Towards Rational Design of Nanoparticle Catalysis in Ionic Liquids
Abstract
:1. Introduction
2. Manipulating the Cation and the Anion
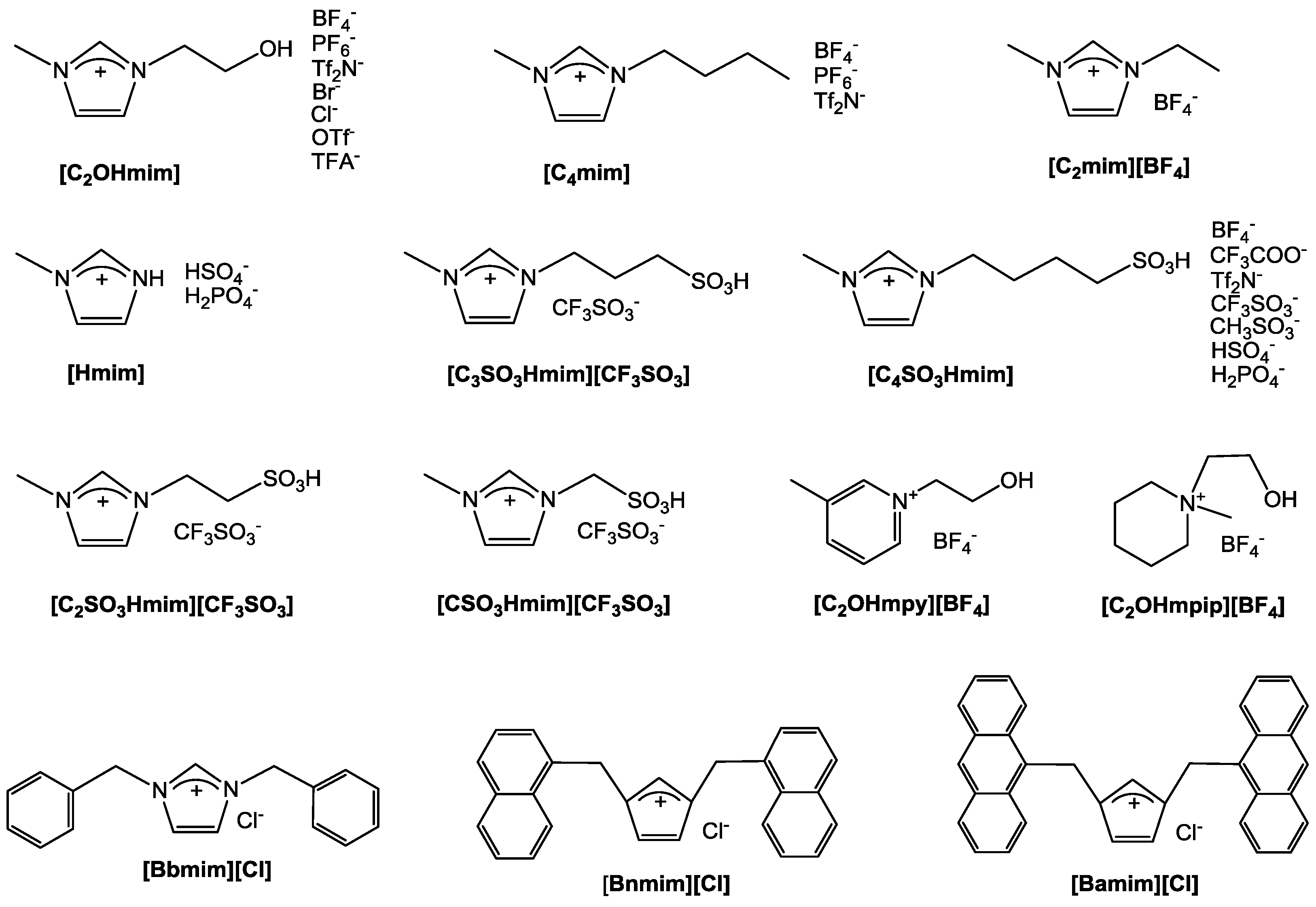
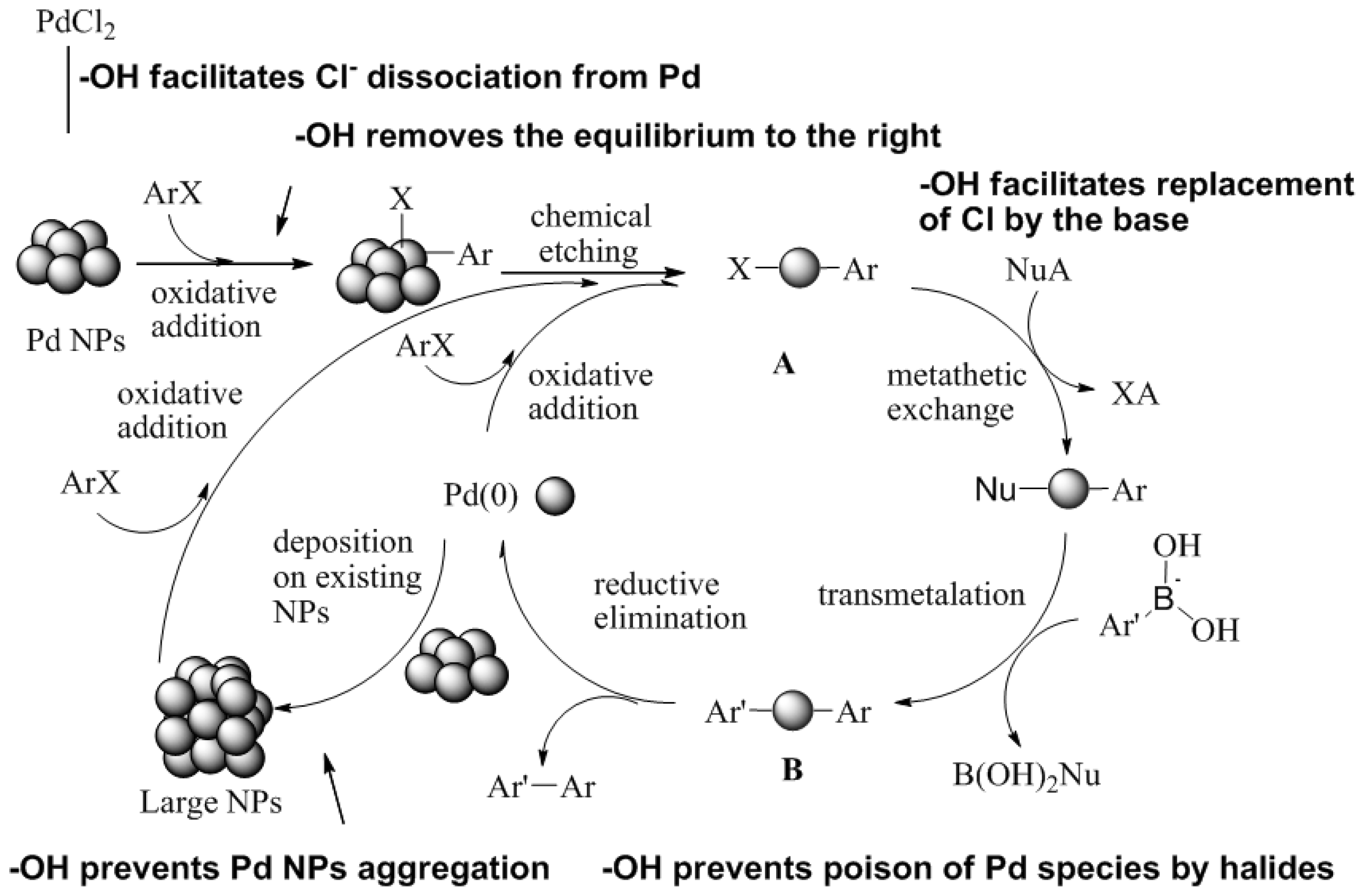
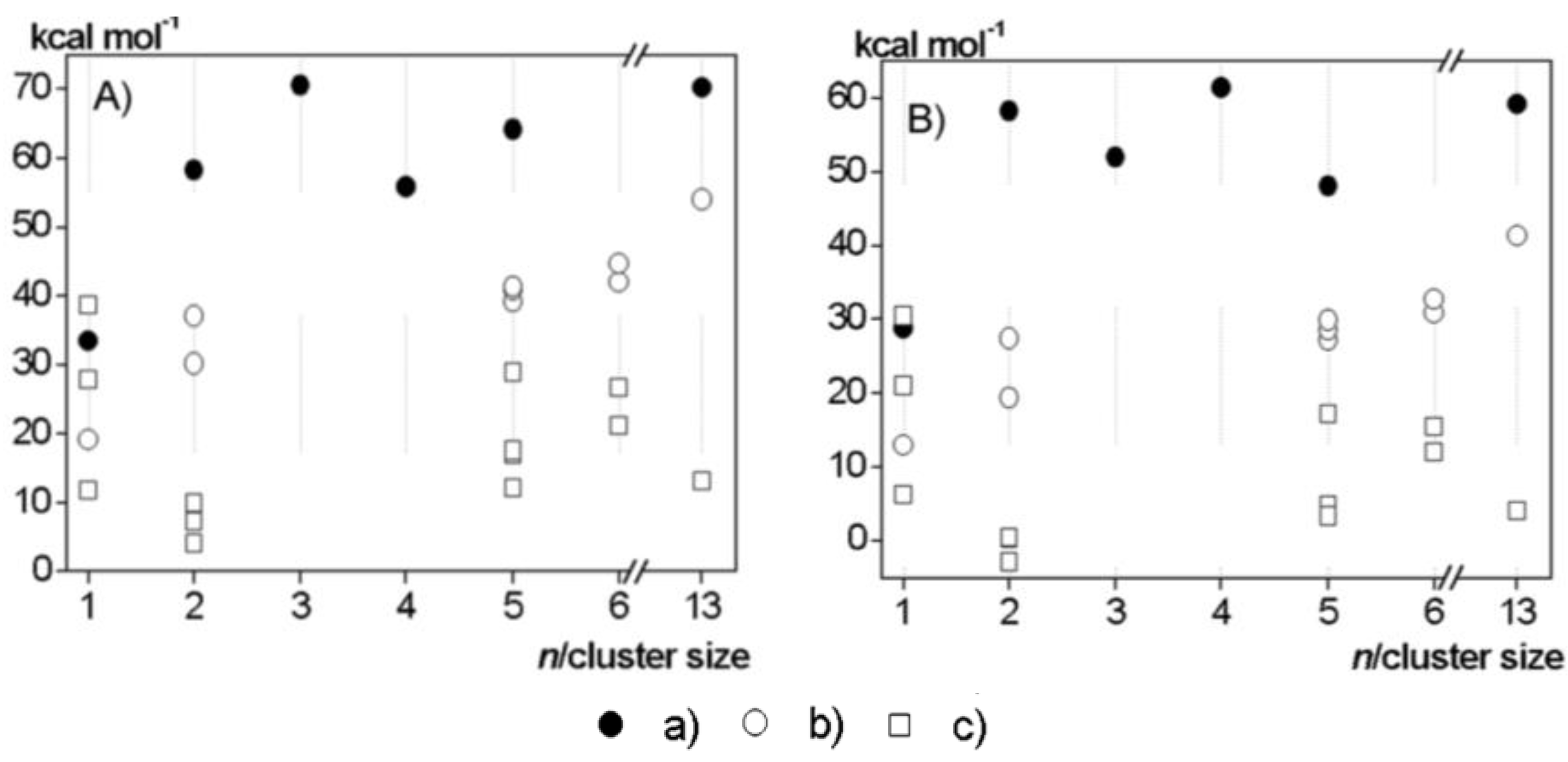
3. Employment of Additional Stabilizer
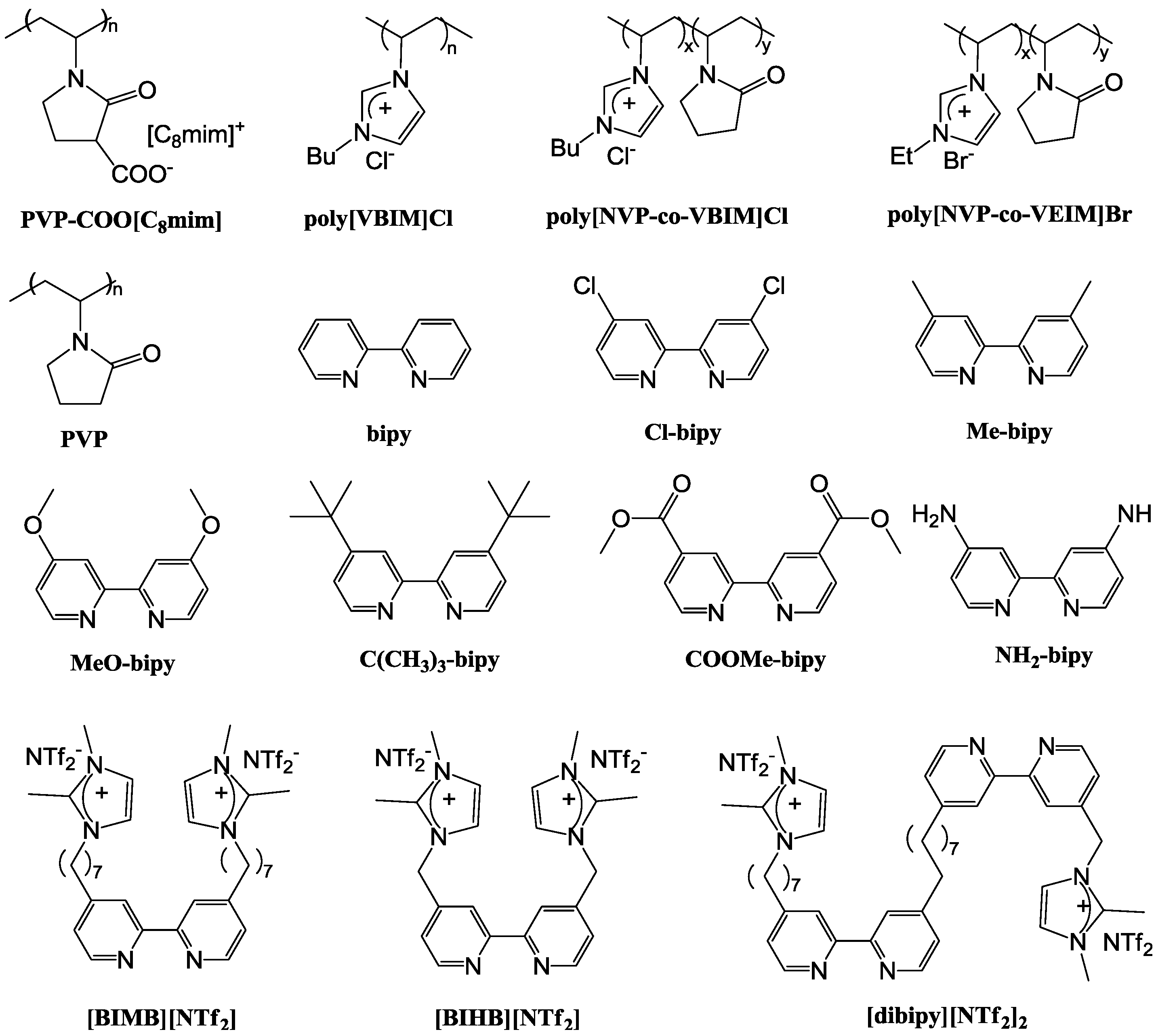
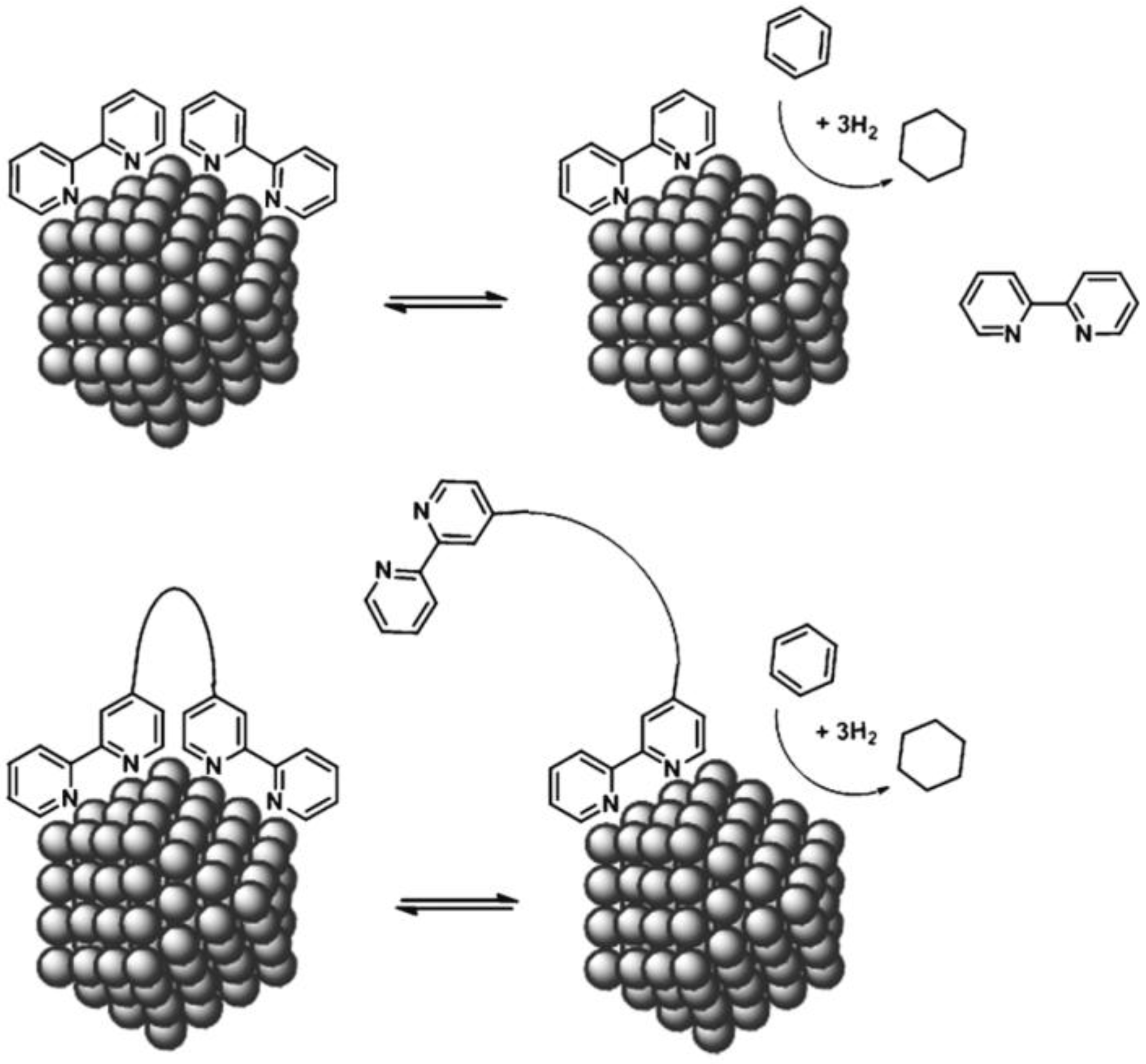
4. Development of Bimetallic NPs
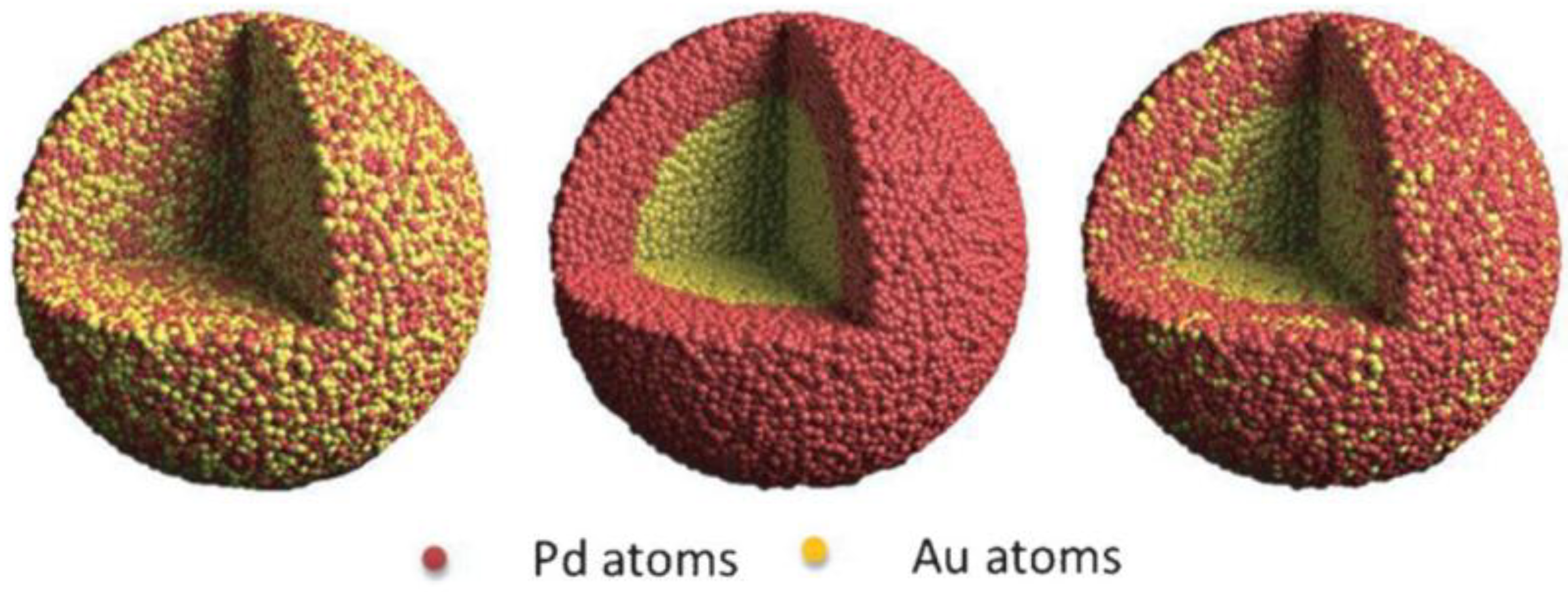
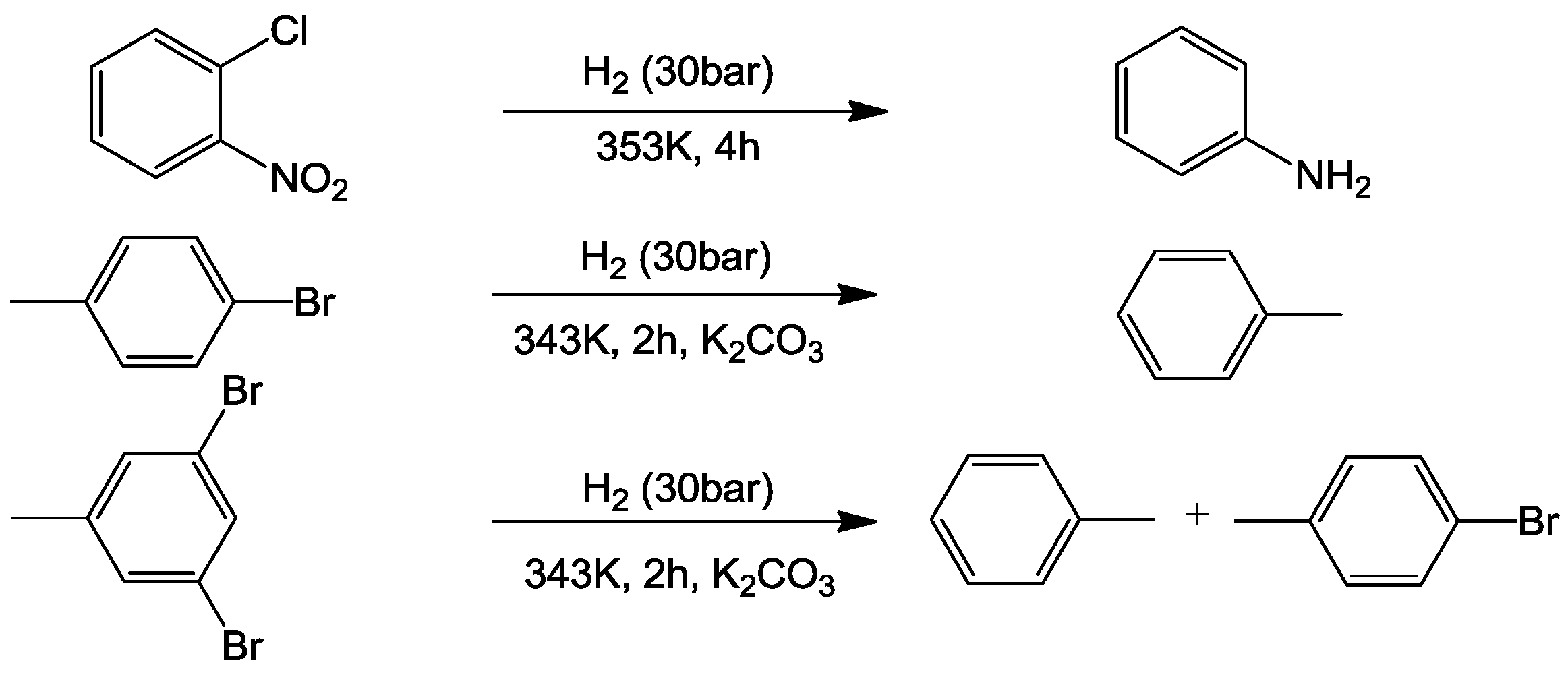
5. Designing of Multifunctional System
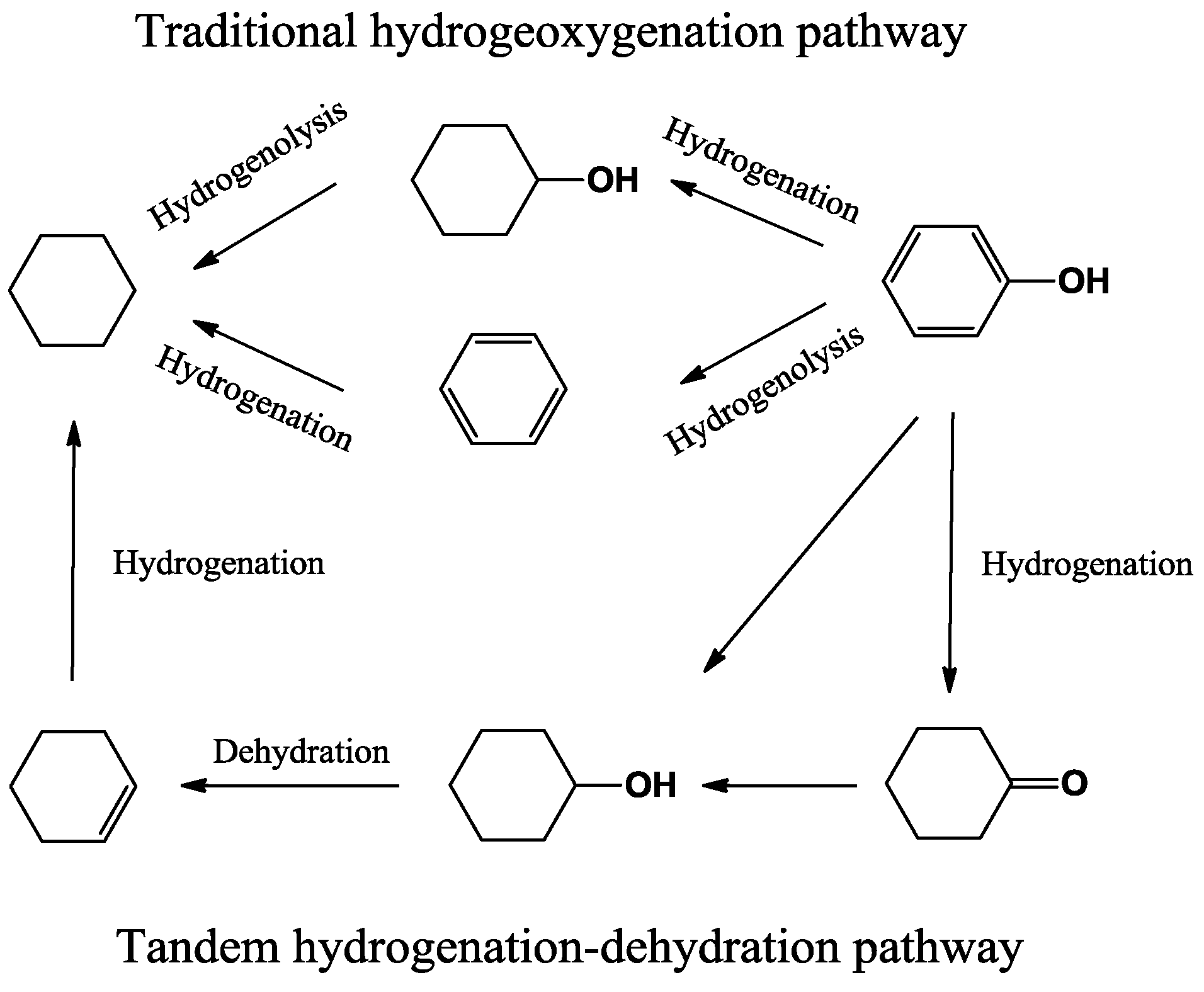
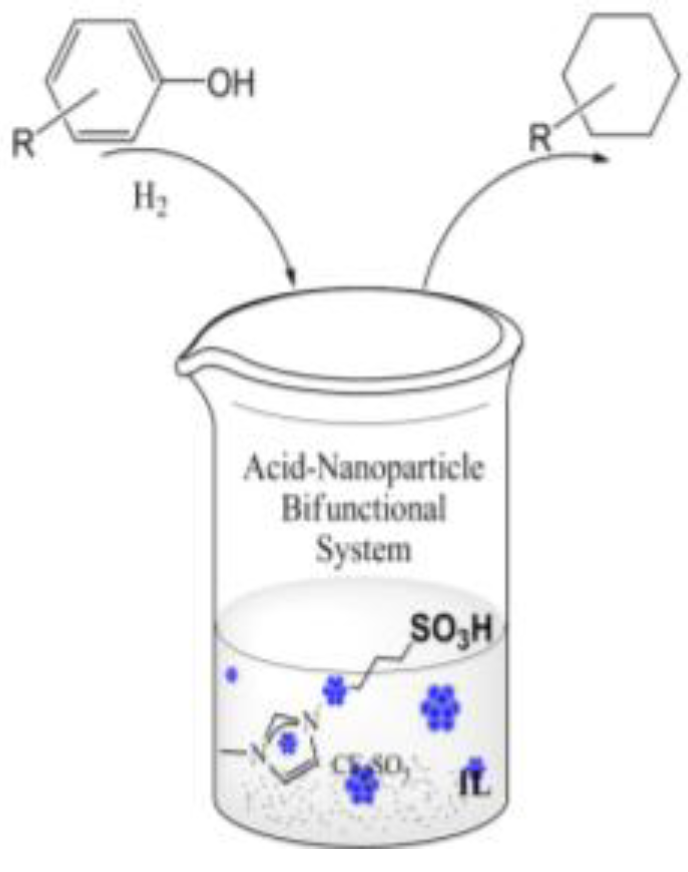
6. Conclusion and Perspectives
Acknowledgments
Conflict of Interest
References
- Dupont, J.; Fonseca, G.S.; Umpierre, A.P.; Fichtner, P.F.P.; Teixeira, S.R. Transition-Metal Nanoparticles in Imidazolium Ionic Liquids: Recycable Catalysts for Biphasic Hydrogenation Reactions. J. Am. Chem. Soc. 2002, 124, 4228–4229. [Google Scholar]
- Lee, J.W.; Shin, J.Y.; Chun, Y.S.; Jang, H.B.; Song, C.E.; Lee, S.-G. Toward Understanding the Origin of Positive Effects of Ionic Liquids on Catalysis: Formation of More Reactive Catalysts and Stabilization of Reactive Intermediates and Transition States in Ionic Liquids. Acc. Chem. Res. 2010, 43, 985–994. [Google Scholar] [CrossRef]
- Ariga, K.; Vinu, A.; Yamauchi, Y.; Ji, Q.; Hill, J.P. Nanoarchitectonics for Mesoporous Materials. Bull. Chem. Soc. Jpn. 2012, 85, 1–32. [Google Scholar] [CrossRef]
- Datta, K.K.R.; Reddy, B.V.S.; Ariga, K.; Vinu, A. Gold Nanoparticles Embedded in a Mesoporous Carbon Nitride Stabilizer for Highly Efficient Three-Component Coupling Reaction. Angew. Chem. Int. Ed. 2010, 49, 5961–5965. [Google Scholar]
- Yan, N.; Zhao, C.; Luo, C.; Dyson, P.J.; Liu, H.; Kou, Y. One-Step Conversion of Cellobiose to C6-Alcohols Using a Ruthenium Nanocluster Catalyst. J. Am. Chem. Soc. 2006, 128, 8714–8715. [Google Scholar]
- Prechtl, M.H.; Scariot, M.; Scholten, J.D.; Machado, G.; Teixeira, S.R.; Dupont, J. Nanoscale Ru(0) particles: Arene hydrogenation catalysts in imidazolium ionic liquids. Inorg. Chem. 2008, 47, 8995–9001. [Google Scholar] [CrossRef]
- Stratton, S.A.; Luska, K.L.; Moores, A. Rhodium nanoparticles stabilized with phosphine functionalized imidazolium ionic liquids as recyclable arene hydrogenation catalysts. Catal. Today 2012, 183, 96–100. [Google Scholar] [CrossRef]
- Roucoux, A.; Nowicki, A.; Philippot, K. Rhodium and Ruthenium Nanoparticles in Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 349–388. [Google Scholar]
- Leger, B.; Denicourt-Nowicki, A.; Olivier-Bourbigou, H.; Roucoux, A. Imidazolium-functionalized bipyridine derivatives: a promising family of ligands for catalytical Rh(0) colloids. Tetrahedron Lett. 2009, 50, 6531–6533. [Google Scholar] [CrossRef]
- Seddon, K.R.; Stark, A. Selective catalytic oxidation of benzyl alcohol and alkylbenzenes in ionic liquids. Green Chem. 2002, 4, 119–123. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, B.; Peng, Y.; Yan, F. Fabrication of ionic liquid-functionalized polypyrrole nanotubes decorated with platinum nanoparticles and their electrocatalytic oxidation of methanol. Chem. Commun. 2011, 47, 2934–2936. [Google Scholar] [CrossRef]
- Balanta, A.; Godard, C.; Claver, C. Pd nanoparticles for C–C coupling reactions. Chem. Soc. Rev. 2011, 40, 4973–4985. [Google Scholar] [CrossRef]
- Fernandez, F.; Cordero, B.; Durand, J.; Muller, G.; Malbosc, F.; Kihn, Y.; Teuma, E.; Gomez, M. Palladium catalyzed Suzuki C-C couplings in an ionic liquid: Nanoparticles responsible for the catalytic activity. Dalton Trans. 2007, 5572–5581. [Google Scholar]
- Durand, J.; Teuma, E.; Malbosc, F.; Kihn, Y.; Gómez, M. Palladium nanoparticles immobilized in ionic liquid: An outstanding catalyst for the Suzuki C–C coupling. Catal. Commun. 2008, 9, 273–275. [Google Scholar]
- Calò, V.; Nacci, A.; Monopoli, A.; Cotugno, P. Palladium-Nanoparticle-Catalysed Ullmann Reactions in Ionic Liquids with Aldehydes as the Reductants: Scope and Mechanism. Chem. Eur. J. 2009, 15, 1272–1279. [Google Scholar]
- Cheng, J.; Tang, L.; Xu, J. An Economical, Green Pathway to Biaryls: Palladium Nanoparticles Catalyzed Ullmann Reaction in Ionic Liquid/Supercritical Carbon Dioxide System. Adv. Synth. Catal. 2010, 352, 3275–3286. [Google Scholar] [CrossRef]
- Xiao, C.-X.; Cai, Z.-P.; Wang, T.; Kou, Y.; Yan, N. Aqueous-phase Fischer-Tropsch synthesis with a ruthenium nanocluster catalyst. Angew. Chem. Int. Ed. 2008, 47, 746–749. [Google Scholar]
- Yinghuai, Z.; Widjaja, E.; Pei Sia, S.L.; Zhan, W.; Carpenter, K.; Maguire, J.A.; Hosmane, N.S.; Hawthorne, M.F. Ruthenium(0) Nanoparticle-Catalyzed Isotope Exchange between 10 B and 11 B Nuclei in Decaborane(14). J. Am. Chem. Soc. 2007, 129, 6507–6512. [Google Scholar]
- Geldbach, T.J.; Zhao, D.; Castillo, N.C.; Laurenczy, G.; Weyershausen, B.; Dyson, P.J. Biphasic Hydrosilylation in Ionic Liquids: A Process Set for Industrial Implementation. J. Am. Chem. Soc. 2006, 128, 9773–9780. [Google Scholar]
- Yinghuai, Z.; Chenyan, K.; Peng, A.T.; Emi, A.; Monalisa, W.; Kui-Jin Louis, L.; Hosmane, N.S.; Maguire, J.A. Catalytic Phenylborylation Reaction by Iridium(0) Nanoparticles Produced from Hydridoiridium Carborane. Inorg. Chem. 2008, 47, 5756–5761. [Google Scholar]
- Lin, Q.; Yang, C.; Jiang, W.; Chen, H.; Li, X. Carbonylation of iodobenzene catalyzed by water-souble palladium–phosphine complexes in ionic liquid. J. Mol. Catal. A 2007, 264, 17–21. [Google Scholar] [CrossRef]
- Fei, Z.; Geldbach, T.J.; Zhao, D.; Dyson, P.J. From dysfunction to bis-function: On the design and applications of functionalized ionic liquids. Chem. Eur. J. 2006, 12, 2122–2130. [Google Scholar]
- Dupont, J.; Scholten, J.D. On the structural and surface properties of transition-metal nanoparticles in ionic liquids. Chem. Soc. Rev. 2010, 39, 1780–1804. [Google Scholar]
- Prechtl, M.H.G.; Campbell, P.S.; Scholten, J.D.; Fraser, G.B.; Machado, G.; Santini, C.C.; Dupont, J.; Chauvin, Y. Imidazolium ionic liquids as promoters and stabilizing agents for the preparation of metal(0) nanoparticles by reduction and decomposition of organometallic complexes. Nanoscale 2010, 2, 2601–2606. [Google Scholar] [CrossRef]
- Prechtl, M.H.G.; Scholten, J.D.; Dupont, J. Carbon-Carbon Cross Coupling Reactions in Ionic Liquids Catalysed by Palladium Metal Nanoparticles. Molecules 2010, 15, 3441–3461. [Google Scholar] [CrossRef]
- Yan, N.; Xiao, C.; Kou, Y. Transition metal nanoparticle catalysis in green solvents. Coord. Chem. Rev. 2010, 254, 1179–1218. [Google Scholar] [CrossRef]
- Luska, K.L.; Moores, A. Functionalized Ionic Liquids for the Synthesis of Metal Nanoparticles and their Application in Catalysis. ChemCatChem 2012, 4, 1534–1546. [Google Scholar] [CrossRef]
- Scholten, J.D.; Leal, B.C.; Dupont, J. Transition Metal Nanoparticle Catalysis in Ionic Liquids. ACS Catal. 2012, 2, 184–200. [Google Scholar] [CrossRef]
- Neouze, M.-A. About the interactions between nanoparticles and imidazolium moieties: emergence of original hybrid materials. J. Mater. Sci. 2010, 20, 9593–9607. [Google Scholar]
- Vollmer, C.; Janiak, C. Naked metal nanoparticles from metal carbonyls in ionic liquids: Easy synthesis and stabilization. Coord. Chem. Rev. 2011, 255, 2039–2057. [Google Scholar] [CrossRef]
- Katsyuba, S.A.; Zvereva, E.E.; Yan, N.; Yuan, X.; Kou, Y.; Dyson, P.J. Rationalization of Solvation and Stabilization of Palladium Nanoparticles in Imidazolium-Based Ionic Liquids by DFT and Vibrational Spectroscopy. ChemPhysChem 2012, 13, 1781–1790. [Google Scholar]
- Zhao, D.; Fei, Z.; Geldbach, T.J.; Scopelliti, R.; Dyson, P.J. Nitrile-Functionalized Pyridinium Ionic Liquids: Synthesis, Characterization, and Their Application in Carbon−Carbon Coupling Reactions. J. Am. Chem. Soc. 2004, 126, 15876–15882. [Google Scholar]
- Schrekker, H.S.; Gelesky, M.A.; Stracke, M.P.; Schrekker, C.M.L.; Machado, G.; Teixeira, S.R.; Rubim, J.C.; Dupont, J. Disclosure of the imidazolium cation coordination and stabilization mode in ionic liquid stabilized gold(0) nanoparticles. J. Colloid Interface Sci. 2007, 316, 189–195. [Google Scholar]
- Yang, X.; Fei, Z.; Geldbach, T.J.; Phillips, A.D.; Hartinger, C.G.; Li, Y.; Dyson, P.J. Suzuki Coupling Reactions in Ether-Functionalized Ionic Liquids: The Importance of Weakly Interacting Cations. Organometallics 2008, 27, 3971–3977. [Google Scholar]
- Zhang, H.; Cui, H. Synthesis and Characterization of Functionalized Ionic Liquid-Stabilized Metal (Gold and Platinum) Nanoparticles and Metal Nanoparticle/Carbon Nanotube Hybrids. Langmuir 2009, 25, 2604–2612. [Google Scholar] [CrossRef]
- Fei, Z.; Zhao, D.; Pieraccini, D.; Ang, W.H.; Geldbach, T.J.; Scopelliti, R.; Chiappe, C.; Dyson, P.J. Development of Nitrile-Functionalized Ionic Liquids for C–C Coupling Reactions: Implication of Carbene and Nanoparticle Catalysts. Organometallics 2007, 26, 1588–1598. [Google Scholar]
- Venkatesan, R.; Prechtl, M.H.G.; Scholten, J.D.; Pezzi, R.P.; Machado, G.; Dupont, J. Palladium nanoparticle catalysts in ionic liquids: Synthesis, characterisation and selective partial hydrogenation of alkynes to Z-alkenes. J. Mater. Sci. 2011, 21, 3030–3036. [Google Scholar]
- Tatumi, R.; Fujihara, H. Remarkably stable gold nanoparticles functionalized with a zwitterionic liquid based on imidazolium sulfonate in a high concentration of aqueous electrolyte and ionic liquid. Chem. Commun. 2005, 83–85. [Google Scholar] [CrossRef]
- Yang, X.; Yan, N.; Fei, Z.; Crespo-Quesada, R.M.; Laurenczy, G.; Kiwi-Minsker, L.; Kou, Y.; Li, Y.; Dyson, P.J. Biphasic Hydrogenation over PVP Stabilized Rh Nanoparticles in Hydroxyl Functionalized Ionic Liquids. Inorg. Chem. 2008, 47, 7444–7446. [Google Scholar] [CrossRef]
- Yan, N.; Yang, X.; Fei, Z.; Li, Y.; Kou, Y.; Dyson, P.J. Solvent-enhanced coupling of sterically hindered reagents and aryl chlorides using functionalized ionic liquids. Organometallics 2009, 28, 937–939. [Google Scholar]
- Mathews, C.J.; Smith, P.J.; Welton, T. Palladium catalysed Suzuki cross-coupling reactions in ambient temperature ionic liquids. Chem. Commun. 2000, 1249–1250. [Google Scholar] [CrossRef]
- Rajagopal, R.; Jarikote, D.V.; Srinivasan, K.V. Ultrasound promoted Suzuki cross-coupling reactions in ionic liquid at ambient conditions. Chem. Commun. 2002, 616–617. [Google Scholar]
- Wang, R.; Twamley, B.; Shreeve, J.N.M. A Highly Efficient, Recyclable Catalyst for C−C Coupling Reactions in Ionic Liquids: Pyrazolyl-Functionalized N-Heterocyclic Carbene Complex of Palladium(II). J. Org. Chem. 2005, 71, 426–429. [Google Scholar]
- Daguenet, C.; Dyson, P.J. Inhibition of Catalytic Activity in Ionic Liquids: Implications for Catalyst Design and the Effect of Cosolvents. Organometallics 2004, 23, 6080–6083. [Google Scholar] [CrossRef]
- Yuan, X.; Yan, N.; Katsyuba, S.A.; Zvereva, E.E.; Kou, Y.; Dyson, P.J. A remarkable anion effect on palladium nanoparticle formation and stabilization in hydroxyl-functionalized ionic liquids. Phys. Chem. Chem. Phys. 2012, 14, 6026–6033. [Google Scholar]
- Song, H.; Yan, N.; Fei, Z.; Kilpin, K.J.; Scopelliti, R.; Li, X.; Dyson, P.J. Evaluation of ionic liquid soluble imidazolium tetrachloropalladate pre-catalysts in Suzuki coupling reactions. Catal. Today 2012, 183, 172–177. [Google Scholar]
- Chiappe, C.; Pieraccini, D.; Zhao, D.; Fei, Z.; Dyson, P.J. Remarkable Anion and Cation Effects on Stille Reactions in Functionalised Ionic Liquids. Adv. Synth. Catal. 2006, 348, 68–74. [Google Scholar] [CrossRef]
- Khare, V.; Li, Z.; Mantion, A.; Ayi, A.A.; Sonkaria, S.; Voelkl, A.; Thunemann, A.F.; Taubert, A. Strong anion effects on gold nanoparticle formation in ionic liquids. J. Mater. Sci. 2010, 20, 1332–1339. [Google Scholar]
- Williams, D.B.; Stoll, M.E.; Scott, B.L.; Costa, D.A.; Oldham, J.W.J. Coordination chemistry of the bis(trifluoromethylsulfonyl)imide anion: molecular interactions in room temperature ionic liquids. Chem. Commun. 2005, 1438–1440. [Google Scholar]
- Yuan, Y.; Yan, N.; Dyson, P.J. Advances in the Rational Design of Rhodium Nanoparticle Catalysts: Control via Manipulation of the Nanoparticle Core and Stabilizer. ACS Catal. 2012, 2, 1057–1069. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, H.-Z.; Yan, N.; Xiao, C.-X.; Mu, X.-D.; Dyson, P.J.; Kou, Y. Ionic-liquid-like copolymer stabilized nanocatalysts in ionic liquids: II. Rhodium-catalyzed hydrogenation of arenes. J. Catal. 2007, 250, 33–40. [Google Scholar]
- Yuan, X.; Yan, N.; Xiao, C.; Li, C.; Fei, Z.; Cai, Z.; Kou, Y.; Dyson, P.J. Highly selective hydrogenation of aromatic chloronitro compounds to aromatic chloroamines with ionic-liquid-like copolymer stabilized platinum nanocatalysts in ionic liquids. Green Chem. 2010, 12, 228–233. [Google Scholar] [CrossRef]
- Zou, M.; Mu, X.; Yan, N.; Kou, Y. Selective hydrogenation of cinnamaldehyde by ionic copolymer-stabilized Pt nanoparticles in ionic liquids. Cuihua Xuebao 2007, 28, 389–391. [Google Scholar]
- Yuan, Y.; Yao, S.; Wang, M.; Lou, S.; Yan, N. Recent Progress in Chemoselective Hydrogenation of α,β-Unsaturated Aldehyde to Unsaturated Alcohol Over Nanomaterials. Curr. Org. Chem. 2013, 17, 400–413. [Google Scholar]
- Yan, N.; Zhang, J.-G.; Tong, Y.; Yao, S.; Xiao, C.; Li, Z.; Kou, Y. Solubility adjustable nanoparticles stabilized by a novel PVP based family: Synthesis, characterization and catalytic properties. Chem. Commun. 2009, 4423–4425. [Google Scholar]
- Yan, N.; Zhang, J.; Yuan, Y.; Chen, G.-T.; Dyson, P.J.; Li, Z.-C.; Kou, Y. Thermoresponsive polymers based on polyvinylpyrrolidone: Applications in nanoparticle catalysis. Chem. Commun. 2010, 46, 1631–1633. [Google Scholar]
- Zhang, J.; Yuan, Y.; Kilpin, K.J.; Kou, Y.; Dyson, P.J.; Yan, N. Thermally responsive gold nanocatalysts based on a modified poly-vinylpyrrolidone. J. Mol. Catal. A 2013, 371, 29–35. [Google Scholar] [CrossRef]
- Chen, G.-T.; Wang, C.-H.; Zhang, J.-G.; Wang, Y.; Zhang, R.; Du, F.-S.; Yan, N.; Kou, Y.; Li, Z.-C. Toward Functionalization of Thermoresponsive Poly(N-vinyl-2-pyrrolidone). Macromolecules 2010, 43, 9972–9981. [Google Scholar]
- Yan, N.; Yuan, Y.; Dyson, P.J. Rhodium nanoparticle catalysts stabilized with a polymer that enhances stability without compromising activity. Chem. Commun. 2011, 47, 2529–2531. [Google Scholar]
- Roucoux, A.; Schulz, J.; Patin, H. Reduced Transition Metal Colloids: A Novel Family of Reusable Catalysts? Chem. Rev. 2002, 102, 3757–3778. [Google Scholar] [CrossRef]
- Yuan, Y.; Yan, N.; Dyson, P.J. pH-Sensitive Gold Nanoparticle Catalysts for the Aerobic Oxidation of Alcohols. Inorg. Chem. 2011, 50, 11069–11074. [Google Scholar] [CrossRef]
- Axet, M.R.; Castillon, S.; Claver, C.; Philippot, K.; Lecante, P.; Chaudret, B. Chiral diphosphite-modified rhodium(0) nanoparticles: Catalyst reservoir for styrene hydroformylation. Eur. J. Inorg. Chem. 2008, 3460–3466. [Google Scholar]
- Salas, G.; Santini, C.C.; Philippot, K.; Colliere, V.; Chaudret, B.; Fenet, B.; Fazzini, P.F. Influence of amines on the size control of in situ synthesized ruthenium nanoparticles in imidazolium ionic liquids. Dalton Trans. 2011, 40, 4660–4668. [Google Scholar]
- Denicourt-Nowicki, A.; Leger, B.; Roucoux, A. N-Donor ligands based on bipyridine and ionic liquids: an efficient partnership to stabilize rhodium colloids. Focus on oxygen-containing compounds hydrogenation. Phys. Chem. Chem. Phys. 2011, 13, 13510–13517. [Google Scholar] [CrossRef]
- Dykeman, R.R.; Yan, N.; Scopelliti, R.; Dyson, P.J. Enhanced rate of arene hydrogenation with imidazolium functionalized bipyridine stabilized rhodium nanoparticle catalysts. Inorg. Chem. 2011, 50, 717–719. [Google Scholar]
- Snelders, D.J.M.; Yan, N.; Gan, W.; Laurenczy, G.; Dyson, P.J. Tuning the Chemoselectivity of Rh Nanoparticle Catalysts by Site-Selective Poisoning with Phosphine Ligands: The Hydrogenation of Functionalized Aromatic Compounds. ACS Catal. 2012, 2, 201–207. [Google Scholar]
- Dykeman, R.R.; Yuan, Y.; Yan, N.; Asakura, H.; Teramura, K.; Tanaka, T.; Dyson, P.J. Rational Design of a Molecular Nanocatalyst-Stabilizer that Enhances both Catalytic Activity and Nanoparticle Stability. ChemCatChem 2012, 4, 1907–1910. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X. Bimetallic Nanoparticles: Kinetic Control Matters. Angew. Chem. Int. Ed. 2012, 51, 3311–3313. [Google Scholar] [CrossRef]
- Okazaki, K.-I.; Kiyama, T.; Hirahara, K.; Tanaka, N.; Kuwabata, S.; Torimoto, T. Single-step synthesis of gold-silver alloy nanoparticles in ionic liquids by a sputter deposition technique. Chem. Commun. 2008, 691–693. [Google Scholar]
- Pearson, A.; O'Mullane, A.P.; Bansal, V.; Bhargava, S.K. Galvanic replacement mediated transformation of Ag nanospheres into dendritic Au-Ag nanostructures in the ionic liquid [BMIM][BF4]. Chem. Commun. 2010, 46, 731–733. [Google Scholar]
- Dash, P.; Scott, R.W.J. 1-Methylimidazole stabilization of gold nanoparticles in imidazolium ionic liquids. Chem. Commun. 2009, 812–814. [Google Scholar] [CrossRef]
- Xiao, F.; Zhao, F.; Mei, D.; Mo, Z.; Zeng, B. Nonenzymatic glucose sensor based on ultrasonic-electrodeposition of bimetallic PtM (M = Ru, Pd and Au) nanoparticles on carbon nanotubes–ionic liquid composite film. Biosens. Bioelectron. 2009, 24, 3481–3486. [Google Scholar] [CrossRef]
- Arquilliere, P.P.; Santini, C.C.; Haumesser, P.H.; Aouine, M. Synthesis of copper and copper-ruthenium nanoparticles in ionic liquids for the metallization of advanced interconnect structures. ECS Trans. 2011, 35, 11–16. [Google Scholar]
- Andanson, J.-M.; Marx, S.; Baiker, A. Selective hydrogenation of cyclohexenone on iron-ruthenium nano-particles suspended in ionic liquids and CO2-expanded ionic liquids. Catal. Sci. Technol. 2012, 2, 1403–1409. [Google Scholar] [CrossRef]
- Dash, P.; Dehm, N.A.; Scott, R.W.J. Bimetallic PdAu nanoparticles as hydrogenation catalysts in imidazolium ionic liquids. J. Mol. Catal. A 2008, 286, 114–119. [Google Scholar] [CrossRef]
- Dash, P.; Miller, S.M.; Scott, R.W.J. Stabilizing nanoparticle catalysts in imidazolium-based ionic liquids: A comparative study. J. Mol. Catal. A 2010, 329, 86–95. [Google Scholar] [CrossRef]
- Hu, B.; Wu, T.; Ding, K.; Zhou, X.; Jiang, T.; Han, B. Seeding Growth of Pd/Au Bimetallic Nanoparticles on Highly Cross-Linked Polymer Microspheres with Ionic Liquid and Solvent-Free Hydrogenation. J. Phys. Chem. C 2010, 114, 3396–3400. [Google Scholar]
- Yuan, X.; Sun, G.; Asakura, H.; Tanaka, T.; Chen, X.; Yuan, Y.; Laurenczy, G.; Kou, Y.; Dyson, P.J.; Yan, N. Development of Palladium Surface-Enriched Heteronuclear Au–Pd Nanoparticle Dehalogenation Catalysts in an Ionic Liquid. Chem. Eur. J. 2013, 19, 1227–1234. [Google Scholar]
- Hutchings, G.J. Nanocrystalline gold and gold-palladium alloy oxidation catalysts: A personal reflection on the nature of the active sites. Dalton Trans. 2008, 5523–5536. [Google Scholar] [CrossRef]
- Yang, X.; Chen, D.; Liao, S.; Song, H.; Li, Y.; Fu, Z.; Su, Y. High-performance Pd–Au bimetallic catalyst with mesoporous silica nanoparticles as support and its catalysis of cinnamaldehyde hydrogenation. J. Catal. 2012, 291, 36–43. [Google Scholar]
- Sheldon, R.A. E factors, green chemistry and catalysis: An odyssey. Chem. Commun. 2008, 3352–3365. [Google Scholar] [CrossRef]
- Pinto, A.C.; Lapis, A.A.M.; da Silva, B.V.; Bastos, R.S.; Dupont, J.; Neto, B.A.D. Pronounced ionic liquid effect in the synthesis of biologically active isatin-3-oxime derivatives under acid catalysis. Tetrahedron Lett. 2008, 49, 5639–5641. [Google Scholar]
- Hajipour, A.R.; Rajaei, A.; Ruoho, A.E. A mild and efficient method for preparation of azides from alcohols using acidic ionic liquid [H-NMP]HSO4. Tetrahedron Lett. 2009, 50, 708–711. [Google Scholar] [CrossRef]
- Harmer, M.A.; Junk, C.P.; Rostovtsev, V.V.; Marshall, W.J.; Grieco, L.M.; Vickery, J.; Miller, R.; Work, S. Catalytic reactions using superacids in new types of ionic liquids. Green Chem. 2009, 11, 517–525. [Google Scholar] [CrossRef]
- Yan, N.; Yuan, Y.; Dykeman, R.; Kou, Y.; Dyson, P.J. Hydrodeoxygenation of Lignin-Derived Phenols into Alkanes by Using Nanoparticle Catalysts Combined with Bronsted Acidic Ionic Liquids. Angew. Chem. Int. Ed. 2010, 49, 5549–5553. [Google Scholar] [CrossRef]
- Yan, N.; Zhao, C.; Dyson, P.J.; Wang, C.; Liu, L.-T.; Kou, Y. Selective degradation of wood lignin over noble-metal catalysts in a two-step process. ChemSusChem 2008, 1, 626–629. [Google Scholar] [CrossRef]
- Zhao, C.; Kou, Y.; Lemonidou, A.A.; Li, X.; Lercher, J.A. Hydrodeoxygenation of bio-derived phenols to hydrocarbons using RANEY Ni and Nafion/SiO2 catalysts. Chem. Commun. 2010, 46, 412–414. [Google Scholar] [CrossRef]
- Zhao, C.; Kou, Y.; Lemonidou, A.A.; Li, X.; Lercher, J.A. Highly Selective Catalytic Conversion of Phenolic Bio-Oil to Alkanes. Angew. Chem. Int. Ed. 2009, 48, 3987–3990. [Google Scholar]
- Thomazeau, C.; Olivier-Bourbigou, H.; Magna, L.; Luts, S.; Gilbert, B. Determination of an Acidic Scale in Room Temperature Ionic Liquids. J. Am. Chem. Soc. 2003, 125, 5264–5265. [Google Scholar] [CrossRef]
- Asakura, H.; Teramura, K.; Shishido, T.; Tanaka, T.; Yan, N.; Xiao, C.X.; Yao, S.Y.; Kou, Y. In situ time-resolved DXAFS study of Rh nanoparticle formation mechanism in ethylene glycol at elevated temperature. Phys. Chem. Chem. Phys. 2012, 14, 2983–2990. [Google Scholar] [CrossRef]
- Yao, S.; Yuan, Y.; Xiao, C.; Li, W.; Kou, Y.; Dyson, P.J.; Yan, N.; Asakura, H.; Teramura, K.; Tanaka, T. Insights into the Formation Mechanism of Rhodium Nanocubes. J. Phys. Chem. C 2012, 116, 15076–15086. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, X.; Yao, Q.; Yu, Y.; Yan, N.; Xie, J. Scalable and Precise Synthesis of Thiolated Au10–12, Au15, Au18, and Au25 Nanoclusters via pH Controlled CO Reduction. Chem. Mater. 2013, 25, 946–952. [Google Scholar] [CrossRef]
- Toikkanen, O.; Ruiz, V.; Rönnholm, G.; Kalkkinen, N.; Liljeroth, P.; Quinn, B.M. Synthesis and Stability of Monolayer-Protected Au38 Clusters. J. Am. Chem. Soc. 2008, 130, 11049–11055. [Google Scholar]
- Qian, H.; Jin, R. Ambient Synthesis of Au144(SR)60 Nanoclusters in Methanol. Chem. Mater. 2011, 23, 2209–2217. [Google Scholar] [CrossRef]
- Jadzinsky, P.D.; Calero, G.; Ackerson, C.J.; Bushnell, D.A.; Kornberg, R.D. Structure of a Thiol Monolayer-Protected Gold Nanoparticle at 1.1 Å Resolution. Science 2007, 318, 430–433. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, B.; Yan, N. Towards Rational Design of Nanoparticle Catalysis in Ionic Liquids. Catalysts 2013, 3, 543-562. https://doi.org/10.3390/catal3020543
Zhang B, Yan N. Towards Rational Design of Nanoparticle Catalysis in Ionic Liquids. Catalysts. 2013; 3(2):543-562. https://doi.org/10.3390/catal3020543
Chicago/Turabian StyleZhang, Bin, and Ning Yan. 2013. "Towards Rational Design of Nanoparticle Catalysis in Ionic Liquids" Catalysts 3, no. 2: 543-562. https://doi.org/10.3390/catal3020543



