Superior MgH2 Kinetics with MgO Addition: A Tribological Effect
Abstract
:1. Introduction
2. Material and Methods
3. Results and Discussions
3.1. Oxidation Effect on Hydrogen Desorption
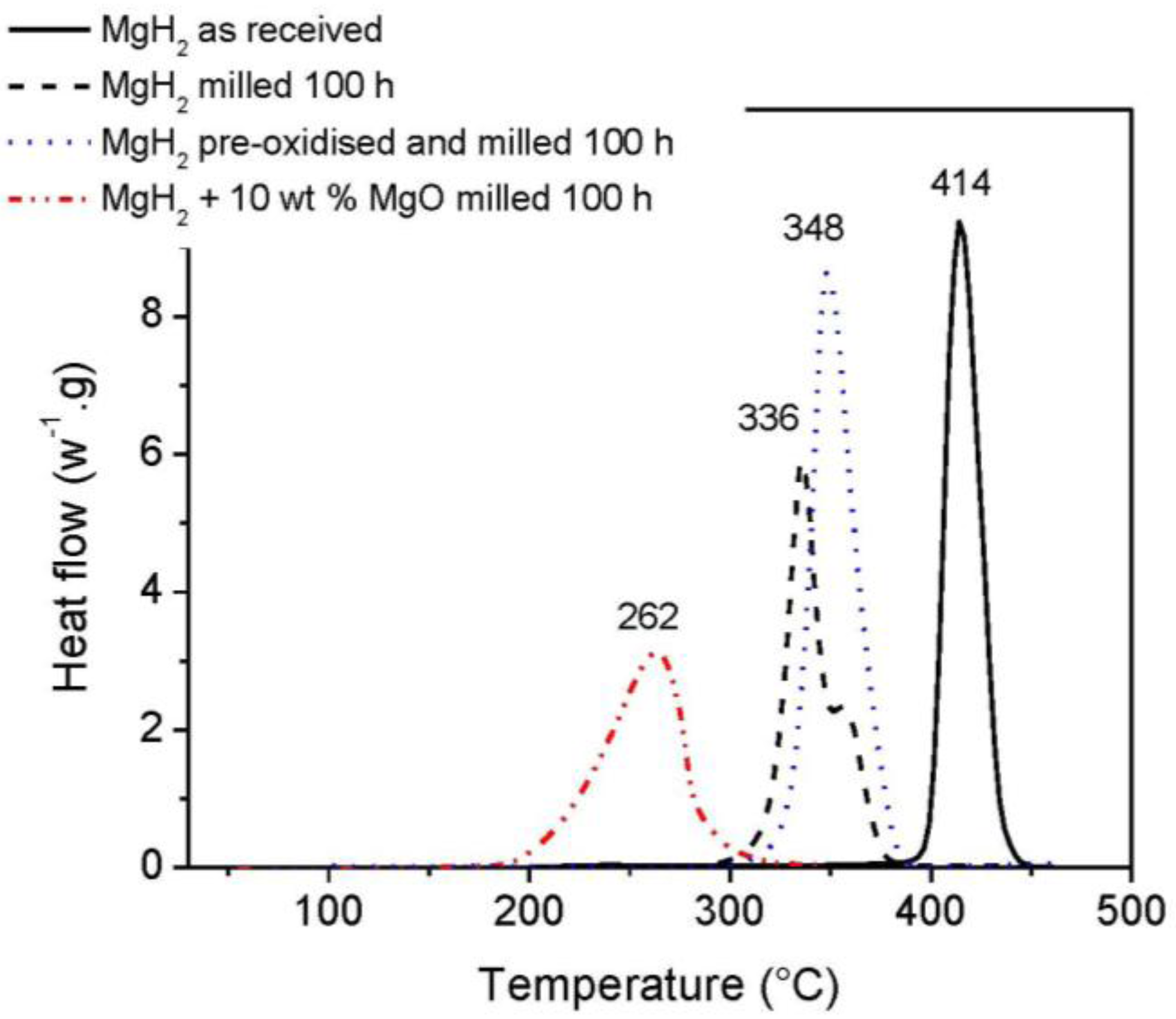
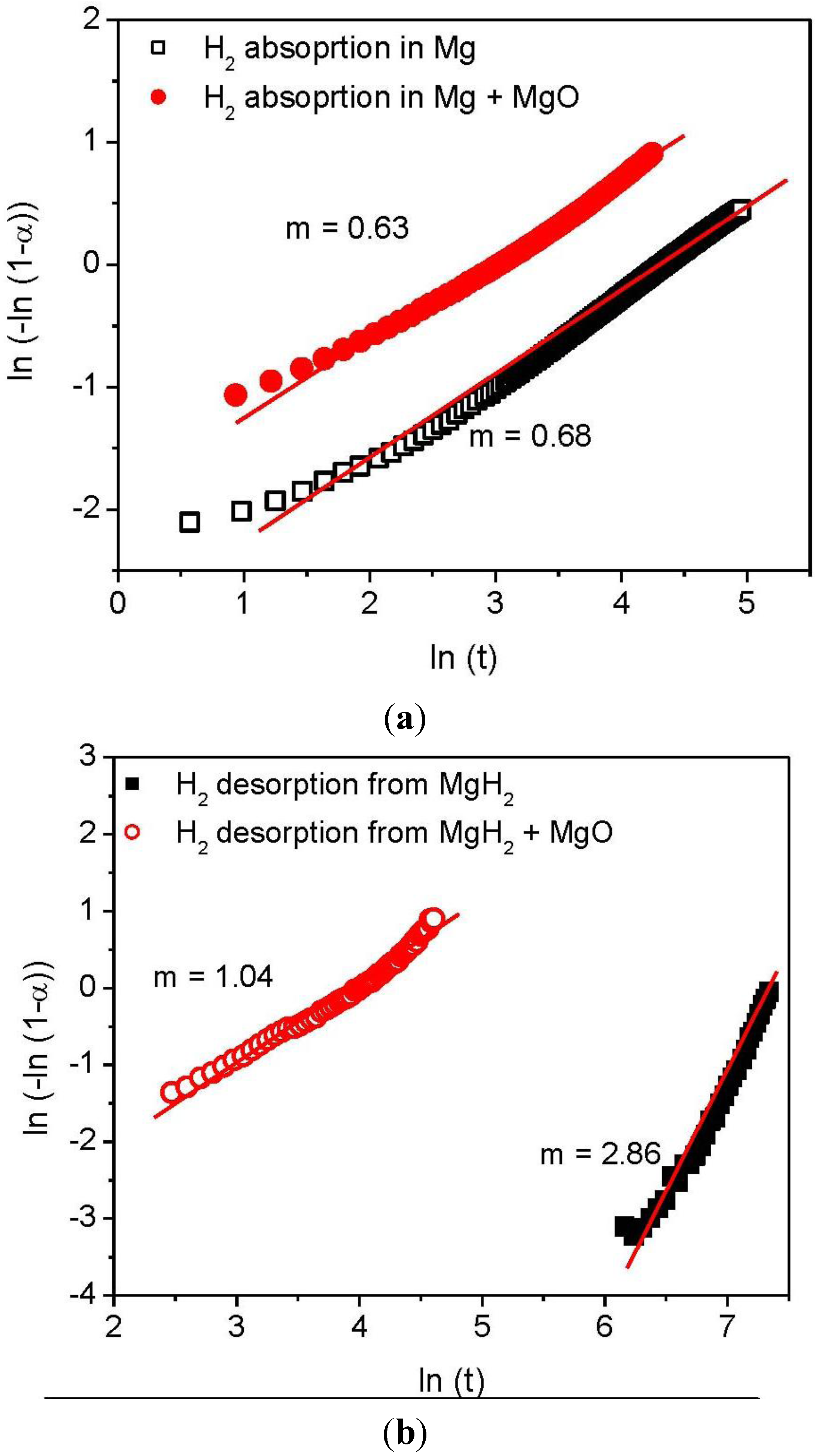
3.2. Kinetics and Rate Limiting Steps
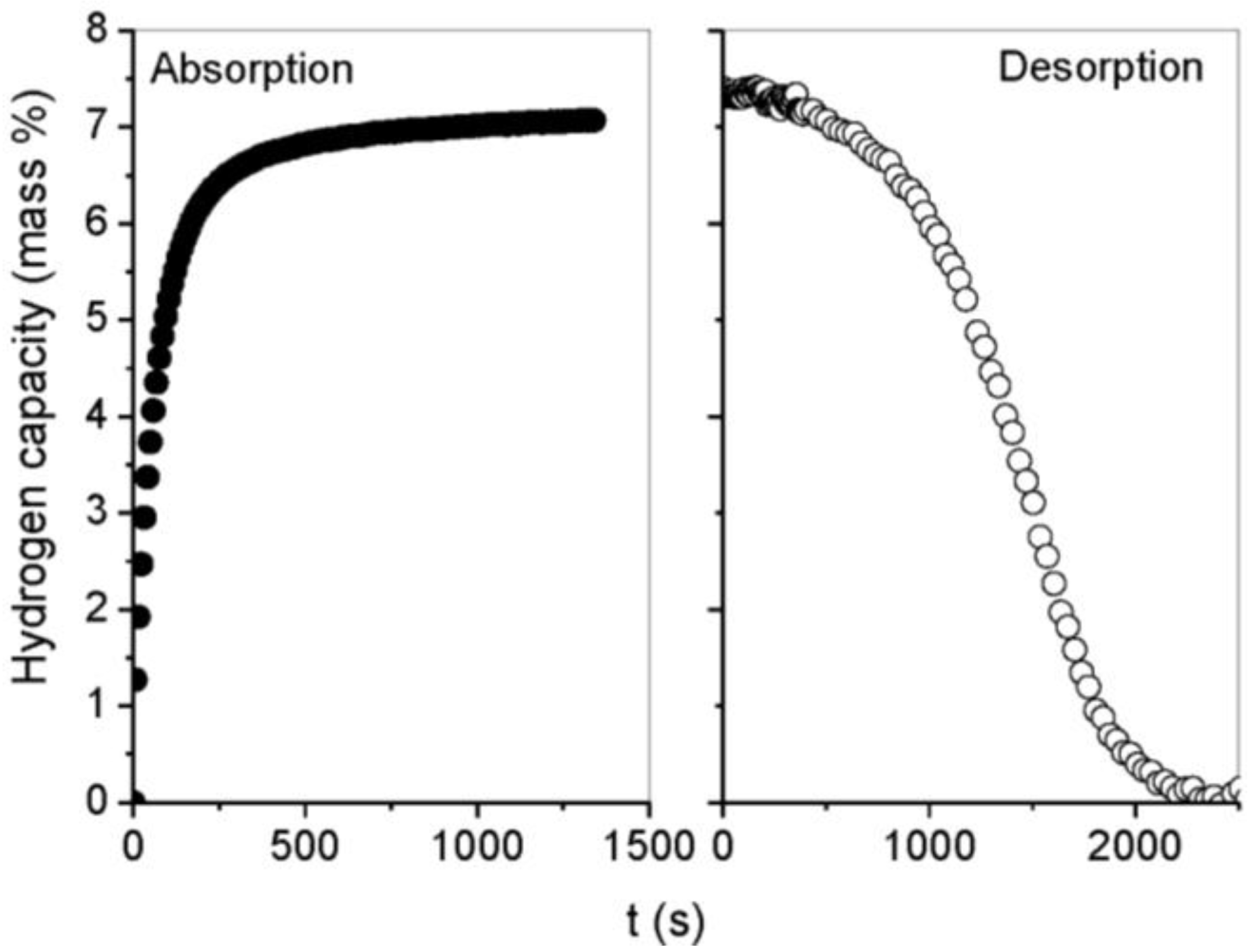
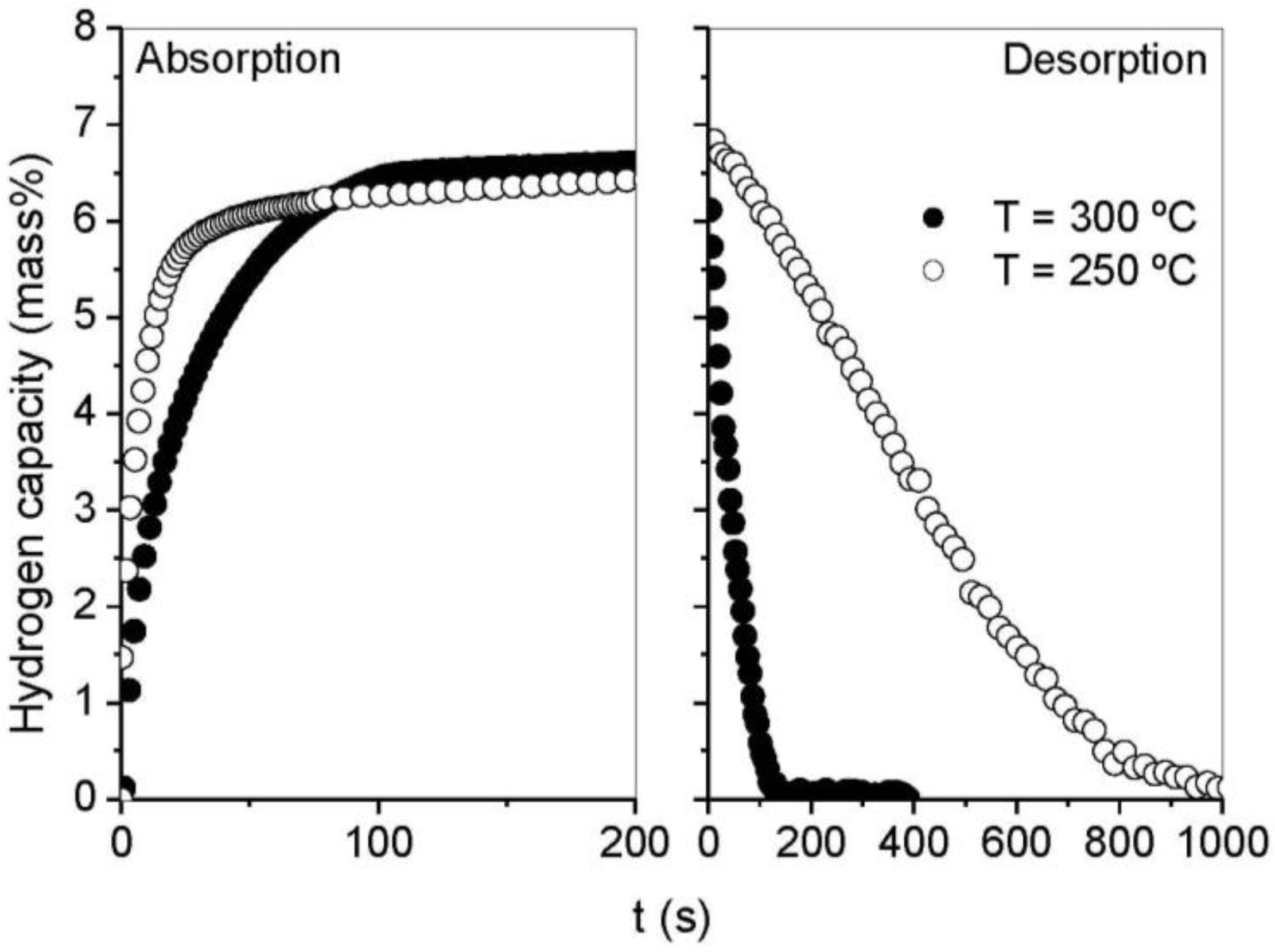

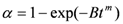
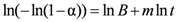
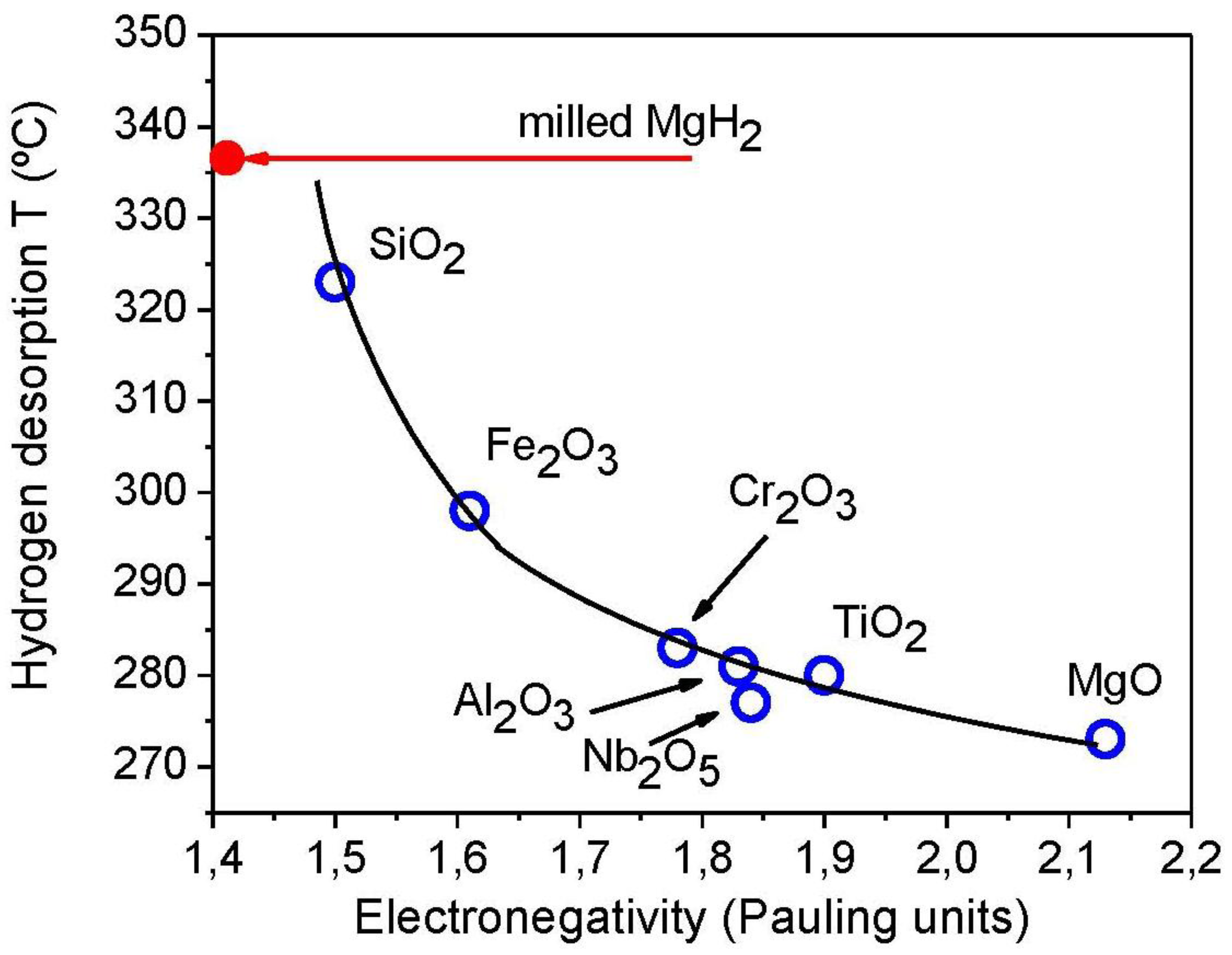
| Mechanism | Functional time dependence G(α) | m |
|---|---|---|
| Surface control | ||
| S1 | α | 1.24 |
| Random nucleation and growth (Avrami equations) | ||
| A1 | −ln(1 − α)1/4 | 4.00 |
| A2 | −ln(1 − α)1/3 | 3.00 |
| A3 | −ln(1 − α)2/5 | 2.50 |
| A4 | −ln(1 − α)1/2 | 2.00 |
| A5 | −ln(1 − α)2/3 | 1.50 |
| Shrinking Core with constant velocity: controlled by interface reaction | ||
| IP2–contracting surface | 1 − (1 − α)1/2 | 1.11 |
| IP3–contracting volume | 1 − (1 − α)1/3 | 1.07 |
| Shrinking Core with decelerating velocity: controlled by diffusion | ||
| D1-1-D diffusion | α2 | 0.62 |
| D2-2-D diffusion | (1 − α)ln(1 − α) + α | 0.57 |
| D3-Jander, 3-D diffusion | (1 −(1 − α)1/3)2 | 0.54 |
| MgH2 [6,32,44] | MgH2 + MgO [32] | MgH2 + Nb2O5 [18,44] | ||
|---|---|---|---|---|
| Particle size (µm) | 1 ± 0.5 | 0.4 ± 0.3 | 0.5 ± 0.3 | |
| Surface area (m2·g−1) | 9.9 | - | 22.4 | |
| Crystallite size (nm) | 79 | 86 | 83 | |
| Absorption at 300 °C (250 °C) | ||||
| Rate constant (s−1) | 0.00068 | 0.0027 (0.0052) | - | |
| Rate limiting step | Diffusion | Diffusion (Diffusion) | Diffusion | |
| Desorption at 300 °C (250 °C) | ||||
| Rate constant (s−1) | 0.00065 | 0.0055 (0.0207) | - | |
| Rate limiting step | Nucleation and growth | Shrinking core controlled by interface(Nucleation and growth) | Shrinking core controlled by interface | |
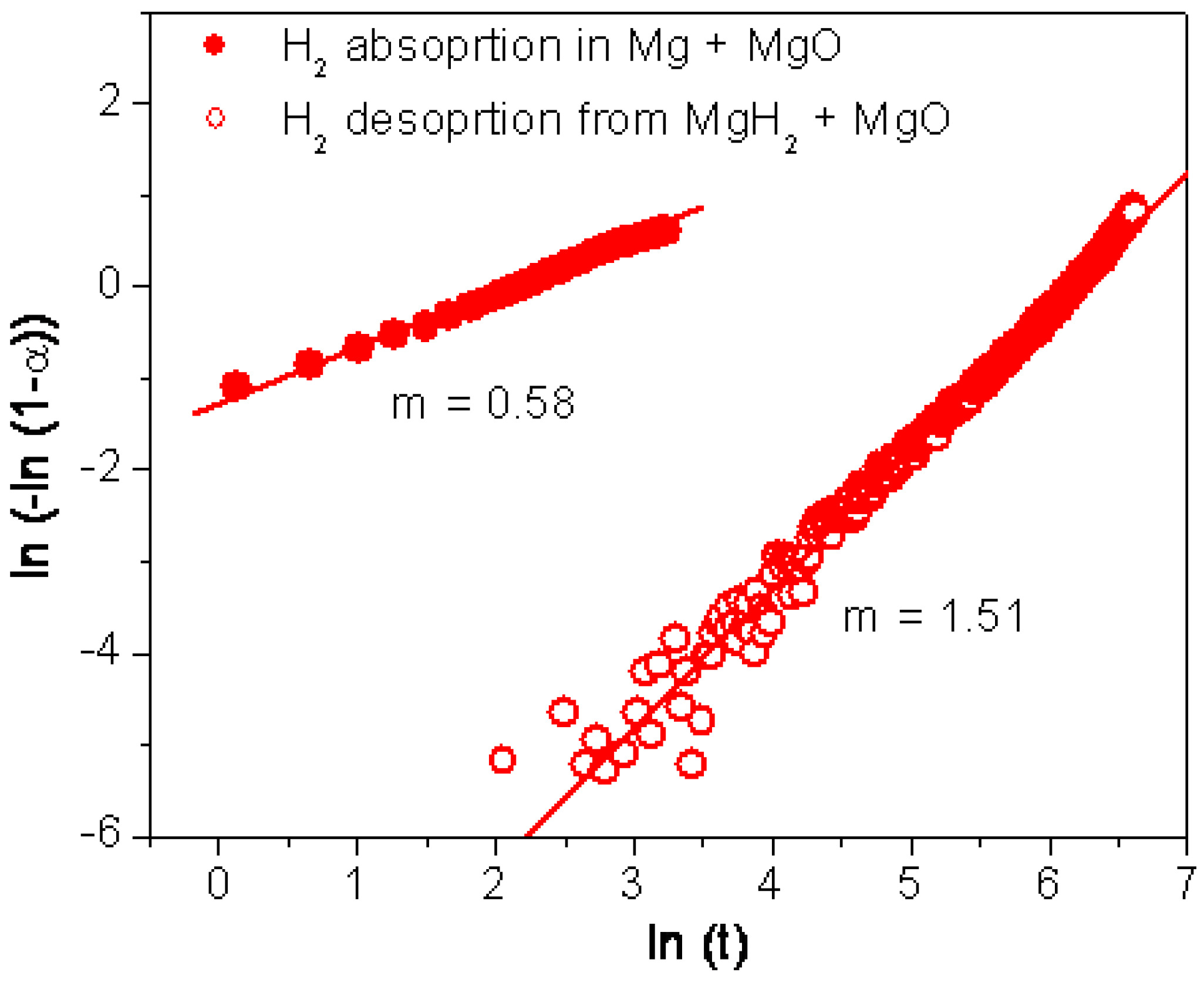
3.3. Rationalisation of the Effect of Oxides
4. Conclusions
Acknowledgments
References
- Niemann, M.U.; Srinivasan, S.S.; Phani, A.R.; Kumar, A.; Goswami, D.Y.; Stefanakos, E.K. Nanomaterials for hydrogen storage applications: A review. J. Nanomater. 2008, 2008, 950967:1–950967:9. [Google Scholar]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydr. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Bogdanovic, B. Catalytic synthesis of organo-lithium and organomagnesium compounds and of lithium and magnesium hydrides—Applications in organic-synthesis and hydrogen storage. Angew. Chem. Int. Ed. 1985, 24, 262–273. [Google Scholar] [CrossRef]
- Huot, J.; Liang, G.; Schulz, R. Mechanically alloyed metal hydride systems. Appl. Phys. Mater. Sci. Proc. 2001, 72, 187–195. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.F.; Ares-Fernandez, J.R. Hydrogen in magnesium: New perspectives toward functional stores. Energy Environ. Sci. 2010, 3, 526–543. [Google Scholar] [CrossRef]
- Huot, J.; Liang, G.; Boily, S.; van Neste, A.; Schulz, R. Structural study and hydrogen sorption kinetics of ball-milled magnesium hydride. J. Alloy. Compd. 1999, 293, 495–500. [Google Scholar] [CrossRef]
- Schulz, R.; Huot, J.; Liang, G.; Boily, S.; Lalande, G.; Denis, M.C.; Dodelet, J.P. Recent developments in the applications of nanocrystalline materials to hydrogen technologies. Mater. Sci. Eng. 1999, 267, 240–245. [Google Scholar] [CrossRef]
- Klassen, T.; Bohn, R.; Fanta, G.; Oelerich, W.; Eigen, N.; Gartner, F.; Aust, E.; Bormann, R.; Kreye, H. Tailoring nanocrystalline materials towards potential applications. Z. Für Metall. 2003, 94, 610–614. [Google Scholar]
- Zaluska, A.; Zaluski, L.; Strom-Olsen, J.O. Nanocrystalline magnesium for hydrogen storage. J. Alloy. Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Friedrichs, O.; Aguey-Zinsou, F.; Fernandez, J.R.A.; Sanchez-Lopez, J.C.; Justo, A.; Klassen, T.; Bormann, R.; Fernández, A. MgH2 with Nb2O5 as additive, for hydrogen storage: Chemical, structural and kinetic behavior with heating. Acta Mater. 2006, 54, 105–110. [Google Scholar]
- Schimmel, H.G.; Johnson, M.R.; Kearley, G.J.; Ramirez-Cuesta, A.J.; Huot, J.; Mulder, F.M. The vibrational spectrum of magnesium hydride from inelastic neutron scattering and density functional theory. Mater. Sci. Eng. 2004, 108, 38–41. [Google Scholar] [CrossRef]
- Schimmel, H.G.; Johnson, M.R.; Kearley, G.J.; Ramirez-Cuesta, A.J.; Huot, J.; Mulder, F.M. Structural information on ball milled magnesium hydride from vibrational spectroscopy and ab-initio calculations. J. Alloy. Compd. 2005, 393, 1–4. [Google Scholar] [CrossRef]
- Huot, J.; Pelletier, J.F.; Lurio, L.B.; Sutton, M.; Schulz, R. Investigation of dehydrogenation mechanism of MgH2-Nb nanocomposites. J. Alloy. Compd. 2003, 348, 319–324. [Google Scholar] [CrossRef]
- Schimmel, H.G.; Huot, J.; Chapon, L.C.; Tichelaar, F.D.; Mulder, F.M. Hydrogen cycling of niobium and vanadium catalyzed nanostructured magnesium. J. Am. Chem. Soc. 2005, 127, 14348–14354. [Google Scholar]
- Oelerich, W.; Klassen, T.; Bormann, R. Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg-based materials. J. Alloy. Compd. 2001, 315, 237–242. [Google Scholar] [CrossRef]
- Song, M.Y.; Bobet, J.L.; Darriet, B. Improvement in hydrogen sorption properties of Mg by reactive mechanical grinding with Cr2O3, Al2O3 and CeO2. J. Alloy. Compd. 2002, 340, 256–262. [Google Scholar] [CrossRef]
- Yan, Y.G.; Chen, Y.G.; Liang, H.; Wu, C.L.; Tao, M.D. Hydrogen storage properties of V30-Ti-Cr-Fe alloys. J. Alloy. Compd. 2007, 427, 110–114. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Kinetic investigation of the effect of milling time on the hydrogen sorption reaction of magnesium catalyzed with different Nb2O5 contents. J. Alloy. Compd. 2006, 407, 249–255. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; van Neste, A.; Schulz, R. Hydrogen storage properties of the mechanically milled MgH2-V nanocomposite. J. Alloy. Compd. 1999, 291, 295–299. [Google Scholar] [CrossRef]
- Bazzanella, N.; Checchetto, R.; Miotello, A. Catalytic effect on hydrogen desorption in Nb-doped microcrystalline MgH2. Appl. Phys. Lett. 2004, 85, 5212–5214. [Google Scholar]
- Dolci, F.; Di Chio, M.; Baricco, M.; Giamello, E. Niobium pentoxide as promoter in the mixed MgH2/Nb2O5 system for hydrogen storage: A multitechnique investigation of the H-2 uptake. J. Mater. Sci. 2007, 42, 7180–7185. [Google Scholar]
- Iizuka, T.; Ogasawara, K.; Tanabe, K. Acidic and catalytic properties of niobium pentaoxide. Bull. Chem. Soc. Jpn. 1983, 56, 2927–2931. [Google Scholar] [CrossRef]
- Ziolek, M.; Sobczak, I.; Lewandowska, A.; Nowak, I.; Decyk, P.; Renn, M.; Jankowska, B. Oxidative properties of niobium-containing mesoporous silica catalysts. Catal. Today 2001, 70, 169–181. [Google Scholar]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Effect of Nb2O5 content on hydrogen reaction kinetics of Mg. J. Alloy. Compd. 2004, 364, 242–246. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Catalytic mechanism of transition-metal compounds on Mg hydrogen sorption reaction. J. Phys. Chem. 2006, 110, 11020–11024. [Google Scholar]
- Friedrichs, O.; Martinez-Martinez, D.; Guilera, G.; Lopez, J.C.S.; Fernandez, A. In situ energy-dispersive XAS and XRD study of the superior hydrogen storage system MgH2/Nb2O5. J. Phys. Chem. 2007, 111, 10700–10706. [Google Scholar]
- Friedrichs, O.; Sanchez-Lopez, J.C.; Lopez-Cartes, C.; Klassen, T.; Bormann, R.; Fernandez, A. Nb2O5 “pathway effect” on hydrogen sorption in Mg. J. Phys. Chem. 2006, 110, 7845–7850. [Google Scholar]
- Aurora, A.; Mancini, M.R.; Gattia, D.M.; Montone, A.; Pilloni, L.; Todini, E.; Antisari, M.V. Microstructural and kinetic investigation of hydrogen sorption reaction of MgH2/Nb2O5 nanopowders. Mater. Manuf. Proc. 2009, 24, 1058–1063. [Google Scholar] [CrossRef]
- Noh, H.; Wang, D.; Luo, S.; Flanagan, T.B.; Balasubramaniam, R.; Sakamoto, Y. Hydrogen bronze formation within Pd/MoO3 composites. J. Phys. Chem. 2004, 108, 310–319. [Google Scholar]
- Sakaguchi, H.; Shirai, H.; Tanaka, H.; Adachi, G.Y. Hydrogen permeation characteristics for oxide/metal multilayered films. Chem. Mater. 1995, 7, 137–141. [Google Scholar] [CrossRef]
- Shirai, H.; Tanaka, H.; Sakaguchi, H.; Adachi, G. Hydrogen permeation characteristics for oxide/metal multilayered films. J. Phys. Chem. 1993, 97, 6007–6010. [Google Scholar]
- Aguey-Zinsou, K.F.; Fernandez, J.R.A.; Klassen, T.; Bormann, R. Using MgO to improve the (de)hydriding properties of magnesium. Mater. Res. Bull. 2006, 41, 1118–1126. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.F.; Ares, J.R.; Klassen, T.; Bormann, R. Production of Magnesium/Magnesium Alloy Powder Hydride Containing Hydrogen Storage Material for Power Supply of Technical Devices, Comprises Grinding the Magnesium Hydride Powder and Diamond Powder under Hydrogen Atmosphere; GKSS Forschungszentrum Geesthacht Gmbh: Geesthacht, Germany, 2006. [Google Scholar]
- Dehouche, Z.; Goyette, J.; Bose, T.K.; Huot, J.; Schulz, R. Sensitivity of nanocrystalline MgH2-V hydride composite to the carbon monoxide during a long-term cycling. Nano Lett. 2001, 1, 175–178. [Google Scholar] [CrossRef]
- Ares, J.R.; Aguey-Zinsou, K.F.; Klassen, T.; Bormann, R. Influence of impurities on the milling process of MgH2. J. Alloy. Compd. 2007, 434, 729–733. [Google Scholar] [CrossRef]
- Friedrichs, O.; Sanchez-Lopez, J.C.; Lopez-Cartes, C.; Dornheim, M.; Klassen, T.; Bormann, R.; Fernández, A. Chemical and microstructural study of the oxygen passivation behaviour of nanocrystalline Mg and MgH2. Appl. Surf. Sci. 2006, 252, 2334–2345. [Google Scholar]
- Borgschulte, A.; Bielmann, M.; Zuttel, A.; Barkhordarian, G.; Dornheim, M.; Bormann, R. Hydrogen dissociation on oxide covered MgH2 by catalytically active vacancies. Appl. Surf. Sci. 2008, 254, 2377–2384. [Google Scholar]
- Paganini, M.C.; Chiesa, M.; Giamello, E.; Coluccia, S.; Martra, G.; Murphy, D.M.; Pacchioni, G. Colour centres at the surface of alkali-earth oxides. A new hypothesis on the location of surface electron traps. Surf. Sci. 1999, 421, 246–262. [Google Scholar] [CrossRef]
- Sterrer, M.; Berger, T.; Diwald, O.; Knozinger, E.; Sushko, P.V.; Shluger, A.L. Chemistry at corners and edges: Generation and adsorption of H atoms on the surface of MgO nanocubes. J. Chem. Phys. 2005, 123, 064714:1–064714:7. [Google Scholar]
- Diwald, O.; Hofmann, P.; Knozinger, E. H-2 chemisorption and consecutive UV stimulated surface reactions on nanostructured MgO. Phys. Chem. Chem. Phys. 1999, 1, 713–721. [Google Scholar]
- Mintz, M.H.; Zeiri, Y. Hydriding kinetics of powders. J. Alloy. Compd. 1995, 216, 159–175. [Google Scholar] [CrossRef]
- Hancock, J.D.; Sharp, J.H. Method of comparing solid-state kinetic data and its application to decomposition of kaolinite, brucite, and BaCO3. J. Am. Ceram. Soc. 1972, 55, 74–77. [Google Scholar] [CrossRef]
- Li, W.Y.; Li, C.S.; Ma, H.; Chen, J. Magnesium nanowires: Enhanced kinetics for hydrogen absorption and desorption. J. Am. Chem. Soc. 2007, 129, 6710–6711. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.F.; Fernandez, J.R.A.; Klassen, T.; Bormann, R. Effect of Nb2O5 on MgH2 properties during mechanical milling. Int. J. Hydr. Energy 2007, 32, 2400–2407. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.F.; Nicolaisen, T.; Fernandez, J.R.A.; Klassen, T.; Bormann, R. Effect of nanosized oxides on MgH2 (de)hydriding kinetics. J. Alloy. Compd. 2007, 434, 738–742. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Erdemir, A. A crystal-chemical approach to lubrication by solid oxides. Tribol. Lett. 2000, 8, 97–102. [Google Scholar] [CrossRef]
- Erdemir, A.; Li, S.H.; Jin, Y.S. Relation of certain quantum chemical parameters to lubrication behavior of solid oxides. Int. J. Mol. Sci. 2005, 6, 203–218. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.F.; Ares-Fernandez, J.R. Synthesis of colloidal magnesium: A near room temperature store for hydrogen. Chem. Mater. 2008, 20, 376–378. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ares-Fernández, J.-R.; Aguey-Zinsou, K.-F. Superior MgH2 Kinetics with MgO Addition: A Tribological Effect. Catalysts 2012, 2, 330-343. https://doi.org/10.3390/catal2030330
Ares-Fernández J-R, Aguey-Zinsou K-F. Superior MgH2 Kinetics with MgO Addition: A Tribological Effect. Catalysts. 2012; 2(3):330-343. https://doi.org/10.3390/catal2030330
Chicago/Turabian StyleAres-Fernández, José-Ramón, and Kondo-Francois Aguey-Zinsou. 2012. "Superior MgH2 Kinetics with MgO Addition: A Tribological Effect" Catalysts 2, no. 3: 330-343. https://doi.org/10.3390/catal2030330





