duan, J.; Jiang, L.; Hu, X.; Zhao, H.; Zhu, J.; Chen, S.; Wang, X. Two-dimensional nanomesh arrays as bifunctional catalysts for N2 electrolysis. ACS Catal. 2020, 10, 11371–11379. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Bai, L.; Moysiadou, A.; Lee, S.; Hu, C.; Liardet, L.; Hu, X. Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: An application-inspired renaissance. J. Am. Chem. Soc. 2018, 140, 7748–7759. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef] [PubMed]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Diaz Morales, O.; Ferrus Suspedra, D.; Koper, M.T.M. The importance of nickel oxyhydroxide deprotonation on its activity towards electrochemical water oxidation. Chem Sci 2016, 7, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Bediako, D.K.; Surendranath, Y.; Nocera, D.G. Mechanistic studies of the oxygen evolution reaction mediated by a nickel–borate thin film electrocatalyst. J. Am. Chem. Soc. 2013, 135, 3662–3674. [Google Scholar] [CrossRef]
- Yeo, B.S.; Bell, A.T. In situ raman study of nickel oxide and gold-supported nickel oxide catalysts for the electrochemical evolution of oxygen. J. Phys. Chem. C 2012, 116, 8394–8400. [Google Scholar] [CrossRef]
- Liu, X.; Ni, K.; Wen, B.; Guo, R.; Niu, C.; Meng, J.; Li, Q.; Wu, P.; Zhu, Y.; Wu, X.; et al. Deep reconstruction of nickel-based precatalysts for water oxidation catalysis. ACS Energy Lett. 2019, 4, 2585–2592. [Google Scholar] [CrossRef]
- Yang, H.Y.; Dai, G.L.; Chen, Z.L.; Wu, J.; Huang, H.; Liu, Y.; Shao, M.W.; Kang, Z.H. Pseudo-periodically coupling Ni-O lattice with Ce-O lattice in ultrathin heteronanowire arrays for efficient water oxidation. Small 2021, 17, 2101727. [Google Scholar] [CrossRef] [PubMed]
- Faisal, F.; Bertram, M.; Stumm, C.; Cherevko, S.; Geiger, S.; Kasian, O.; Lykhach, Y.; Lytken, O.; Mayrhofer, K.J.J.; Brummel, O.; et al. Atomically defined Co3O4(111) thin films prepared in ultrahigh vacuum: Stability under electrochemical conditions. J. Phys. Chem. C 2018, 122, 7236–7248. [Google Scholar] [CrossRef]
- Stumm, C.; Bertram, M.; Kastenmeier, M.; Speck, F.D.; Sun, Z.Z.; Rodríguez-Fernández, J.; Lauritsen, J.V.; Mayrhofer, K.J.J.; Cherevko, S.; Brummel, O.; et al. Structural dynamics of ultrathin cobalt oxide nanoislands under potential control. Adv. Funct. Mater. 2021, 31, 2009923. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Deng, X.Y.; Sorescu, D.C.; Nguyen-Phan, T.D.; Wang, C.J.; Marin, C.M.; Stavitski, E.; Waluyo, I.; Hunt, A. Edge-enhanced oxygen evolution reactivity at ultrathin, Au-supported Fe2O3 electrocatalysts. ACS Catal. 2019, 9, 5375–5382. [Google Scholar] [CrossRef]
- Deng, X.Y.; Sorescu, D.C.; Waluyo, I.; Hunt, A.; Kauffman, D.R. Bulk vs intrinsic activity of NiFeOx electrocatalysts in the oxygen evolution reaction: The influence of catalyst loading, morphology, and support material. ACS Catal. 2020, 10, 11768–11778. [Google Scholar] [CrossRef]
- Hannemann, H.; Ventrice, C.A.; Bertrams, T.; Brodde, A.; Neddermeyer, H. Scanning tunneling microscopy on the growth of ordered NiO layers on Au(111). Phys. Status Solidi A 1994, 146, 289–297. [Google Scholar] [CrossRef]
- Zhao, S.Q.; Lin, L.; Huang, W.G.; Zhang, R.K.; Wang, D.Q.; Mu, R.T.; Fu, Q.; Bao, X.H. Design of lewis pairs via interface engineering of oxide-metal composite catalyst for water activation. J. Phys. Chem. Lett 2021, 12, 1443–1452. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W.; Chen, M.; Wan, H. Reaction of propane with the ordered NiO/Rh(111) studied by XPS and leiss. Appl. Surf. Sci. 2018, 439, 569–576. [Google Scholar] [CrossRef]
- Al-Kuhaili, M.F.; Ahmad, S.H.A.; Durrani, S.M.A.; Faiz, M.M.; Ul-Hamid, A. Application of nickel oxide thin films in NiO/Ag multilayer energy-efficient coatings. Mater. Sci. Semicond. Process. 2015, 39, 84–89. [Google Scholar] [CrossRef]
- Fester, J.; Makoveev, A.; Grumelli, D.; Gutzler, R.; Sun, Z.; Rodríguez-Fernández, J.; Kern, K.; Lauritsen, J.V. The structure of the cobalt oxide/Au catalyst interface in electrochemical water splitting. Angew. Chem. Int. Ed. 2018, 57, 11893–11897. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Kim, D.Y.; Lee, S. Excellent oxygen evolution reaction of activated carbon-anchored NiO nanotablets prepared by green routes. Nanomaterials 2020, 10, 1382. [Google Scholar] [CrossRef] [PubMed]
- Corby, S.; Tecedor, M.-G.; Tengeler, S.; Steinert, C.; Moss, B.; Mesa, C.A.; Heiba, H.F.; Wilson, A.A.; Kaiser, B.; Jaegermann, W.; et al. Separating bulk and surface processes in NiOx electrocatalysts for water oxidation. Sustain. Energy Fuels 2020, 4, 5024–5030. [Google Scholar] [CrossRef]
- Zhu, D.; Guo, C.; Liu, J.; Wang, L.; Du, Y.; Qiao, S.Z. Two-dimensional metal–organic frameworks with high oxidation states for efficient electrocatalytic urea oxidation. Chem. Commun. 2017, 53, 10906–10909. [Google Scholar] [CrossRef] [PubMed]
- Yeo, B.S.; Bell, A.T. Enhanced activity of gold-supported cobalt oxide for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 2011, 133, 5587–5593. [Google Scholar] [CrossRef]
- Bajdich, M.; García-Mota, M.; Vojvodic, A.; Nørskov, J.K.; Bell, A.T. Theoretical investigation of the activity of cobalt oxides for the electrochemical oxidation of water. J. Am. Chem. Soc. 2013, 135, 13521–13530. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Xiao, W.; Wu, H.J.; Liu, X.M.; Zang, W.J.; Zhang, H.; Ding, J.; Feng, Y.P.; Pennycook, S.J.; Wang, J. Hollow Mo-doped Cop nanoarrays for efficient overall water splitting. Nano Energy 2018, 48, 73–80. [Google Scholar] [CrossRef]
- Ventrice, C.A.; Bertrams, T.; Hannemann, H.; Brodde, A.; Neddermeyer, H. Stable reconstruction of the polar (111) surface of NiO on Au(111). Phys. Rev. B 1994, 49, 5773–5776. [Google Scholar] [CrossRef]
- Okada, K.; Kotani, A. Multiplet splitting in Ni 2p X-ray photoemission spectra of Ni dihalides. J. Phys. Soc. Jpn. 1991, 60, 772–775. [Google Scholar] [CrossRef]
- Alders, D.; Voogt, F.C.; Hibma, T.; Sawatzky, G.A. Nonlocal screening effects in 2p X-ray photoemission spectroscopy of NiO(100). Phys. Rev. B 1996, 54, 7716–7719. [Google Scholar] [CrossRef]
- Caffio, M.; Cortigiani, B.; Rovida, G.; Atrei, A.; Giovanardi, C.; di Bona, A.; Valeri, S. Ultrathin nickel oxide films grown on Ag(001): A study by XPS, LEIS and LEED intensity analysis. Surf. Sci. 2003, 531, 368–374. [Google Scholar] [CrossRef]
- Peck, M.A.; Langell, M.A. Comparison of nanoscaled and bulk NiO structural and environmental characteristics by XRD, XAFS, and XPS. Chem. Mater. 2012, 24, 4483–4490. [Google Scholar] [CrossRef]
- Roy, C.; Sebok, B.; Scott, S.B.; Fiordaliso, E.M.; Sorensen, J.E.; Bodin, A.; Trimarco, D.B.; Damsgaard, C.D.; Vesborg, P.C.K.; Hansen, O.; et al. Impact of nanoparticle size and lattice oxygen on water oxidation on NiFeOxHy. Nat. Catal. 2018, 1, 820–829. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Li, Z.S.; Li, B.L.; Yu, M.; Yu, C.L.; Shen, P.K. Amorphous metallic ultrathin nanostructures: A latent ultra-high-density atomic-level catalyst for electrochemical energy conversion. Int. J. Hydrogen Energy 2022, 47, 26956–26977. [Google Scholar] [CrossRef]
- Liang, Q.H.; Brocks, G.; Bieberle-Hutter, A. Oxygen evolution reaction (OER) mechanism under alkaline and acidic conditions. J. Phys. Energy 2021, 3, 026001. [Google Scholar] [CrossRef]

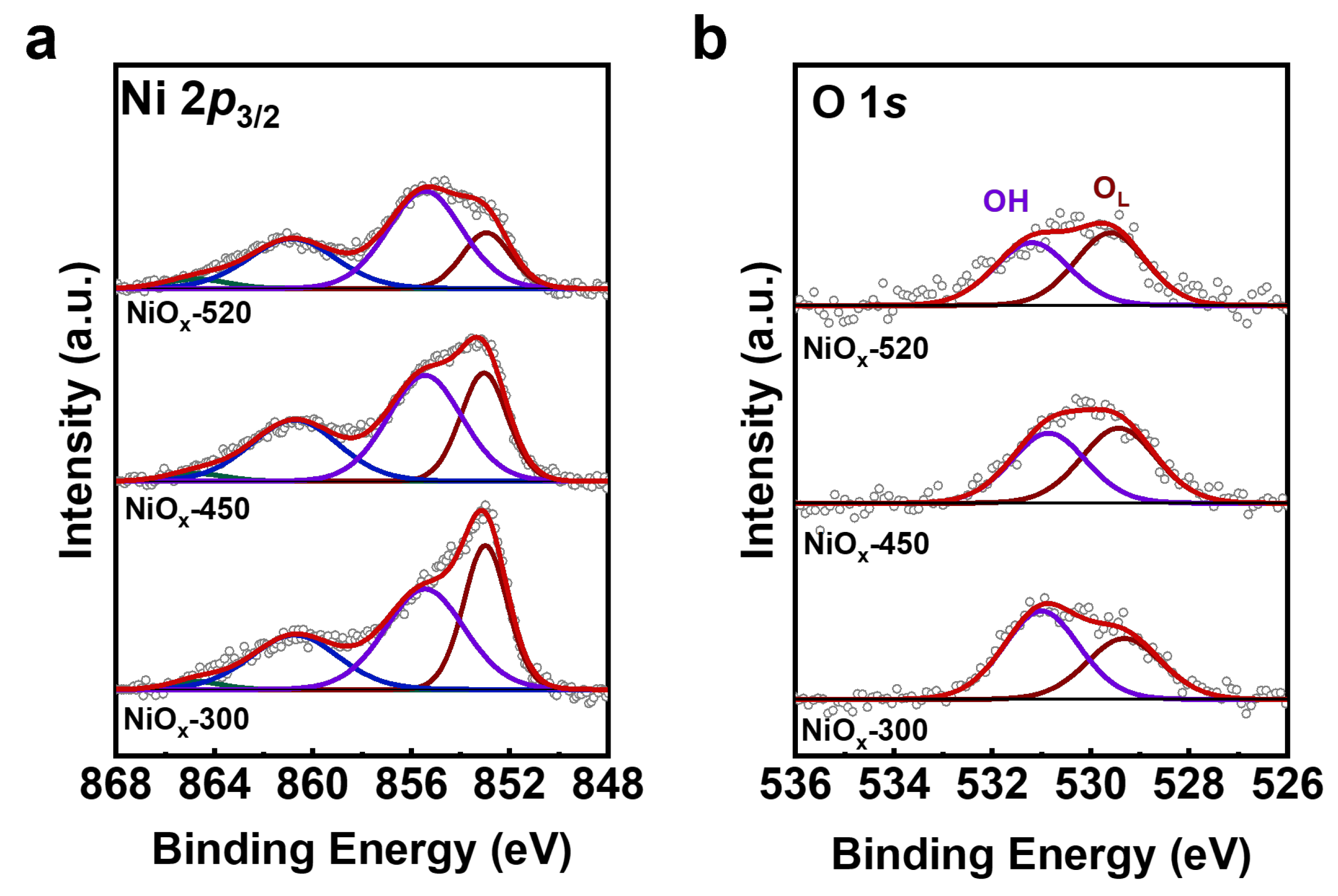
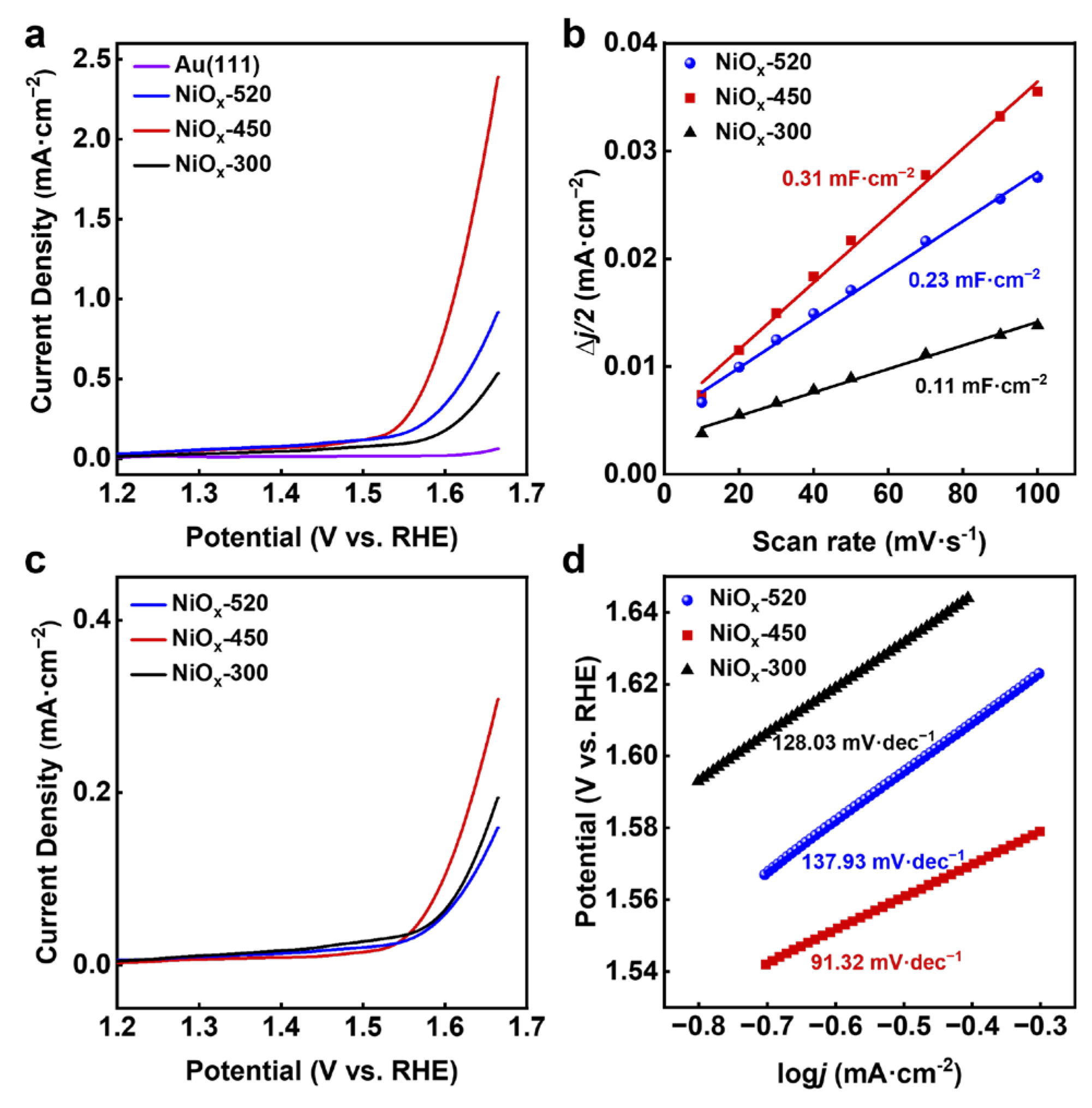
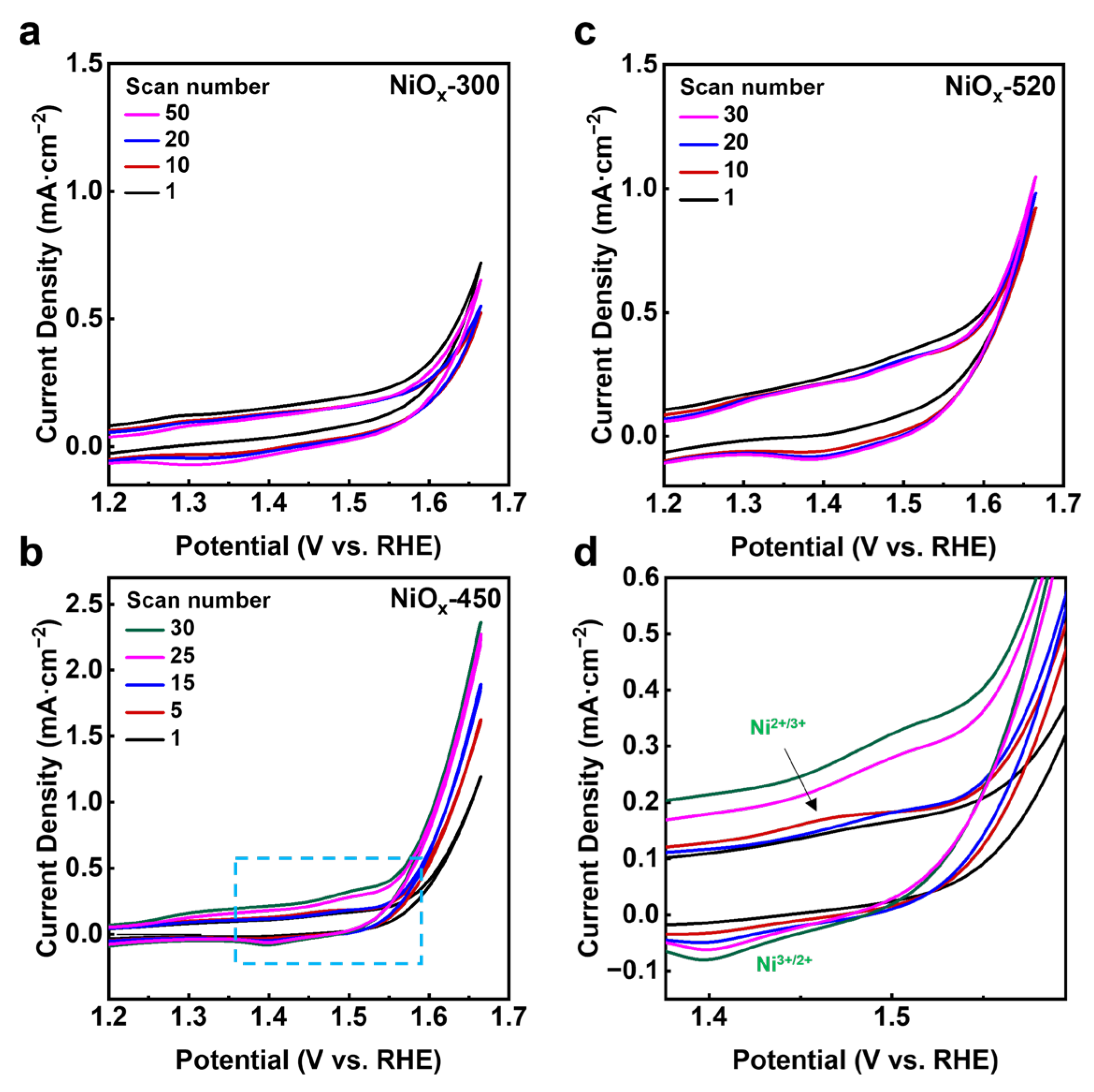
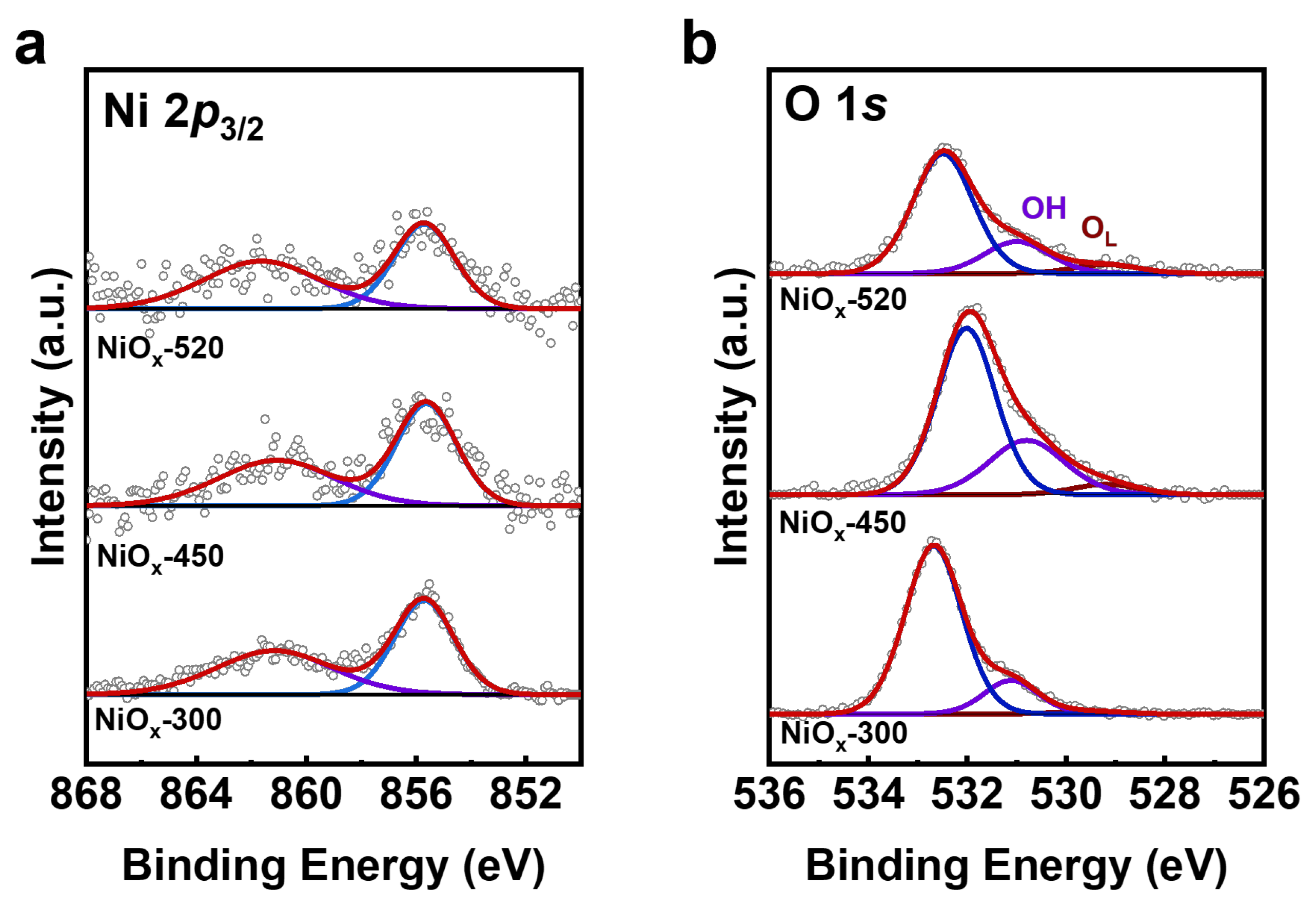

| Catalyst | I(OH)/I(OL) | I (855.2 eV)/I (853.4 eV) |
|---|---|---|
| NiOx-300 | 1.311 | 1.233 |
| NiOx-450 | 1.191 | 2.093 |
| NiOx-520 | 1.185 | 3.038 |
| Catalyst | I (Ni 2p3/2)/I (Au 4f7/2) (Pre-Reaction) | I (Ni 2p3/2)/I (Au 4f7/2) (Post-Reaction) | Current Density at 1.665 V (mA·cm−2) | Normalized Current Density at 1.665 V (mA·cm−2) |
|---|---|---|---|---|
| NiOx-300 | 0.0534 | 0.0252 | 0.533 | 21.15 |
| NiOx-450 | 0.0521 | 0.0305 | 2.390 | 78.36 |
| NiOx-520 | 0.0412 | 0.0235 | 0.917 | 39.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, H.; Wang, R.; Lv, J.; Huang, W.; Guo, C.; Yang, F. Enhancing Oxygen Evolution Reaction with Two-Dimensional Nickel Oxide on Au (111). Catalysts 2024, 14, 284. https://doi.org/10.3390/catal14050284
Zhang H, Zhang H, Wang R, Lv J, Huang W, Guo C, Yang F. Enhancing Oxygen Evolution Reaction with Two-Dimensional Nickel Oxide on Au (111). Catalysts. 2024; 14(5):284. https://doi.org/10.3390/catal14050284
Chicago/Turabian StyleZhang, Handing, Haoyu Zhang, Ruijing Wang, Jiayu Lv, Wugen Huang, Chenyan Guo, and Fan Yang. 2024. "Enhancing Oxygen Evolution Reaction with Two-Dimensional Nickel Oxide on Au (111)" Catalysts 14, no. 5: 284. https://doi.org/10.3390/catal14050284





