Application of Mesoporous/Hierarchical Zeolites as Catalysts for the Conversion of Nitrogen Pollutants: A Review
Abstract
:1. Mesoporous/Hierarchical Zeolites in Catalysis
| Short Description | Treatment/Synthesis Conditions | Remarks | References |
|---|---|---|---|
| Removal of the framework Si. | Treatment with organic or inorganic bases or a combination of both of them in strictly defined conditions (base concentration, process duration, and temperature). | It strongly depends on the Si/Al ratio. Partial amorphisation and crystallinity drop can be observed. Results in a wide pore size distribution. This may lead to Al debris on the surface of the zeolite that can be removed by subsequent acid treatment (‘acid wash’). Simple and cheap. | [27,28,29,30] |
| Removal of framework Al. | Steam treatment (at temp. > 500 °C), acid treatment (also in combination with microwave irradiation), or heat treatment for strictly defined conditions (acid concentration, process duration, and temperature). | Partial amorphisation and crystallinity drop can be observed. Results in a wide pore size distribution. Increases the stability of zeolite steam/heat stability. The steam treatment causes the deposition of extra-framework Al species on the zeolite surface, which can be removed by subsequent acid treatment (‘acid wash’). Simple and cheap. | [29,30,31] |
| Partial dissolution of zeolite and recrystallisation. | Dissolution in alkaline media or alternatively depolymerization with glycerol, followed by recrystallisation under hydrothermal treatment in the presence of meso-directing surfactant. | Zeolite crystals are embedded in the walls of the mesoporous material. Strong zeolitic acidity can be preserved. | [15,32,33] |
| Delamination or pillaring. | Expansion of the space between layers by additional surfactants and modification of the alignment of sheets by delamination (acidification and ultrasound treatment) or pillarization (e.g., SiO2, TiO2, or Al2O3 pillars). | It can be applied only to layered zeolites. Mesopores are generated between the stacked layers. Demanding, laborious, with few step modifications. | [34,35,36] |
| During the synthesis, despite the zeolite SDA, a second template of mesopores is also used. | Addition of a soft mesopore template during zeolite synthesis, such as surfactants, polymers, and organosilanes. | Known also as the dual-template method. The mesopore template is removed by calcination. Often, specially designed templates (not available commercially), make the process complex and costly. | [37,38,39] |
| The zeolitic phase crystallises around the hard (solid) template (porogen). | Addition of a hard mesopore template (e.g., silica, carbon, starch, and polystyrene) in the form of particles, fibres, or nanotubes. | Known also as the dual-template method. Easy control of mesopore sizes. The hard template has to be removed by dissolution, burning (often requires high temperatures), or consumed during the synthesis. | [39,40,41,42] |
| Crystallisation under controlled conditions. | Self-assembly of crystals to create a mesoporous structure under controlled conditions of the nucleation, crystal growth, and aggregation stages. | Easy, one-step synthesis method without the use of mesopore structure-directing agent (SDA), which makes it economically and environmentally friendly. | [39,42,43,44,45] |
| Size of the zeolite crystals in the nanometric range. | Synthesis of nanocrystals with the use of zeolite size-confining methods (seed-induced methods, use of microwaves, or ultrasonic irradiation), or reduction in the crystal size by milling. | Superior properties are characteristic of nanomaterials resulting from the high ratio between the external surface area of grains and their volume (short diffusion paths, enhanced accessibility to active sites, etc.). | [46,47] |
| Recrystallisation of mesoporous material in the presence of zeolite SDA. | Hydrothermal treatment of mesoporous material in the presence of a micropore structure-directing agent (SDA). | The risk of mesopore network collapse and formation of single zeolite crystals. | [32,48,49,50] |
| Thin zeolitic shell surrounding a hollow core. | Zeolitic shells can be prepared by the assembly of nanozeolites on a macrotemplate (e.g., polymer beds or mesoporous silica spheres), followed by hydrothermal and thermal treatment. | The size of macropores can be modified depending on the diameter of the macrotemplate. The mechanical stability depends on the thickness of the zeolitic shell. | [51,52,53] |
| Application of 3D printing technology to construct hierarchical zeolites. | Formation of hierarchical zeolite by 3D printing technology without the use of a binder. | Precise control of connectivity, proportion, and distribution of micro- and mesopores. | [54,55,56] |
2. Selective Catalytic Reduction of NOx with Ammonia (NH3-SCR)
3. Selective Catalytic Oxidation of Ammonia (NH3-SCO)
4. N2O Decomposition
5. Other Reactions
6. Summary
7. Future Perspectives and Challenges
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Weissenberger, T.; Machoke, A.G.F.; Reiprich, B.; Schwieger, W. Preparation and Potential Catalytic Applications of Hierarchically Structured Zeolites with Macropores. Adv. Mater. Interfaces 2021, 8, 2001653–2001672. [Google Scholar] [CrossRef]
- Kerstens, D.; Smeyers, B.; Van Waeyenberg, J.; Zhang, Q.; Yu, J.; Sels, B.F. State of the Art and Perspectives of Hierarchical Zeolites: Practical Overview of Synthesis Methods and Use in Catalysis. Adv. Mater. 2020, 32, 2004690–2004737. [Google Scholar] [CrossRef] [PubMed]
- Vu, X.H.; Armbruster, U.; Martin, A. Micro/Mesoporous Zeolitic Composites: Recent Developments in Synthesis and Catalytic Applications. Catalysts 2016, 6, 183–206. [Google Scholar] [CrossRef]
- Hartmann, M.; Thommes, M.; Schwieger, M. Hierarchically-Ordered Zeolites: A Critical Assessment. Adv. Mater. Interfaces 2021, 8, 2001841–2001879. [Google Scholar] [CrossRef]
- Liu, Z.; Hua, Y.; Wang, J.; Dong, X.; Tian, Q.; Han, Y. Recent progress in the direct synthesis of hierarchical zeolites: Synthetic strategies and characterization methods. Mater. Chem. Front. 2017, 1, 2195–2212. [Google Scholar] [CrossRef]
- Lopez-Orozco, S.; Inayat, A.; Schwab, A.; Selvam, T.; Schwieger, W. Zeolitic materials with hierarchical porous structures. Adv. Mater. 2011, 23, 2602–2615. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Parmentier, T.E.; De Jong, K.P.; Zečević, J. Tailoring and visualizing the pore architecture of hierarchical zeolites. Chem. Soc. Rev. 2015, 44, 7234–7261. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Kim, Y.; Kwon, S.; Na, K. Synthesis of Mesoporous Zeolites and Their Opportunities in Heterogeneous Catalysis. Catalysts 2021, 11, 1541. [Google Scholar] [CrossRef]
- Farsana, O.P.; Kumari, P. Hierarchical Assembly of Zeolites: A Present Scenario. Eng. Sci. 2023, 21, 781. [Google Scholar]
- Degnan, T.F. Applications of zeolites in petroleum refining. Top. Catal. 2000, 13, 349–356. [Google Scholar] [CrossRef]
- Verboekend, D.; Vilé, G.; Pérez-Ramírez, J. Hierarchical Y and USY Zeolites Designed by Post-Synthetic Strategies. Adv. Funct. Mater. 2012, 22, 916–928. [Google Scholar] [CrossRef]
- García-Martínez, J.; Li, K.; Krishnaiah, G. A mesostructured Y zeolite as a superior FCC catalyst—From lab to refinery. Chem. Commun. 2012, 48, 11841–11843. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Valla, J.; García-Martínez, J. Realizing the Commercial Potential of Hierarchical Zeolites: New Opportunities in Catalytic Cracking. ChemCatChem 2014, 6, 46–66. [Google Scholar] [CrossRef]
- Čejka, J.; Mintova, S. Perspectives of Micro/Mesoporous Composites in Catalysis. Catal. Rev. 2007, 49, 457–509. [Google Scholar] [CrossRef]
- Ivanova, I.I.; Knyazeva, E.E. Micro-mesoporous materials obtained by zeolite recrystallization: Synthesis, characterization and catalytic applications. Chem. Soc. Rev. 2013, 42, 3671–3688. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Chmielarz, L. Advances in synthesis of zeolitic materials with hierarchical porous structure for application in environmental catalysis. In Advances in Materials Science Research; NOVA Science Publisher: Hauppauge, NY, USA, 2016; Volume 22, ISBN 978-1-63483-759-0. [Google Scholar]
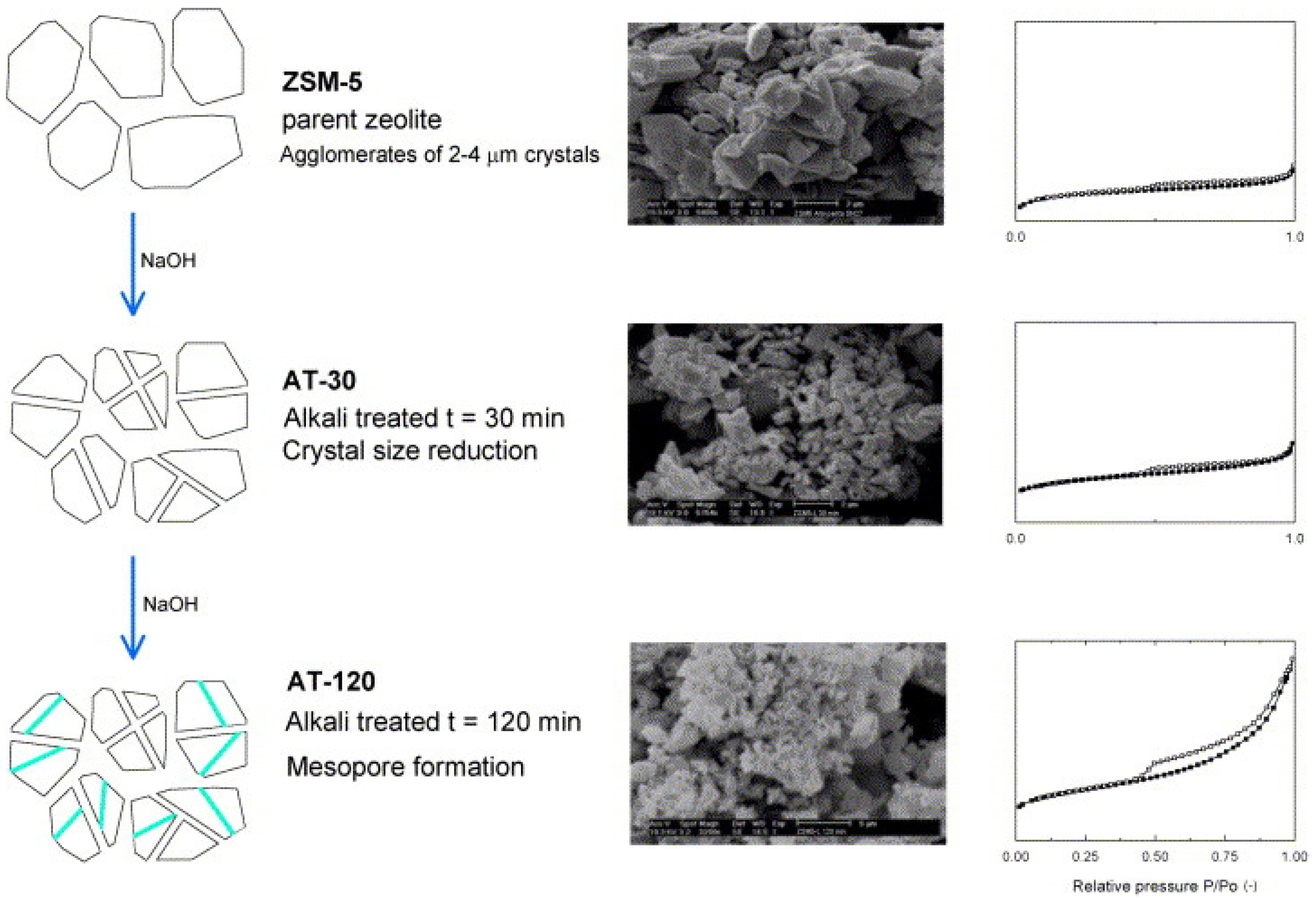
- Rutkowska, M.; Macina, D.; Piwowarska, Z.; Gajewska, M.; Díaz, U.; Chmielarz, L. Hierarchically structured ZSM-5 obtained by optimized mesotemplate-free method as active catalyst for methanol to DME conversion. Catal. Sci. Technol. 2016, 6, 4849–4862. [Google Scholar] [CrossRef]
- Rutkowska, M.; Macina, D.; Mirocha-Kubień, N.; Piwowarska, Z.; Chmielarz, L. Hierarchically structured ZSM-5 obtained by desilication as new catalyst for DME synthesis from methanol. Appl. Catal. B Environ. 2015, 174, 336–343. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Kong, F.; Zhou, R. Low-temperature VOCs oxidation performance of Pt/zeolites catalysts with hierarchical pore structure. J. Environ. Sci. 2023, 124, 505–512. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Shi, Y.; Zhou, R. Synergistic effect of Pt nanoparticles and micro-mesoporous ZSM-5 in VOCs low-temperature removal. J. Environ. Sci. 2021, 107, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.S.; Taarning, E.; Egeblad, K.; Christensen, C.H. Catalysis with hierarchical zeolites. Catal. Today 2011, 168, 3–16. [Google Scholar] [CrossRef]
- Aguirre-Cruz, G.; Legorreta-Garcia, F.; Aguirre-Cruz, G.; Stanciu, L.; Aguirre-Alvarez, G. Synthesis of hierarchical silica zeolites for heterogenous catalysis and adsorption. Microporous Mesoporous Mater. 2022, 345, 112274. [Google Scholar] [CrossRef]
- Li, C.; Sun, P.; Li, F. Hierarchical zeolites-confined metal catalysts and their enhanced catalytic performances. Chem. Asian J. 2021, 16, 2795–2805. [Google Scholar] [CrossRef] [PubMed]
- Mardiana, S.; Azhari, N.J.; Ilmi, T.; Kadja, G.T.M. Hierarchical zeolite for biomass conversion to biofuel: A review. Fuel 2022, 309, 122119. [Google Scholar] [CrossRef]
- Martins, A.; Nunes, N.; Carvalho, A.P.; Martins, L.M.D.R.S. Zeolites and related materials as catalyst supports for hydrocarbon oxidation reactions. Catalysts 2022, 12, 154. [Google Scholar] [CrossRef]
- Miao, C.; Wang, L.; Zhou, S.; Yu, D.; Zhang, C.; Gao, S.; Yu, X.; Zhao, Z. Preparation of Mesoporous Zeolites and Their Applications in Catalytic Elimination of Atmospheric Pollutants. Catalysts 2024, 14, 75. [Google Scholar] [CrossRef]
- Verboekend, D.; Pérez-Ramírez, J. Design of hierarchical zeolite catalysts by desilication. Catal. Sci. Technol. 2011, 1, 879–890. [Google Scholar] [CrossRef]
- Groen, J.C.; Moulijn, J.A.; Pérez-Ramírez, J. Desilication: On the controlled generation of mesoporosity in MFI zeolites. J. Mater. Chem. 2006, 16, 2121–2131. [Google Scholar] [CrossRef]
- Silaghi, M.-C.; Chizallet, C.; Raybaud, P. Challenges on molecular aspects of dealumination and desilication of zeolites. Microporous Mesoporous Mater. 2014, 191, 82–96. [Google Scholar] [CrossRef]
- Qin, Z.; Gilson, J.-P.; Valtchev, V. Mesoporous zeolites by fluoride etching. Curr. Opin. Chem. Eng. 2015, 8, 1–6. [Google Scholar] [CrossRef]
- Müller, M.; Harvey, G.; Prins, R. Comparison of the dealumination of zeolites beta, mordenite, ZSM-5 and ferrierite by thermal treatment, leaching with oxalic acid and treatment with SiCl4 by 1H, 29Si and 27Al MAS NMR. Microporous Mesoporous Mater. 2000, 34, 135–147. [Google Scholar] [CrossRef]
- Mumtaz, F.; Irfan, M.F.; Usman, M.R. Synthesis methods and recent advances in hierarchical zeolites: A brief review. J. Iran. Chem. Soc. 2021, 18, 2215–2229. [Google Scholar] [CrossRef]
- De Oliveira Jardim, E.; Serrano, E.; Martínez, J.C.; Linares, N.; García-Martínez, J. Consecutive surfactant-templating opens up new possibilities for hierarchical zeolites. Cryst. Growth Des. 2020, 20, 515–520. [Google Scholar] [CrossRef]
- Roth, W.J.; Gil, B.; Makowski, W.; Marszalek, B.; Eliášová, P. Layer like porous materials with hierarchical structure. Chem. Soc. Rev. 2016, 45, 3400–3438. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Čejka, J. Two-dimensional zeolites: Current status and perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef]
- Ramos, F.S.O.; Pietre, M.K.; Pastore, H.O. Lamellar zeolites: An oxymoron? RSC Adv. 2013, 3, 2084–2111. [Google Scholar] [CrossRef]
- Hong, M.; Dong, L.; Yang, S. Organic Mesopore Generating Agents (OMeGAs) for Hierarchical Zeolites: Combining Functions on Multiple Scales. ChemNanoMat 2019, 5, 869–877. [Google Scholar] [CrossRef]
- Sachse, A.; García-Martínez, J. Surfactant-Templating of Zeolites: From Design to Application. Chem. Mater. 2017, 29, 3827–3853. [Google Scholar] [CrossRef]
- Egeblad, K.; Christensen, C.H.; Kustova, M.; Christensen, C.H. Templating mesoporous zeolites. Chem. Mater. 2008, 20, 946–960. [Google Scholar] [CrossRef]
- Janssen, A.H.; Schmidt, I.; Jacobsen, C.J.H.; Koster, A.J.; de Jong, K.P. Exploratory study of mesopore templating with carbon during zeolite synthesis. Microporous Mesoporous Mater. 2003, 65, 59–75. [Google Scholar] [CrossRef]
- Zhu, K.; Egeblad, K.; Christensen, C.H. Tailoring the porosity of hierarchical zeolites by carbon-templating. Stud. Surf. Sci. Catal. 2008, 174, 285–288. [Google Scholar]
- Demikhova, N.R.; Rubtsova, M.I.; Kireev, G.A.; Cherednichenko, K.A.; Vinokurov, V.A.; Glotov, A.P. Micro-mesoporous catalysts based on ZSM-5 zeolite synthesized from natural clay nanotubes: Preparation and application in the isomerization of C-8 aromatic fraction. Chem. Eng. J. 2023, 453, 139581. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, M.; Li, S.; Yang, C.; Shi, L.; Zhou, S.; Yu, G.; Ge, L.; Yu, X.; Liu, Z.; et al. Gamma-ray irradiation to accelerate crystallization of mesoporous zeolites. Angew. Chem. Int. Ed. 2020, 59, 11325–11329. [Google Scholar] [CrossRef]
- Maghfirah, A.; Ilmi, M.M.; Fajar, A.T.N.; Kadja, G.T.M. A review on the green synthesis of hierarchically porous zeolite. Mater. Today Chem. 2020, 17, 100348–100369. [Google Scholar] [CrossRef]
- Shestakova, D.O.; Sashkina, K.A.; Parkhomchuk, E.V. Template-Free Synthesis of Hierarchical Zeolite ZSM-5. Pet. Chem. 2019, 59, 838–844. [Google Scholar] [CrossRef]
- Wakihara, T.; Tatami, J. Top-down tuning of nanosized zeolites by bead-milling and recrystallization. J. Jpn. Pet. Inst. 2013, 56, 206–213. [Google Scholar] [CrossRef]
- Tosheva, L.; Valtchev, V.P. Nanozeolites: Synthesis, crystallization mechanism, and applications. Chem. Mater. 2005, 17, 2494–2513. [Google Scholar] [CrossRef]
- Fang, Y.; Hu, H. An ordered mesoporous aluminosilicate with completely crystalline zeolite wall structure. J. Am. Chem. Soc. 2006, 128, 10636–10637. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, M.J.; Kooyman, P.J.; Van der Waal, J.C.; Rigutto, M.S.; Peters, J.A.; Van Bekkum, H. Partial transformation of MCM-41 material into zeolites: Formation of nanosized MFI type crystallites. Chem. Mater. 2001, 13, 683–687. [Google Scholar] [CrossRef]
- Huang, L.; Guo, W.; Deng, P.; Xue, Z.; Li, Q. Investigation of Synthesizing MCM-41/ZSM-5 Composites. J. Phys. Chem. B 2000, 104, 2817–2823. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Dong, M.; Fan, S.; Zhao, T.; Wang, J.; Fan, W. Strategies to control zeolite particle morphology. Chem. Soc. Rev. 2019, 48, 885–907. [Google Scholar] [CrossRef]
- Wang, K.; Dong, M.; Li, J.; Liu, P.; Zhang, K.; Wang, J.; Fan, W. Facile fabrication of ZSM-5 zeolite hollow spheres for catalytic conversion of methanol to aromatics. Catal. Sci. Technol. 2017, 7, 560–564. [Google Scholar] [CrossRef]
- Wang, X.D.; Yang, W.L.; Tang, Y.; Wang, Y.J.; Fu, S.K.; Gao, Z. Fabrication of hollow zeolite spheres. Chem. Commun. 2000, 21, 2161–2162. [Google Scholar] [CrossRef]
- Merilaita, N.; Vastamäki, T.; Ismailov, A.; Levänen, E.; Järveläinen, M. Stereolithography as a manufacturing method for a hierarchically porous ZSM-5 zeolite structure with adsorption capabilities. Ceram. Int. 2021, 47, 10742–10748. [Google Scholar] [CrossRef]
- Magzoub, F.; Li, X.; Lawson, S.; Rezaei, F.; Rownaghi, A.A. 3D-printed HZSM-5 and 3D-HZM5@SAPO-34 structured monoliths with controlled acidity and porosity for conversion of methanol to dimethyl either. Fuel 2020, 280, 118628–118634. [Google Scholar] [CrossRef]
- Halevi, O.; Chen, T.-Y.; Lee, P.S.; Magdassi, S.; Hriljac, J.A. Nuclear wastewater decontamination by 3D-Printed hierarchical zeolite monoliths. RSC Adv. 2020, 10, 5766–5776. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, L.; Dziembaj, R. Modified layered silicas as catalysts for conversion of nitrogen pollutants in flue gases—A review. Catalysts 2021, 11, 644. [Google Scholar] [CrossRef]
- Jabłońska, M. Recent progress in the selective catalytic reduction of NOx with NH3 on Cu-SAPO-34 catalysts. Mol. Catal. 2022, 518, 112111. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, Y.; Cavataio, G.; McCabe, R.W.; Fu, L.; Li, J. Characterization of commercial Cu-SSZ-13 and Cu-SAPO-34 catalysts with hydrothermal treatment for NH3-SCR of NOx in diesel exhaust. Chem. Eng. J. 2013, 225, 323–330. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Zhao, Z.; Liao, J.; Chen, C.; Li, Q. Recent progress of metal-exchanged zeolites for selective catalytic reduction of NOx with NH3 in diesel exhaust. Fuel 2021, 305, 121482. [Google Scholar] [CrossRef]
- Shan, Y.; Du, J.; Yu, Y.; Shan, W.; Shi, X.; He, H. Precise control of post-treatment significantly increases hydrothermal stability of in-situ synthesized cu-zeolites for NH3-SCR reaction. Appl. Catal. B Environ. 2020, 266, 118655. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Weng, D.; Si, Z.; Ran, R. Evolution of copper species on Cu/SAPO-34 SCR catalysts upon hydrothermal aging. Catal. Today 2017, 281, 596–604. [Google Scholar] [CrossRef]
- Paolucci, C.; Khurana, I.; Parekh, A.A.; Li, S.; Shih, A.J.; Li, H.; Albarracin-Caballero, J.D.; Yezerets, A.; Miller, J.T.; Delgass, W.N.; et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 2017, 357, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Lian, Z.; Zhang, Y.; Shan, W.; He, H. The promotional effect of H2 reduction treatment on the low-temperature NH3-SCR activity of Cu/SAPO-18. Appl. Surf. Sci. 2019, 483, 536–544. [Google Scholar] [CrossRef]
- Xia, Y.; Zhan, W.; Guo, Y.; Guo, Y.; Lu, G. Fe-Beta zeolite for selective catalytic reduction of NOx with NH3: Influence of Fe content. Chin. J. Catal. 2016, 37, 2069–2078. [Google Scholar] [CrossRef]
- Oord, R.; ten Have, I.C.; Arends, J.M.; Hendriks, F.C.; Schmidt, J.; Lezcano-Gonzalez, I.; Weckhuysen, B.M. Enhanced activity of desilicated Cu-SSZ-13 for the selective catalytic reduction of NOx and its comparison with steamed Cu-SSZ-13. Catal. Sci. Technol. 2017, 7, 3851–3862. [Google Scholar] [CrossRef]
- Rutkowska, M.; Pacia, I.; Basąg, S.; Kowalczyk, A.; Piwowarska, Z.; Duda, M.; Tarach, K.A.; Góra-Marek, K.; Michalik, M.; Díaz, U.; et al. Catalytic performance of commercial Cu-ZSM-5 zeolite modified by desilication in NH3-SCR and NH3-SCO processes. Microporous Mesoporous Mater. 2017, 246, 193–206. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Verboekend, D.; Bonilla, A.; Abelló, S. Zeolite catalysts with tunable hierarchy factor by pore-growth moderators. Adv. Funct. Mater. 2009, 19, 3972–3979. [Google Scholar] [CrossRef]
- Ma, J.; Weng, D.; Wu, X.; Si, Z.; Wu, Z. Highly dispersed iron species created on alkali-treated zeolite for ammonia SCR. Prog. Nat. Sci. 2013, 23, 493–500. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.; Li, J. Design and synthesis of core-shell structured meso-Cu-SSZ-13@mesoporous aluminosilicate catalyst for SCR of NOx with NH3: Enhancement of activity, hydrothermal stability and propene poisoning resistance. Appl. Catal. B Environ. 2016, 195, 48–58. [Google Scholar] [CrossRef]
- Liu, J.; Yu, F.; Liu, J.; Cui, L.; Zhao, Z.; Li, Y.; Wei, Y.; Sun, Q. Synthesis and kinetics investigation of meso-microporous Cu-SAPO-34 catalysts for the selective catalytic reduction of NO with ammonia. J. Environ. Sci. 2016, 48, 45–58. [Google Scholar] [CrossRef]
- Peng, C.; Yan, R.; Peng, H.; Mi, Y.; Liang, J.; Liu, W.; Wang, X.; Song, G.; Wu, P.; Liu, F. One-pot synthesis of layered mesoporous ZSM-5 plus Cu ion-exchange: Enhanced NH3-SCR performance on Cu-ZSM-5 with hierarchical pore structures. J. Hazard. Mater. 2020, 385, 121593. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, Z.; Wang, X.; Tong, Y.; Yuan, F.; Zhu, Y. One-pot synthesis of Cu/SAPO-34 with hierarchical pore using cupric citrate as a copper source for excellent NH3-SCR of NO performance. ChemCatChem 2020, 12, 4871–4878. [Google Scholar] [CrossRef]
- Li, R.; Wang, P.; Ma, S.; Yuan, F.; Li, Z.; Zhu, Y. Excellent selective catalytic reduction of NOx by NH3 over Cu/SAPO-34 with hierarchical pore structure. Chem. Eng. J. 2020, 379, 122376. [Google Scholar] [CrossRef]
- Liang, J.; Tao, J.; Mi, Y.; Liu, W.; Wang, Z.; Li, Z.; Wu, D.; Wu, P.; Peng, H. Unraveling the boosting low-temperature performance of ordered mesoporous Cu-SSZ-13 catalyst for NOx reduction. Chem. Eng. J. 2021, 409, 128238. [Google Scholar] [CrossRef]
- Święs, A.; Kowalczyk, A.; Rutkowska, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. Ferrierite and Its Delaminated and Silica-Intercalated Forms Modified with Copper as Effective Catalysts for NH3-SCR Process. Catalysts 2020, 10, 734. [Google Scholar] [CrossRef]
- Rutkowska, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. Cu and Fe modified derivatives of 2D MWW-type zeolites (MCM-22, ITQ-2 and MCM-36) as new catalysts for DeNOx process. Appl. Catal. B Environ. 2015, 168, 531–539. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, B.; Lv, N.; Wang, T.; Bi, X.; Zhu, H.; Yuan, P.; Bai, Z.; Cui, Q.; Bao, X. Direct synthesis of hierarchical FeCu-ZSM-5 zeolite with wide temperature window in selective catalytic reduction of NO by NH3. ChemCatChem 2019, 11, 4744–4754. [Google Scholar] [CrossRef]
- Jabłońska, M.; Robles, A.M. A Comparative Mini-Review on Transition Metal Oxides Applied for the Selective Catalytic Ammonia Oxidation (NH3-SCO). Materials 2022, 15, 4770. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, L.; Jabłońska, M. Advances in selective catalytic oxidation of ammonia to dinitrogen: A review. RSC Adv. 2015, 5, 43408–43431. [Google Scholar] [CrossRef]
- Lan, T.; Zhao, Y.; Deng, J.; Zhang, J.; Shi, L.; Zhang, D. Selective catalytic oxidation of NH3 over noble metal-based catalysts: State of the art and future prospects. Catal. Sci. Technol. 2020, 10, 5792–5810. [Google Scholar] [CrossRef]
- Jabłońska, M. Progress on Selective Catalytic Ammonia Oxidation (NH3-SCO) over Cu Containing Zeolite-Based Catalysts. ChemCatChem 2020, 12, 4490–4500. [Google Scholar] [CrossRef]
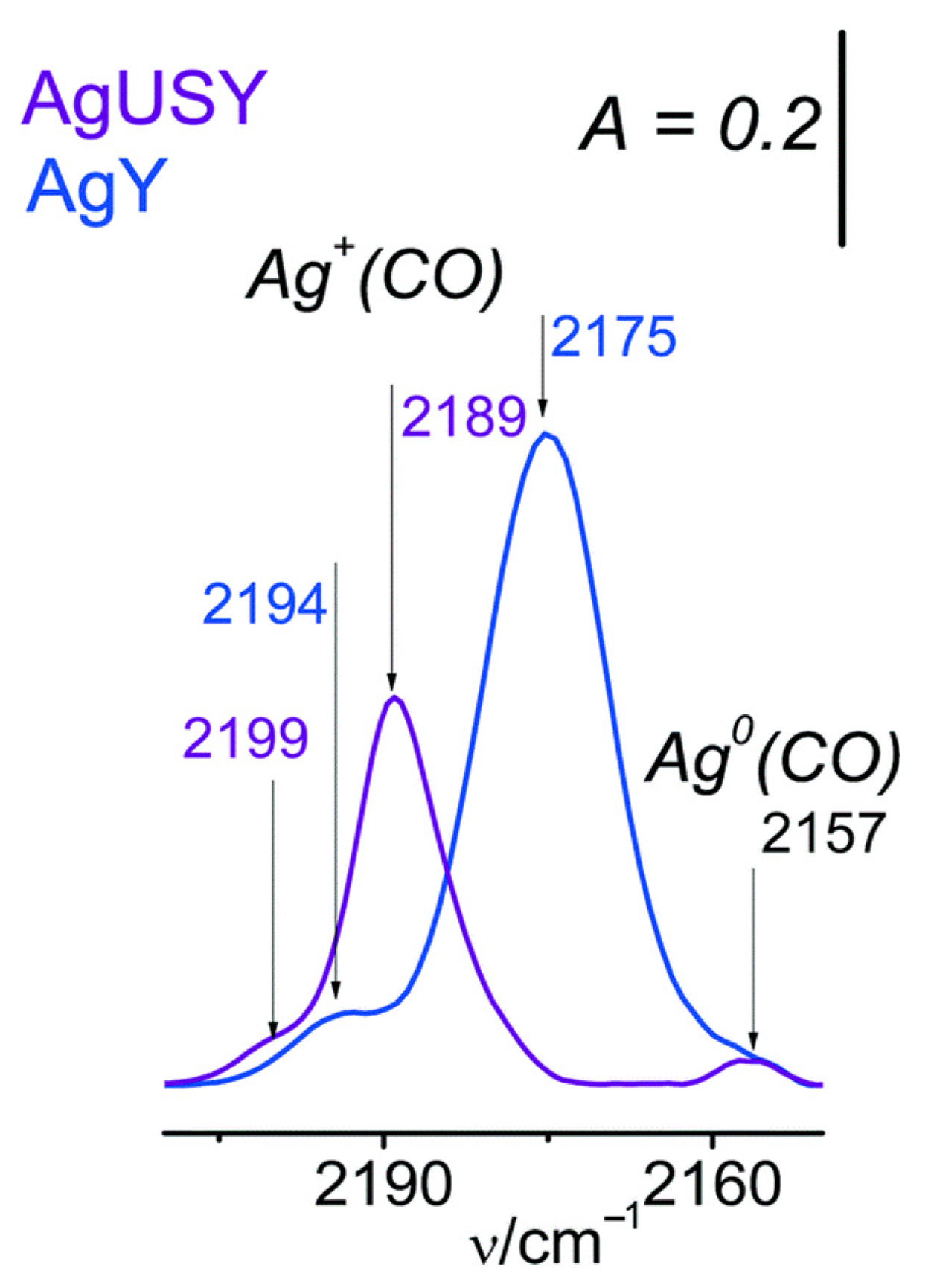
- Góra-Marek, K.; Tarach, K.; Piwowarska, Z.; Łaniecki, M.; Chmielarz, L. Ag-loaded zeolites Y and USY as the catalysts for selective ammonia oxidation. Catal. Sci. Technol. 2016, 6, 1651–1660. [Google Scholar] [CrossRef]
- Góra-Marek, K.; Brylewska, K.; Tarach, K.; Rutkowska, M.; Jabłońska, M.; Choi, M.; Chmielarz, L. IR studies of Fe modified ZSM-5 zeolites of diverse mesopore topologies in the terms of their catalytic performance in NH3-SCR and NH3-SCO processes. Appl. Catal. B Environ. 2015, 179, 589–598. [Google Scholar] [CrossRef]
- Borcuch, A.; Rutkowska, M.; Marzec, A.; Kowalczyk, A.; Michalik, M.; Moreno, J.M.; Díaz, U.; Chmielarz, L. Selective ammonia oxidation over ZSM-5 zeolite: Impact of catalyst’s support porosity and type of deposited iron species. Catal. Today 2020, 348, 223–229. [Google Scholar] [CrossRef]
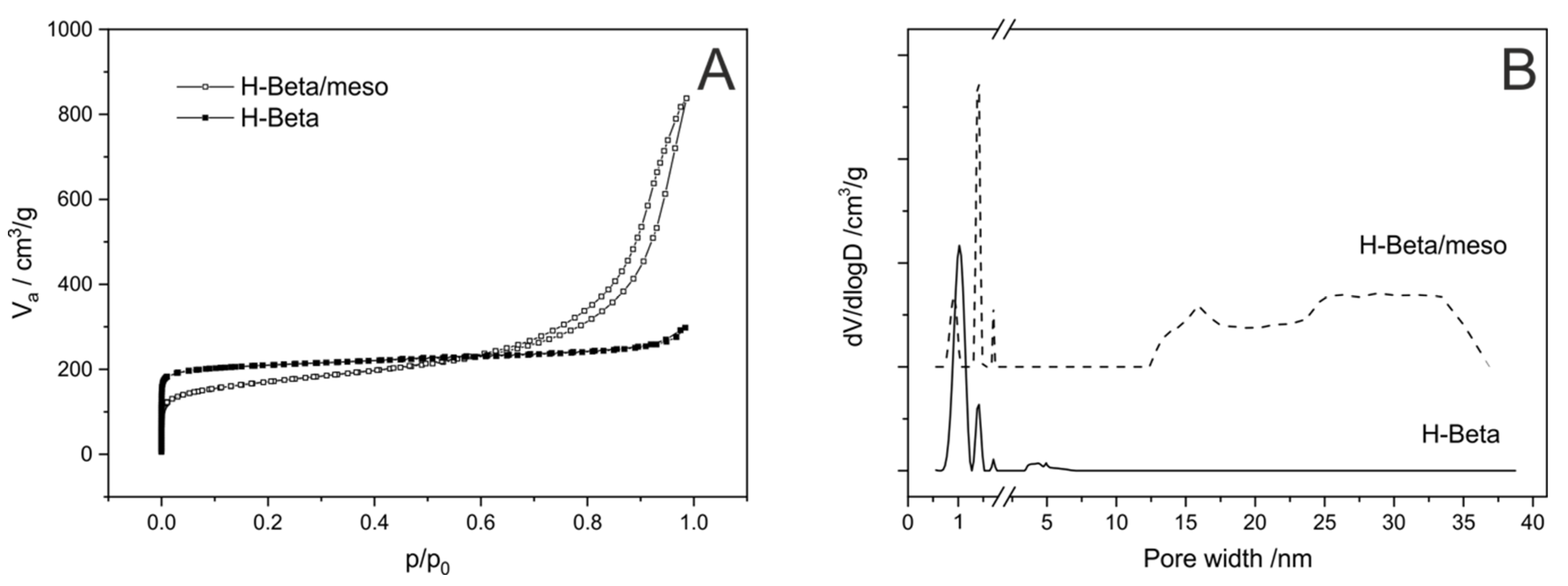
- Rutkowska, M.; Duda, M.; Macina, D.; Górecka, S.; Dębek, R.; Moreno, J.M.; Díaz, U.; Chmielarz, L. Mesoporous Beta zeolite functionalisation with FexCry oligocations; catalytic activity in the NH3-SCO process. Microporous Mesoporous Mater. 2019, 278, 1–13. [Google Scholar] [CrossRef]
- Święs, A.; Rutkowska, M.; Kowalczyk, A.; Díaz, U.; Palomares, A.E.; Chmielarz, L. Ferrierite and its delaminated forms modified with copper as effective catalysts for NH3-SCO process. Materials 2020, 13, 4885. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.; Wang, D.; Hong, Z.; Qu, C.; Li, X. Hollow ZSM-5 zeolite encapsulated Ag nanoparticles for SO2-resistant selective catalytic oxidation of ammonia to nitrogen. Sep. Purif. Technol. 2019, 209, 1016–1026. [Google Scholar] [CrossRef]
- Kapteijn, F.; Rodriguez-Mirasol, J.; Moulijn, J.A. Heterogeneous catalytic decomposition of nitrous oxide. Appl. Catal. B Environ. 1996, 9, 25–64. [Google Scholar] [CrossRef]
- Liu, Z.; He, F.; Ma, L.; Peng, S. Recent advances in catalytic decomposition of N2O on noble metal and metal oxide catalysts. Catal. Surv. Asia 2016, 20, 121–132. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, Y.; Liu, G.; Chen, J.; Peng, Y.; Liu, B.; Li, J. Nature of active Fe species and reaction mechanism over high-efficiency Fe/CHA catalysts in catalytic decomposition of N2O. J. Catal. 2020, 392, 322–335. [Google Scholar] [CrossRef]
- Cao, H.; Yuan, X.; Du, J.; Ren, M.; Wang, Y.; Wang, X.; Shan, Y.; He, H. High-temperature treatment promotes N2O decomposition over Fe-impregnated Al-rich SSZ-13 zeolites. Catal. Today 2024, 433, 114670. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, N.; Lei, Z.; Chen, B. Selective transformation of various nitrogen-containing exhaust gases toward N2 over zeolite catalysts. Chem. Rev. 2016, 116, 3658–3721. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Q.; He, C.; Ma, C.; Cheng, J.; Liu, Z.; Hao, Z. Decomposition of nitrous oxide over Co-zeolite catalysts: Role of zeolite structure and active site. Catal. Sci. Technol. 2012, 2, 1249–1258. [Google Scholar] [CrossRef]
- Melián-Cabrera, I.; Espinosa, S.; Groen, J.C.; v/d Linden, B.; Kapteijn, F.; Moulijn, J.A. Utilizing full-exchange capacity of zeolites by alkaline leaching: Preparation of Fe-ZSM5 and application in N2O decomposition. J. Catal. 2006, 238, 250–259. [Google Scholar] [CrossRef]
- Wu, M.; Chen, X.; Zhong, L.; Wang, H.; Zhang, X.; Shen, Q.; Wei, W.; Sun, Y. Comparison of alkaline pre-treatment of MFI zeolites for N2O decomposition: Different zeolite sources. Greenh. Gases Sci. Technol. 2016, 6, 710–723. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, L.; Wu, M.; He, C.; Wei, W.; Sun, N.; Sun, Y. Postsynthesis of mesoporous ZSM-5 zeolites with TPAOH-assisted desilication and determination of activity performance in N2O decomposition. J. Porous Mater. 2017, 24, 759–767. [Google Scholar] [CrossRef]
- Groen, J.C.; Brückner, A.; Berrier, E.; Maldonado, L.; Moulijn, J.A.; Pérez-Ramírez, J. Iron site modification upon alkaline treatment of Fe-ZSM-5 zeolites—Opportunities for improved N2O decomposition activity. J. Catal. 2006, 243, 212–216. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, L.; Wu, M.; Wang, H.; Sun, N.; Wei, W.; Sun, Y. High-silica nanoflower hierarchical Fe-MFI with excellent catalytic performance for N2O decomposition. Mater. Res. Bull. 2017, 87, 1–5. [Google Scholar] [CrossRef]
- Liu, G.; Zhuang, J.; Yang, F.; Xiao, Q.; Zhong, Y.; Zhu, W. N2O direct decomposition over hierarchical FeZSM-5 catalysts. Adv. Mater. Res. 2012, 518–523, 2593–2596. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Kapteijn, F.; Groen, J.C.; Doménech, A.; Mul, G.; Moulijn, J.A. Steam-activated FeMFI zeolites. Evolution of iron species and activity in direct N2O decomposition. J. Catal. 2003, 214, 33–45. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J. Active iron sites associated with the reaction mechanism of N2O conversions over steam-activated FeMFI zeolites. J. Catal. 2004, 227, 512–522. [Google Scholar] [CrossRef]
- Zou, W.; Xie, P.; Hua, W.; Wang, Y.; Kong, D.; Yue, Y.; Ma, Z.; Yang, W.; Gao, Z. Catalytic decomposition of N2O over Cu-ZSM-5 nanosheets. J. Mol. Catal. A Chem. 2014, 394, 83–88. [Google Scholar] [CrossRef]
- Rutkowska, M.; Chmielarz, L.; Macina, D.; Piwowarska, Z.; Dudek, B.; Adamski, A.; Witkowski, S.; Sojka, Z.; Obalová, L.; Van Oers, C.J.; et al. Catalytic decomposition and reduction of N2O over micro-mesoporous materials containing Beta zeolite nanoparticles. Appl. Catal. B Environ. 2014, 146, 112–122. [Google Scholar] [CrossRef]
- Rutkowska, M.; Piwowarska, Z.; Micek, E.; Chmielarz, L. Hierarchical Fe-, Cu- and Co-Beta zeolites obtained by mesotemplate-free method. Part I: Synthesis and catalytic activity in N2O decomposition. Microporous Mesoporous Mater. 2015, 209, 54–65. [Google Scholar] [CrossRef]
- Rutkowska, M.; Jankowska, A.; Różycka-Dudek, E.; Dubiel, W.; Kowalczyk, A.; Piwowarska, Z.; Llopis, S.; Díaz, U.; Chmielarz, L. Modification of MCM-22 Zeolite and Its Derivatives with Iron for the Application in N2O Decomposition. Catalysts 2020, 10, 1139. [Google Scholar] [CrossRef]
- Jankowska, A.; Kowalczyk, A.; Rutkowska, M.; Mozgawa, W.; Gil, B.; Chmielarz, L. Silica and silica–titania intercalated MCM-36 modified with iron as catalysts for selective reduction of nitrogen oxides—The role of associated reactions. Catal. Sci. Technol. 2020, 10, 7940–7954. [Google Scholar] [CrossRef]
- Kustova, M.Y.; Rasmussen, S.B.; Kustov, A.L.; Christensen, C.H. Direct NO decomposition over conventional and mesoporous Cu-ZSM-5 and Cu-ZSM-11 catalysts: Improved performance with hierarchical zeolites. Appl. Catal. B Environ. 2006, 67, 60–67. [Google Scholar] [CrossRef]
- Konysheva, K.M.; Boichuk, T.M.; Shvets, O.V. Effect of structural, size, and acid characteristics of hierarchical BEA and MOR zeolites on their activity in the catalytic reduction of N2O and NO by propylene. Theor. Exp. Chem. 2016, 52, 90–96. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Kapteijn, F.; Schöffel, K.; Moulijn, J.A. Formation and control of N2O in nitric acid production: Where do we stand today? Appl. Catal. B Environ. 2003, 44, 117–151. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Zhuang, J.; Xiao, Q.; Zhong, Y.; Zhu, W. Direct oxidation of benzene to phenol by N2O over meso-Fe-ZSM-5 catalysts obtained via alkaline post-treatment. Catal. Sci. Technol. 2011, 1, 1250–1255. [Google Scholar] [CrossRef]
- Li, L.; Meng, Q.; Wen, J.; Wang, J.; Tu, G.; Xu, C.; Zhang, F.; Zhong, Y.; Zhu, W.; Xiao, Q. Improved performance of hierarchical Fe-ZSM-5 in the direct oxidation of benzene to phenol by N2O. Microporous Mesoporous Mater. 2016, 227, 252–257. [Google Scholar] [CrossRef]
- Koekkoek, A.J.J.; Kim, W.; Degirmenci, V.; Xin, H.; Ryoo, R.; Hensen, E.J.M. Catalytic performance of sheet-like Fe/ZSM-5 zeolites for the selective oxidation of benzene with nitrous oxide. J. Catal. 2013, 299, 81–89. [Google Scholar] [CrossRef]
- Xin, H.; Koekkoek, A.; Yang, Q.; van Santen, R.; Li, C.; Hensen, E.J.M. A hierarchical Fe/ZSM-5 zeolite with superior catalytic performance for benzene hydroxylation to phenol. Chem. Commun. 2009, 7590–7592. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramírez, J.; Kondratenko, E.V. Steam-activated FeMFI zeolites as highly efficient catalysts for propane and N2O valorisation via oxidative conversions. Chem. Commun. 2003, 2152–2153. [Google Scholar] [CrossRef]
- Wei, W.; Moulijn, J.A.; Mul, G. FAPO and Fe-TUD-1: Promising catalysts for N2O mediated selective oxidation of propane? J. Catal. 2009, 262, 1–8. [Google Scholar] [CrossRef]

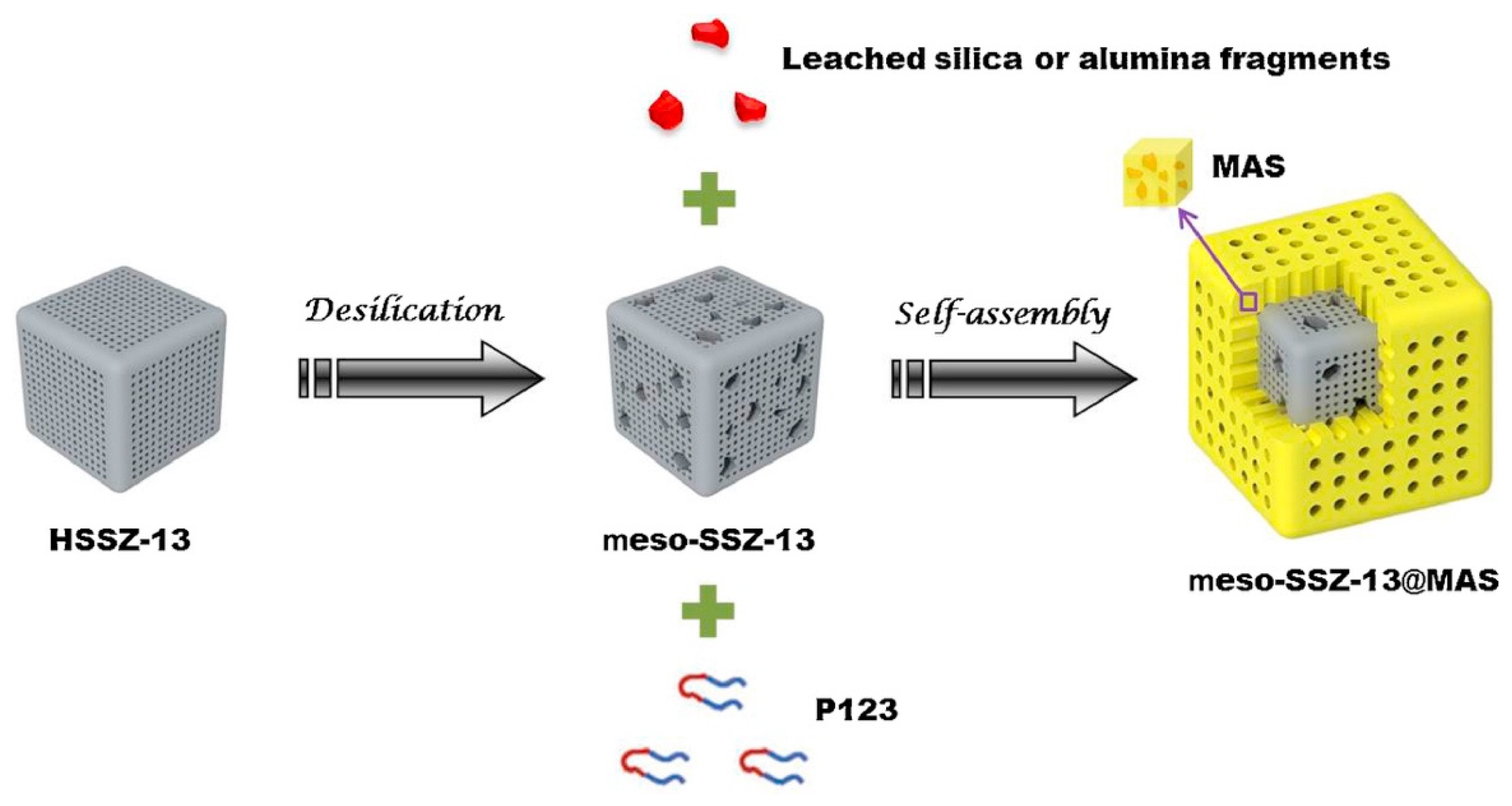
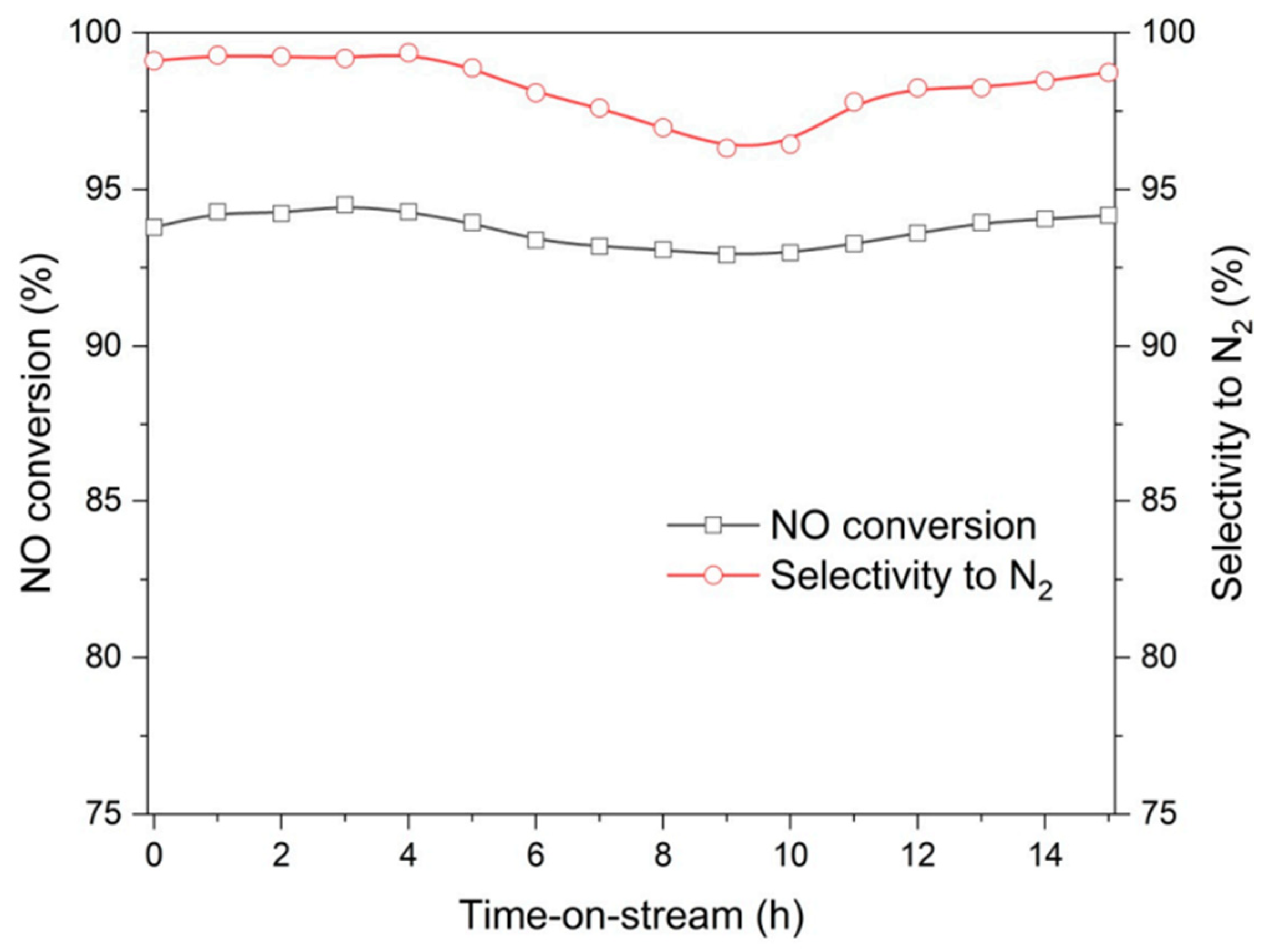
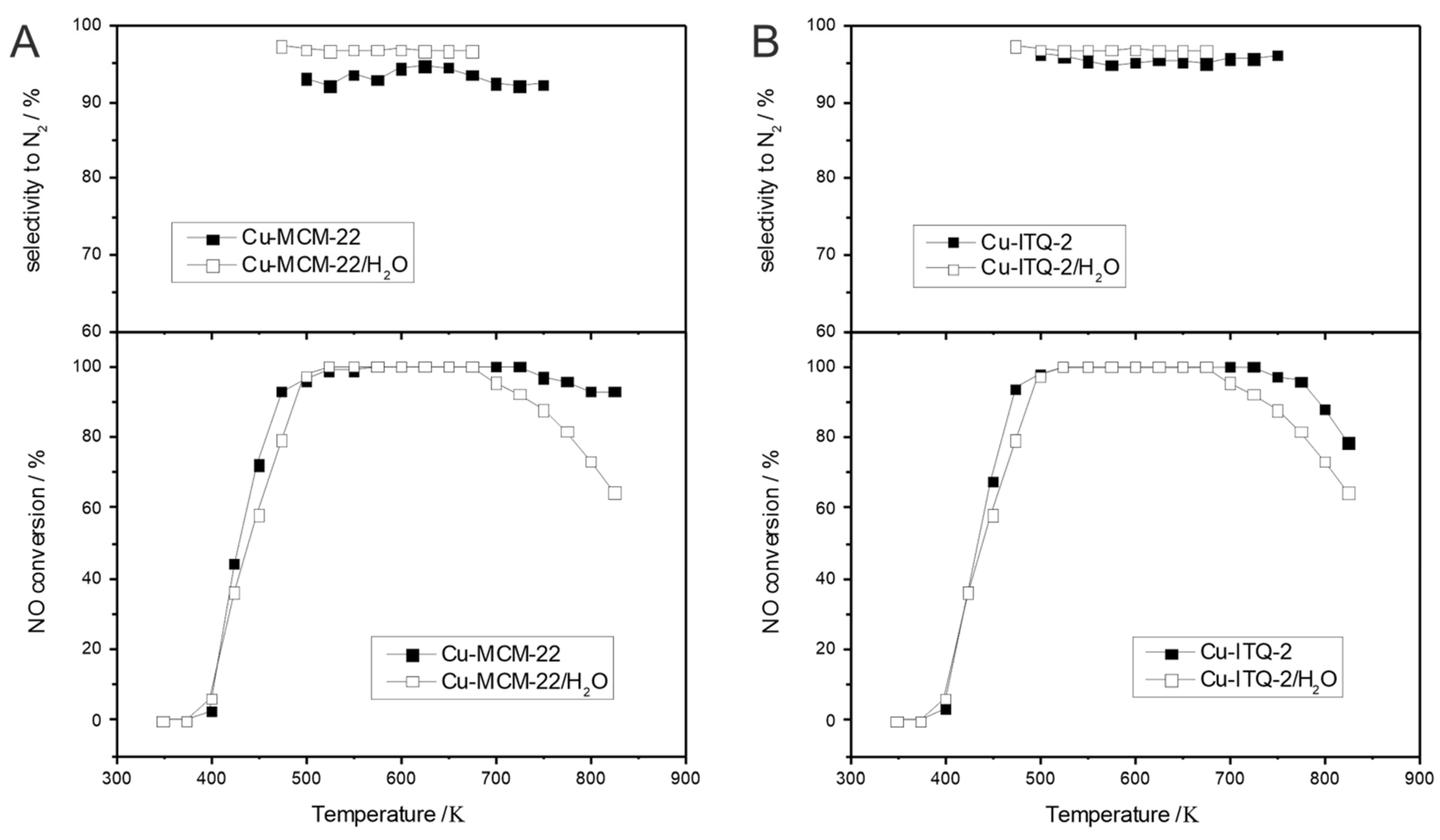





| Topology | Zeolite | Modification | Result | References |
|---|---|---|---|---|
| CHA | Cu-SSZ-13 | Desilication | Increased activity, especially at low temperatures, <200 °C. | [66] |
| CHA | Cu-SSZ-13 | Desilication/core–shell | Increased activity, high copper distribution, better hydrothermal stability, and resistance to propene poisoning. | [70] |
| CHA | Cu-SAPO-34 | Soft-templating | Increased activity, enhanced redox properties, and improved diffusion. | [71] |
| CHA | Cu-SAPO-34 Cu-SSZ-13 | Hard-templating | High activity in a broad temperature range, resistance to the presence of H2O and SO2 and to NH4NO3 formation. Improved reactant diffusion. | [73,74,75] |
| FER | Cu-ITQ-6 Cu-ITQ-36 | Delamination/pillaring | Increased activity, high copper distribution, and very good stability. | [76] |
| MFI | Cu-ZSM-5 | Desilication | Increased activity, higher surface acidity, higher amount of introduced Cu, and enhanced redox properties. | [67] |
| MFI | Cu-ZSM-5 | Soft-templating | Increased activity, enhanced redox properties, better NO adsorption capacity, and good resistance for SO2. | [72] |
| MFI | Fe-ZSM-5 FeCu-ZSM-5 | Desilication, spherical (template-free) | Increased activity, high dispersion of iron. Iron and copper are mainly in the form of isolated cations Fe3+ and Cu2+. | [69,78] |
| MWW | Cu-ITQ-2 Cu-MCM-36 Fe-ITQ-2 | Delamination/pillaring | Higher activity of Cu-modified samples and very good hydrothermal stability. | [77] |
| Topology | Zeolite | Modification | Result | References |
|---|---|---|---|---|
| BEA | FexCry-Beta | Template-free | Slightly increased activity in the case of Cr-modified samples with a simultaneous decrease in selectivity to N2. Lower activity in the case of Fe-modified samples. | [86] |
| FAU | Ag-Y | Dealumination | Increased activity, better dispersion of smaller Ag particles, resistance to the presence of H2O and SO2, and good long-term stability. | [83] |
| FER | Cu-Al-ITQ-6 | Delamination | Increased activity, high selectivity to N2, high dispersion of copper species (good balance between monomeric and aggregated Cu species). | [87] |
| MFI | Ag-ZSM-5 | Hollow | Better long-term stability and resistance against Ag sintering and leaching from the sample. Very good SO2 and H2O resistance. | [88] |
| MFI | Fe-ZSM-5 | Desilication | Increased activity, high dispersion of iron. | [84] |
| MFI | Fe-ZSM-5 | Desilication | Slightly higher NH3 conversion, good stability during 60 h of catalytic reaction, and higher amount of introduced iron. | [85] |
| MFI | Fe-ZSM-5 | Desilication | Lower activity and lower content of oligomeric iron species than in conventional zeolite. | [69] |
| MFI | Fe-ZSM-5 | Soft-templating | Increased activity, high dispersion of iron. | [84] |
| MFI | Cu-ZSM-5 | Desilication | Increased activity and surface acidity, better reducibility of copper species. | [67] |
| Topology | Zeolite | Modification | Result | References |
|---|---|---|---|---|
| BEA | Fe-Beta | Template-free, hard-templating | Improved accessibility to ion exchange positions, and better distribution of iron species. | [105,106] |
| MFI | Cu-ZSM-5 | Nanosheets | Higher activity, better reducibility of Cu+ species. More facile desorption of oxygen. Better stability. | [103] |
| MFI | Fe-ZSM-5 | Desilication | Higher activity, complete ion exchange, and increased accessibility to ion exchange positions. | [95,96,97] |
| MFI | Fe-ZSM-5 | Desilication of Fe-sample | Higher activity, higher concentration of Fe2+, enhanced N2O activation, and better O2 desorption. | [98] |
| MFI | Fe-ZSM-5 | Template-free | Improved diffusion and higher iron loading. Higher activity in comparison to conventional catalysts. | [99] |
| MFI | Fe-ZSM-5 | Soft-templating, spherical | Improved mass transfer, and higher activity in comparison to conventional zeolite. | [100] |
| MFI | Fe-ZSM-5 | Steaming, desilication | Higher activity, higher content of active iron species, and improved mass transport. | [101,102] |
| MWW | Fe-ITQ-2 Fe-MCM-36 | Delamination, intercalation | Lower activity compared to conventional MCM-22 zeolite. Partially destroyed zeolitic structure (lower crystallinity, lower volume of micropores). | [106,107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutkowska, M.; Chmielarz, L. Application of Mesoporous/Hierarchical Zeolites as Catalysts for the Conversion of Nitrogen Pollutants: A Review. Catalysts 2024, 14, 290. https://doi.org/10.3390/catal14050290
Rutkowska M, Chmielarz L. Application of Mesoporous/Hierarchical Zeolites as Catalysts for the Conversion of Nitrogen Pollutants: A Review. Catalysts. 2024; 14(5):290. https://doi.org/10.3390/catal14050290
Chicago/Turabian StyleRutkowska, Małgorzata, and Lucjan Chmielarz. 2024. "Application of Mesoporous/Hierarchical Zeolites as Catalysts for the Conversion of Nitrogen Pollutants: A Review" Catalysts 14, no. 5: 290. https://doi.org/10.3390/catal14050290







