2.1.1. Copolymerization Using Cp'TiX2(Y)–Cocatalyst Systems
Modified half-titanocenes of the type Cp’TiX
2(Y) (Cp’ = cyclopentadienyl group; X = halogen, alkyl; Y = anionic ligand, such as
O-2,6-
iPr
2C
6H
3, N=C
tBu
2, N=PR
3) have been known to exhibit unique characteristics as olefin polymerization catalysts [
15,
16,
17,
18]. In particular, Cp’TiCl
2(OAr) (OAr =
O-2,6-
iPr
2C
6H
3) not only showed high activities for ethylene polymerization [
80], but also exhibited efficient comonomer incorporations for copolymerization of ethylene with α-olefin [
81,
82,
83], as well as with styrene [
52,
53,
54,
55,
56] in the presence of MAO (
Scheme 1).
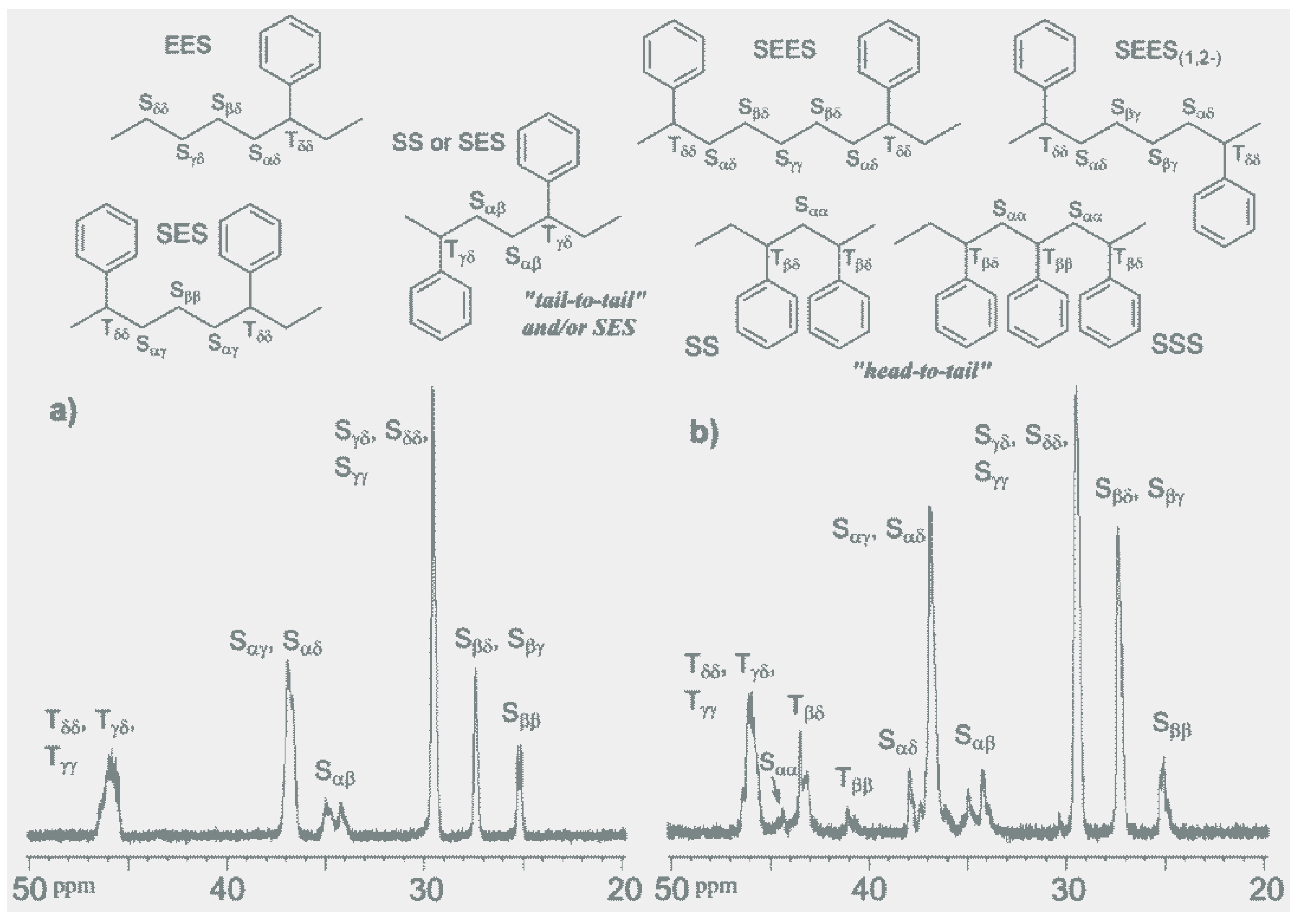
Figure 1.
13C NMR spectra (in CDCl
3 at 60 °C) of poly(ethylene-
co-styrene)s (methylene and methine region, tetrahydrofuran (THF) soluble fraction) [
53]. (
a) catalyst = [Me
2Si(C
5Me
4)(N
tBu)]TiCl
2, styrene content 32.7 mol%; (
b) catalyst = (1,2,3-Me
3C
5H
2)TiCl
2(
O-2,6-
iPr
2C
6H
3), styrene content 38.8 mol%. The peak at 30.5 ppm is due to the impurity (2,6-di-
tert-butyl-
p-cresol) added.
Figure 1.
13C NMR spectra (in CDCl
3 at 60 °C) of poly(ethylene-
co-styrene)s (methylene and methine region, tetrahydrofuran (THF) soluble fraction) [
53]. (
a) catalyst = [Me
2Si(C
5Me
4)(N
tBu)]TiCl
2, styrene content 32.7 mol%; (
b) catalyst = (1,2,3-Me
3C
5H
2)TiCl
2(
O-2,6-
iPr
2C
6H
3), styrene content 38.8 mol%. The peak at 30.5 ppm is due to the impurity (2,6-di-
tert-butyl-
p-cresol) added.
Scheme 1.
Effective catalyst precursors for random ethylene/styrene copolymerization [
52,
53,
54,
55,
56].
Scheme 1.
Effective catalyst precursors for random ethylene/styrene copolymerization [
52,
53,
54,
55,
56].
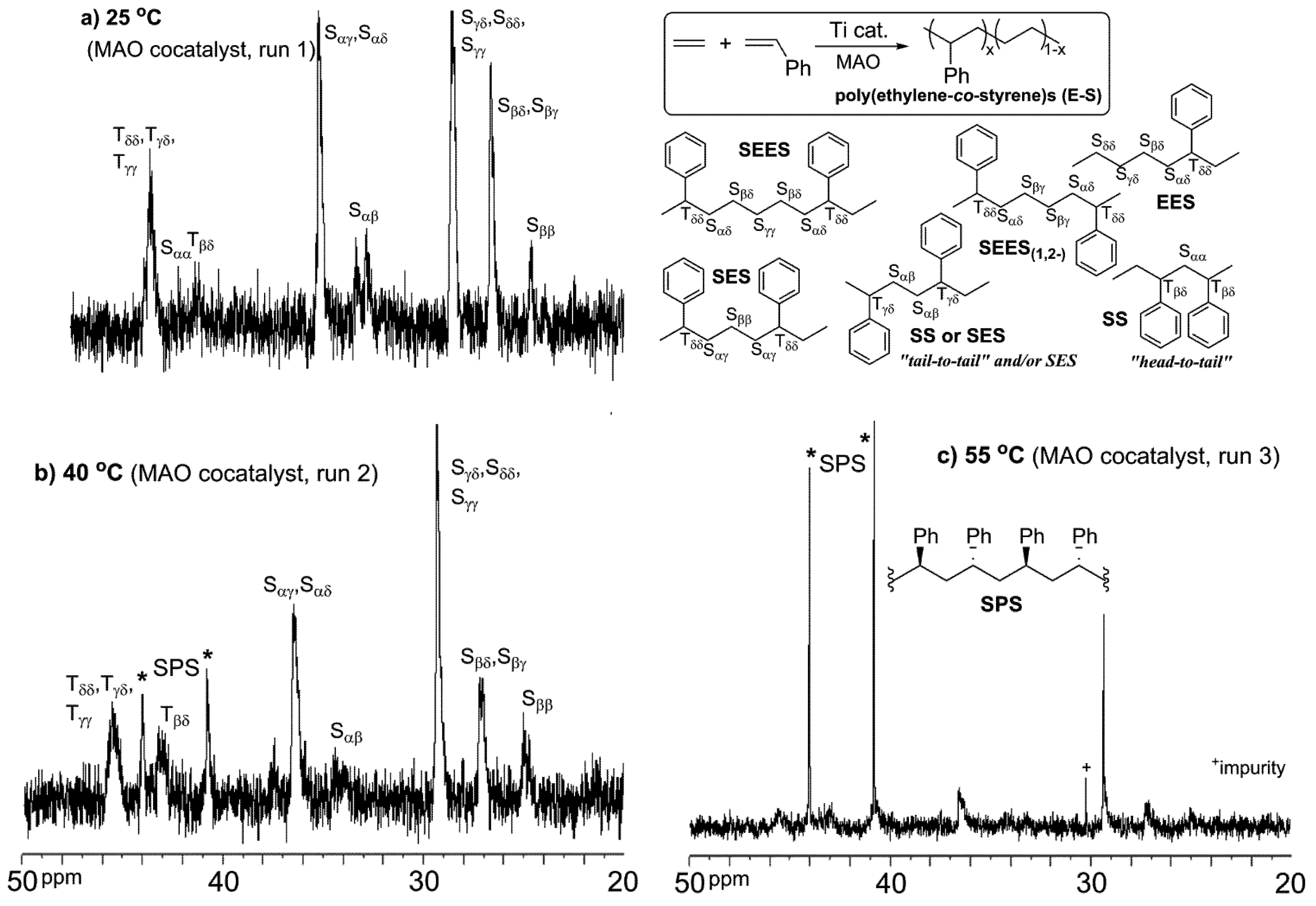
As summarized in
Table 1, Cp'TiCl
2(OAr) (Cp' = 1,2,3-Me
3C
5H
2 (
1), 1,3-Me
2C
5H
3 (
2),
tBuC
5H
4 (
3)
etc.; Ar = 2,6-
iPr
2C
6H
3) exhibited remarkable activities for ethylene/styrene copolymerization in the presence of MAO [
52,
53], whereas the Cp* analogue (
4), which exhibited remarkable activities and comonomer incorporations in ethylene/α-olefin copolymerization [
80,
81,
82,
83], exhibited lower activities than
1–
3 (
Table 1) [
52,
53,
54,
55,
56]. The resultant polymers are poly(ethylene-
co-styrene)s exclusively without by-producing polyethylene and/or syndiotactic polystyrene [
52,
53]. The resultant copolymers possessed not only relatively high molecular weights with unimodal molecular weight distributions, but also a single composition, as confirmed by DSC thermograms, CFC and GPC/FT-IR [
53]. The activities by
1-
3 decreased slightly with an increase in the styrene concentration, whereas the styrene contents in the copolymers increased upon increasing the styrene concentration. Styrene incorporations with aryloxo analogues is more efficient than that with [Me
2Si(C
5Me
4)(N
tBu)]TiCl
2 (
5). An analysis of the microstructure of the resultant poly(ethylene-
co-styrene)s by
13C NMR spectroscopy (
Figure 1) indicated that the resultant copolymer prepared by
1 possesses resonances ascribed to two and three styrene units connected via head-to-tail coupling [at δ = 40.7–41.0 ppm (T
ββ), 43.1–45 ppm (S
αα and T
βδ), respectively], in addition to the resonances ascribed to tail-to-tail coupling of a styrene unit or head-to-head bridged by an intervening ethylene unit (S
αβ, at δ = 34.3 and 35.1 ppm). This is especially interesting in contrast to the results with the linked half-titanocene (
5).
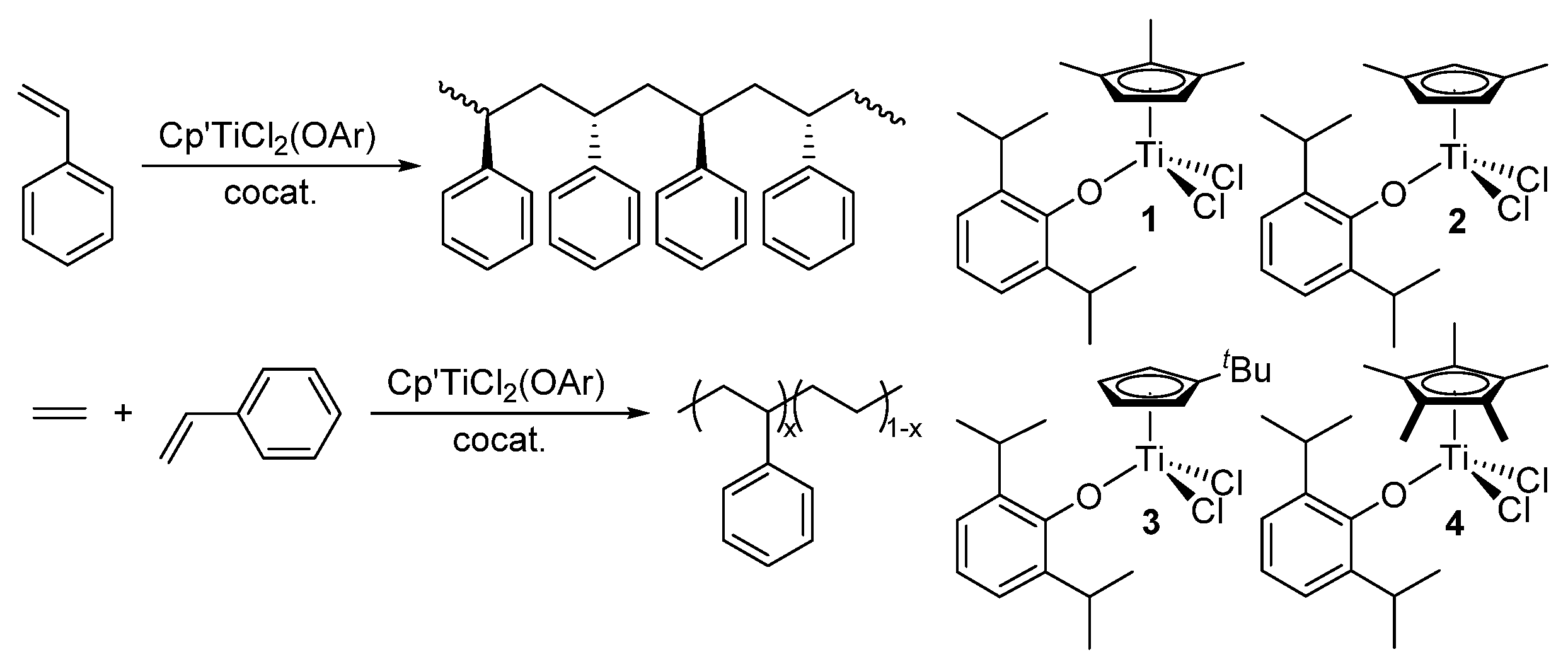
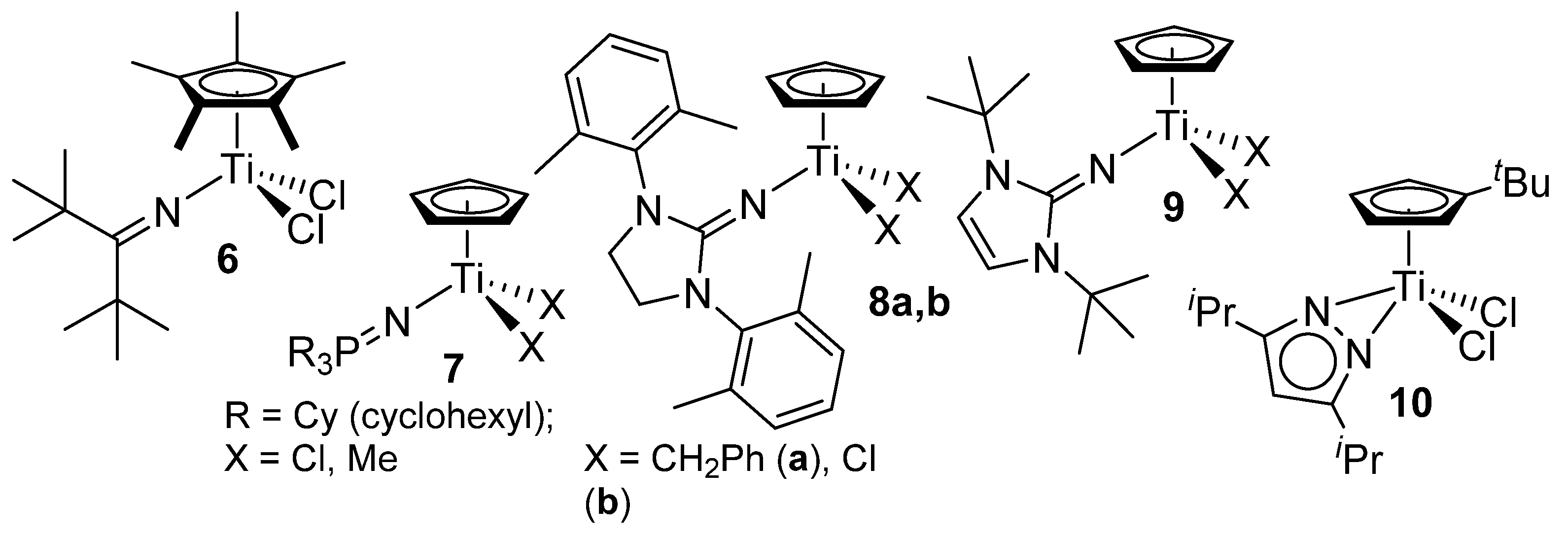
Copolymerization with the other modified half-titanocenes has also been reported (
Scheme 2). Ethylene/styrene copolymerization by Cp*TiCl
2(N=C
tBu
2) (
6) took place in a living manner in the presence of MAO cocatalyst, although the homopolymerization of ethylene and styrene did not proceed in a living manner [
57]. No styrene repeating units were observed in the resultant copolymers, which suggest that a certain degree of styrene insertion inhibits chain transfer in this catalysis. The living nature was maintained under various conditions (Al/Ti molar ratios, ethylene pressure, styrene concentrations, temperature) [
58].
Table 1.
Ethylene/styrene copolymerization by Cp’TiCl
2(OAr) [Cp’ = 1,2,3-Me
3C
5H
2 (
1), 1,3-Me
2C
5H
3 (
2),
tBuC
5H
4 (
3); OAr = O-2,6-
iPr
2C
6H
3] or [Me
2Si(C
5Me
4)(N
tBu)]TiCl
2 (
5)–MAO catalyst systems [
52,
53].
a
Table 1.
Ethylene/styrene copolymerization by Cp’TiCl2(OAr) [Cp’ = 1,2,3-Me3C5H2 (1), 1,3-Me2C5H3 (2), tBuC5H4 (3); OAr = O-2,6-iPr2C6H3] or [Me2Si(C5Me4)(NtBu)]TiCl2 (5)–MAO catalyst systems [52,53].a
| Complexes | Styrene | Activity b | THF soluble (E/S copolymer) |
|---|
| | /mL | | Content c /wt.% | Mw d× 10−4 | Mw/Mn d | Styrene /mol% e |
|---|
| (1,2,3-Me3C5H2)TiCl2(OAr) (1) | 3 | 4100 | 99.1 | 17.0 | 1.6 | 26.0 |
| (1,2,3-Me3C5H2)TiCl2(OAr) (1) | 5 | 3070 | 98.3 | 11.0 | 1.7 | 38.8 |
| (1,2,3-Me3C5H2)TiCl2(OAr) (1) | 10 | 2720 | 97.8 | 6.6 | 1.6 | 51.2 |
| (1,3-Me2C5H3)TiCl2(OAr) (2) | 3 | 3670 | 97.1 | 6.4 | 1.8 | 32.3 |
| (1,3-Me2C5H3)TiCl2(OAr) (2) | 5 | 4280 | 98.2 | 6.0 | 2.1 | 38.5 |
| (1,3-Me2C5H3)TiCl2(OAr) (2) | 10 | 4140 | 98.2 | 3.7 | 1.6 | 49.0 |
| (tBuC5H4)TiCl2(OAr) (3) | 5 | 2180 | 99.6 | 5.9 | 1.7 | 37.6 |
| (tBuC5H4)TiCl2(OAr) (3) | 10 | 1840 | 98.7 | 3.5 | 2.2 | 51.2 |
| [Me2Si(C5Me4)(NtBu)]TiCl2 (5) | 10 | 5630 | 99.6 | 18.0 | 1.8 | 32.7 |
Scheme 2.
Other half-titanocenes employed for ethylene/styrene copolymerization [
48,
49,
50,
51].
Scheme 2.
Other half-titanocenes employed for ethylene/styrene copolymerization [
48,
49,
50,
51].
Copolymerization with CpTiX
2(N=PCy
3) (
7, X = Cl, Me)–cocatalyst (MAO, borates) systems proceeded with remarkable catalytic activities [at 60–90 °C, ethylene 70 psi (4.76 atmv)], however, styrene incorporation seemed less efficient than with either aryloxo analogues (
1–
3) or linked half-titanocene (
5) [styrene content: 33.4–61.4 wt% (<27.6 mol%)] [
60,
84]. Half-titanocene-containing imidazolidin-2-iminato ligand (
8a) also exhibited remarkable catalytic activity under certain conditions, but the styrene content in the resultant copolymer was low [ethylene 5 bar, styrene/toluene = 20/210 mL, 80 °C; activity = 1960 kg-polymer/mol-Ti·h,
Mw = 1.79×10
5,
Mw/
Mn = 2.1, styrene 10 wt% (2.9 mol%)] [
59]. However, the copolymerization using the dichloro- analogue (
8b) afforded a mixture of PE and the copolymer confirmed by DSC thermograms [
61]. The imidazolin-2-iminato analogue (
9) showed moderate catalytic activities and afforded the copolymers exclusively under the optimized conditions: the copolymerization proceeded in a quasi living manner, as observed in the copolymerization by
6 [
63]. The pyrazolato analogue (
10) also showed the similar copolymerization behavior to those observed by
6,
9, although the activities were low [
62]. Microstructure analysis results indicate that the resultant poly(ethylene-
co-styrene)s prepared by
9,
10 possessed resonances ascribed to isolated and alternating styrene inserted units. On the basis of these experiments, it seems likely that an isolated (and/or alternating) insertion of styrene plays a role for the (quasi) living polymerization to disturb the propagated chain from β-H elimination [
57,
58,
62,
63].
On the basis of the above results, modified half-titanocenes, Cp’TiX2(Y), especially the aryloxo modified half-titanocenes, are better catalyst precursors for the synthesis of ethylene/styrene copolymers, and both the activity and the styrene incorporation are highly affected by the kind of ligands used (both cyclopentadienyl and anionic donor).
2.1.2. Role of Anionic Donor Ligand (Y) and Mechanistic Considerations
Three half-titanocene complexes containing Cp* ligand of type, Cp*TiX
2(Y) [X = Cl, Y = N= C
tBu
2 (
6),
O-2,6-
iPr
2C
6H
3 (
4), Cl (
11); X = Me, Y =
O-2,6-
iPr
2C
6H
3 (
12), Me (
13)]–cocatalyst systems were used for the copolymerization to explore the effect of anionic donor ligands under the same conditions (
Table 2,
Table 3) [
58,
85,
86]. The ketimide analogue (
6)–MAO catalyst showed moderate catalytic activities, and the styrene content increased at higher temperature. The resultant copolymers prepared with
6 even at 55 and 70 °C still possessed relatively low PDI values, suggesting that the living nature was maintained under these conditions (
Table 2). In contrast, the polymerizations by the aryloxide analogue (
4) gave copolymers with high styrene contents (31.9–34.3 mol%), and a significant increase in the activity was not observed at high temperature. The resultant copolymers possessed lower
Mn values with unimodal, rather large PDI values (
Mw/
Mn = 1.50–1.62), which strongly suggests that a chain transfer reaction occurred to some degree. Note that the polymers prepared with the trichloride analogue (
11) showed bimodal molecular weight distributions consisting of a mixture of PE and SPS, and the proportion of SPS increased at high temperature, due to an increase in the activity for syndiospecific styrene polymerization [
58,
87,
88].
Table 2.
Copolymerization of ethylene with styrene by Cp
*TiCl
2(Y) [Y = N= C
tBu
2 (
6),
O-2,6-
iPr
2C
6H
3 (OAr,
4), Cl (
11)]–MAO catalyst systems [
58]
a.
Table 2.
Copolymerization of ethylene with styrene by Cp*TiCl2(Y) [Y = N= CtBu2 (6), O-2,6-iPr2C6H3 (OAr, 4), Cl (11)]–MAO catalyst systems [58] a.
| Catalysts | Temp. | Composition (%) c | Activity d | Styrene cont.e | Mn f | Mw/Mn f |
|---|
| Y | /°C | E-S | PE | SPS | | /mol% | ×10−4 | |
|---|
| N=CtBu2 (6) | 25 | >99 | trace | trace | 396 | 7.4 | 9.5 | 1.18 |
| N=CtBu2 (6) | 40 | >99 | trace | trace | 790 | 9.3 | 14.4 | 1.28 |
| N=CtBu2 (6) | 55 | >99 | trace | trace | 1110 | 10.4 | 19.7 | 1.31 |
| N=CtBu2 (6) | 70 | >99 | trace | trace | 1260 | 12.2 | 16.3 | 1.57 |
| OAr (4) | 25 | >99 | trace | trace | 504 | 31.9 | 9.28 | 1.62 |
| OAr (4) | 40 | >98 | trace | trace | 660 | 34.3 | 9.79 | 1.5 |
| Cl (11) | 25 | trace | 86.8 | 13.2 | 250 | >99 g | 5.85 | 1.26 |
| | | | | | | - | 0.29 | 2.69 |
| Cl (11) | 40 | trace | 81.6 | 18.4 | 280 | >99 g | 5.07 | 1.31 |
| | | | | | | - | 0.31 | 1.75 |
| Cl (11) | 55 | trace | 69.8 | 30.2 | 260 | >99 g | 3.56 | 1.48 |
| | | | | | | - | 0.21 | 1.77 |
Copolymerization using the aryloxo-dimethyl analogue (
12)–MAO or [PhN(H)Me
2][B(C
6F
5)
4] (AFPB) catalyst system afforded the copolymer (
Table 3); no distinct differences in the microstructures were seen between MAO and AFPB in the
13C NMR spectra. In contrast, note that the polymer prepared with the Cp*TiMe
3 (
13)–AFPB catalyst was PE (containing a trace amount of the copolymer with low styrene content) or the copolymer with an extremely low styrene content, whereas the copolymerization in the presence of MAO afforded a mixture of PE and SPS, as seen with the trichloride analogue (
11). The fact that no SPS was formed in polymerization with
13–AFPB catalyst was analogous to the fact that Cp*Ti(CH
2Ph)
3–AFPB catalyst did not afford SPS in an attempted styrene polymerization (under dark conditions), and only poly(propylene-
co-styrene) oligomer was formed in the propylene/styrene copolymerization [
89]. These results strongly suggest that cationic Ti(IV) species play an important key role in ethylene polymerization, as well as ethylene/styrene copolymerization. These results also suggest that another catalytically-active species [likely Ti(III)] for syndiospecific styrene polymerization is formed in the presence of MAO [
58].
Table 3.
Ethylene/styrene copolymerization by Cp*TiMe
2(
O-2,6-
iPr
2C
6H
3) (
12), Cp*TiMe
3 (
13)–MAO or [PhN(H)Me
2][B(C
6F
5)
4] (AFPB)–catalyst systems [
58]
a.
Table 3.
Ethylene/styrene copolymerization by Cp*TiMe2(O-2,6-iPr2C6H3) (12), Cp*TiMe3 (13)–MAO or [PhN(H)Me2][B(C6F5)4] (AFPB)–catalyst systems [58] a.
| Cat. (μmol) | Time | Composition (%) b | Activity c | styrene cont.d | Mne | Mw/Mn e |
|---|
| | /min | E-S | PE | SPS | | /mol% | ×10−4 | |
|---|
| 12/MAO (2.0) | 10 | 99 | trace | trace | 519 | 30.5 | 5.34 | 2.05 |
| 12/AFPB (5.0) | 20 | 99 | trace | trace | 79.2 | 46.7 | 2.81 | 2.16 |
| 13/MAO (2.0) | 10 | trace | 68.6 | 31.4 | 366 | >99 | 8.66 | 1.37 |
| | | | | | | - | 0.81 | 2.33 |
| 13/AFPB (10.0) | 20 | trace | 99 | trace | 43.8 | trace | 0.5 | 3.53 |
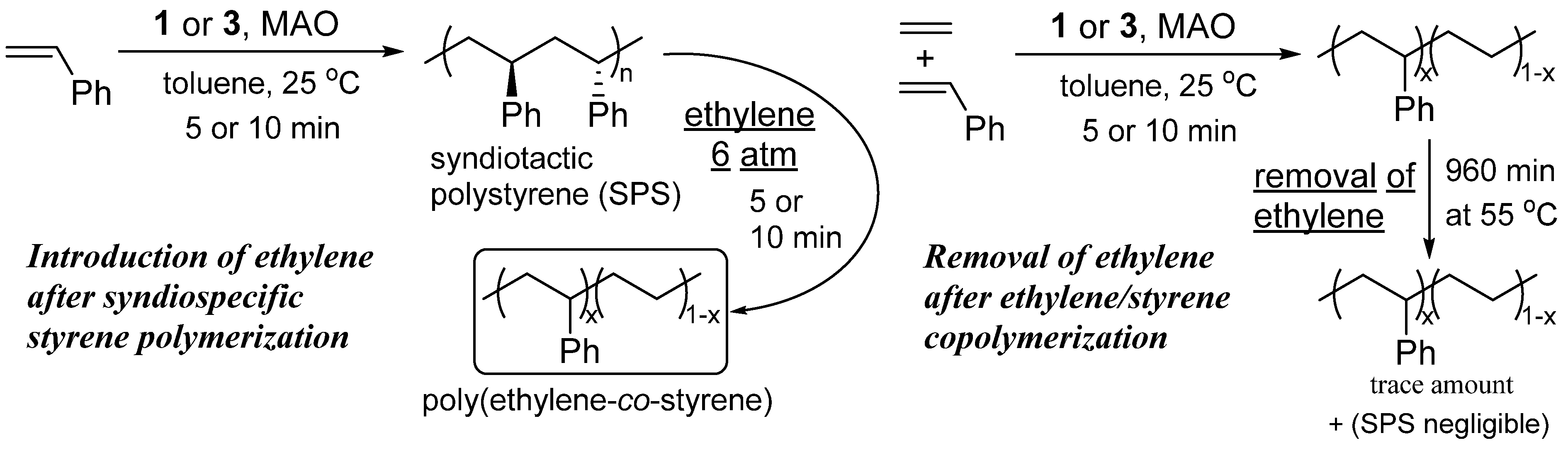
The exclusive formation of copolymers without formation of SPS as a by-product was observed with the introduction of ethylene into a solution of syndiospecific styrene polymerization using Cp’TiCl
2(O-2,6-
iPr
2C
6H
3)–MAO catalysts (
Scheme 3) [
90]. Moreover, the activities and the
Mw values, as well as the styrene contents in the latter copolymerizations, were identical to those in independent runs (
Table 4), clearly indicating that the catalytically active species for the syndiospecific styrene polymerization can be tuned to the active species for copolymerization by exposure of ethylene.
Scheme 3.
Step (co)polymerization of ethylene with styrene [
90].
Scheme 3.
Step (co)polymerization of ethylene with styrene [
90].
Taking into account these findings [
58,
87,
88,
91], it is thus highly assumed that the cationic Ti(IV) species, [Cp’TiR(Y)]
+, likely play a role in copolymerization, and the active species containing an anionic ancillary donor ligand [assumed to be neutral Ti(III), Cp’TiR(Y)] proposed by Tomotsu
et al. [
37,
38] plays a role in syndiospecific styrene polymerization (
Scheme 4) [
90]. These results should also explain the reported finding that the catalytic activities and molecular weight of the resultant syndiotactic polystyrene in styrene polymerization using Cp'TiX
2(Y)–cocatalyst systems were highly dependent upon the anionic donor ligand (Y), regardless of the kind of the cocatalyst used [
90]. These proposals are in contrasts to the hypothesis that cationic Ti(III) species, [Cp'Ti(R)(styrene)]
+, play a role as the catalytically-active species for the styrene polymerization using Cp’TiX
3 [
89,
92]. This hypothesis should help to explain why polystyrene structures in the resultant copolymers prepared with Cp'TiCl
2(Y)–MAO catalysts are atactic [
52,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62,
63].
Table 4.
Two step ethylene/styrene (co)polymerization by Cp
'TiCl
2(
O-2,6-
iPr
2C
6H
3) [Cp' = 1,2,4-Me
3C
5H
2 (
1),
tBuC
5H
4 (
3)]–MAO catalysts [
90]
a.
Table 4.
Two step ethylene/styrene (co)polymerization by Cp'TiCl2(O-2,6-iPr2C6H3) [Cp' = 1,2,4-Me3C5H2 (1), tBuC5H4 (3)]–MAO catalysts [90] a.
| catalysts | Ethylene b | Time. | Copolymersc | SPSd |
|---|
| Cp' | 1st+2nd | 1st+2nd | Yield | Mwe | Mw/ | Styrene f | Yield | Mwe | Mw/ |
|---|
| | /atm. | /min | /mg | ×10−4 | Mne | /mol% | /mg | ×10−4 | Mne |
|---|
| 1,2,4-Me3C5H2 (1) | 0 | 5 | - | | | | 45 | 24.1 | 1.82 |
| 1,2,4-Me3C5H2 (1) | 0 | 10 | - | | | | 175 | 24.6 | 2.07 |
| 1,2,4-Me3C5H2 (1) | 6 | 5 | 440 | 12.4 | 1.80 | 48.3 | trace | | |
| 1,2,4-Me3C5H2 (1) | 6 | 10 | 593 | 11.5 | 1.54 | 50.4 | trace | | |
| tBuC5H4 (3) | 0 | 5 | - | | | | 242 | 17.2 | 2.70 |
| tBuC5H4 (3) | 6 | 5 | 574 | 12.7 | 1.85 | 44.7 | trace | | |
| 1,2,4-Me3C5H2 (1) | 0+6 | 10+5 | 340 | 10.7 | 2.54 | 48.3 | 140 | 21.9 | 2.53 |
| 1,2,4-Me3C5H2 (1) | 0+6 | 10+10 | 577 | 10.8 | 1.92 | 49.8 | 157 | 25.7 | 2.07 |
| tBuC5H4 (3) | 0+6 | 5+5 | 360 | 13.2 | 2.36 | 45.3 | 221 | 16.8 | 2.51 |
Scheme 4.
Proposed active species in two polymerization using half-titanocenes [
90].
Scheme 4.
Proposed active species in two polymerization using half-titanocenes [
90].