Real-Time Raman Monitoring during Photocatalytic Epoxidation of Cyclohexene over V-Ti/MCM-41 Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
| Entry | Nomenclatures | V:Ti:Si atomic weight ratio a (nominal) | V loading b (wt.%) | Ti loading b (wt.%) | V:Ti:Si atomic weight ratio c (ICP-AES) | ||||
|---|---|---|---|---|---|---|---|---|---|
| V | Ti | Si | V | Ti | Si | ||||
| 1 | V2Ti0/MCM-41 | 2 | 0 | 100 | 0.36 | 0 | 1.7 | 0 | 100 |
| 2 | V1Ti1/MCM-41 | 1 | 1 | 100 | 0.18 | 0.29 | 0.9 | 1.3 | 100 |
| 3 | V0.25Ti2/MCM-41 | 0.25 | 2 | 100 | 0.04 | 0.53 | 0.22 | 2.3 | 100 |
| 4 | V0.05Ti4/MCM-41 | 0.05 | 4 | 100 | 0.01 | 0.96 | 0.06 | 4.2 | 100 |
| 5 | V0Ti5/MCM-41 | 0 | 5 | 100 | 0 | 1.12 | 0 | 4.9 | 100 |
| Entry | Catalysts | Specific surface area (m2/g) | BJH average pore diameter (nm) | Band gap (eV) |
|---|---|---|---|---|
| 1 | V2Ti0/MCM-41 | 856 | 3.0 | 3.1 |
| 2 | V1Ti1/MCM-41 | 815 | 3.0 | 3.4 |
| 3 | V0.25Ti2/MCM-41 | 891 | 2.9 | 4.5 |
| 4 | V0.05Ti4/MCM-41 | 841 | 2.9 | 4.4 |
| 5 | V0Ti5/MCM-41 | 850 | 2.9 | 4.3 |




2.2. Liquid-Phase Epoxidation of Cyclohexene
| Entry | Conditions | Conversion of cyclohexene (%) | ||
|---|---|---|---|---|
| Catalyst | UV irradiation | Temp. (°C) | ||
| 1 | NO | NO | 80 | 5.5 |
| 2 | NO | YES | 60 | 3.4 |
| 3 | V0.25Ti2/MCM-41 | NO | 60 | 0.9 |
| 4 | MCM-41 | YES | 60 | 0.9 |
2.2.1. Effect of V-Ti/Si Weight Ratio on the Photocatalytic Epoxidation
| Entry | Catalysts | V/Ti ratio | Conversion of cyclohexene (%) |
|---|---|---|---|
| 1 | V2Ti0/MCM-41 | - | 37 |
| 2 | V1Ti1/MCM-41 | 1 | 44 |
| 3 | V0.25Ti2/MCM-41 | 0.125 | 35 |
| 4 | V0.05Ti4/MCM-41 | 0.0125 | 38 |
| 5 | V0Ti5/MCM-41 | - | 47 |
2.2.2. Catalytic and Photocatalytic Activity Comparison on V0.25Ti2/MCM-41 Catalyst

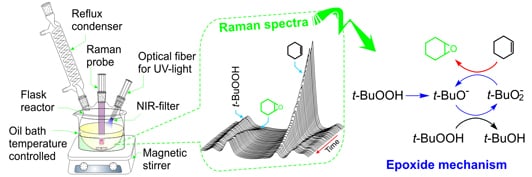
2.3. Real-time Raman Monitoring of Cyclohexene Epoxidation


2.4. Proposed Photo-Mechanism for the Formation of Cyclohexene Epoxidation

3. Experimental Section
3.1. Preparation of Catalysts

3.2. Characterization of Catalysts
3.3. Liquid-Phase Photocatalytic Epoxidation of Cyclohexene

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oyama, S.T. Rates, Kinetics, and Mechanisms of Epoxidation: Homogeneous, Heterogeneous, and Biological Routes. In Mechanisms in Homogeneous and Heterogeneous Epoxidation Catalysis; Oyama, S.T., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 3–99. [Google Scholar]
- Nguyen, V.-H.; Chan, H.-Y.; Wu, J.C.S.; Bai, H. Direct gas-phase photocatalytic epoxidation of propylene with molecular oxygen by photocatalysts. Chem. Eng. J. 2012, 179, 285–294. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Chan, H.-Y.; Wu, J. Synthesis, characterization and photo-epoxidation performance of Au-loaded photocatalysts. J. Chem. Sci. 2013, 125, 859–867. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Wu, J.C.S.; Bai, H. Temperature effect on the photo-epoxidation of propylene over V-Ti/MCM-41 photocatalyst. Catal. Commun. 2013, 33, 57–60. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Lin, S.D.; Wu, J.C.-S.; Bai, H. Artificial sunlight and ultraviolet light induced photo-epoxidation of propylene over V-Ti/MCM-41 photocatalyst. Beilstein J. Nanotechnol. 2014, 5, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-H.; Lin, S.D.; Wu, J.C.S.; Bai, H. Influence of co-feeds additive on the photo-epoxidation of propylene over V-Ti/MCM-41 photocatalyst. Catal. Today 2015, 245, 186–191. [Google Scholar] [CrossRef]
- Kim, I.; Kim, S.M.; Ha, C.-S.; Park, D.-W. Synthesis and cyclohexene oxide/carbon dioxide copolymerizations of zinc acetate complexes bearing bidentate pyridine-alkoxide ligands. Macromol. Rapid Commun. 2004, 25, 888–893. [Google Scholar] [CrossRef]
- Anaya de Parrodi, C.; Juaristi, E. Chiral 1,2-amino alcohols and 1,2-diamines derived from cyclohexene oxide: recent applications in asymmetric synthesis. Synlett 2006, 2006, 2699–2715. [Google Scholar]
- Gago, S.; Rodríguez-Borges, J.E.; Teixeira, C.; Santos, A.M.; Zhao, J.; Pillinger, M.; Nunes, C.D.; Petrovski, Ž.; Santos, T.M.; Kühn, F.E.; et al. Synthesis, characterization and catalytic studies of bis(chloro)dioxomolybdenum(VI)-chiral diimine complexes. J. Mol. Catal. A 2005, 236, 1–6. [Google Scholar] [CrossRef]
- Li, G.; Xu, M.; Larsen, S.C.; Grassian, V.H. Photooxidation of cyclohexane and cyclohexene in BaY. J. Mol. Catal. A 2003, 194, 169–180. [Google Scholar] [CrossRef]
- Larsen, R.G.; Saladino, A.C.; Hunt, T.A.; Mann, J.E.; Xu, M.; Grassian, V.H.; Larsen, S.C. A Kinetic Study of the Thermal and Photochemical Partial Oxidation of Cyclohexane with Molecular Oxygen in Zeolite Y. J. Catal. 2001, 204, 440–449. [Google Scholar] [CrossRef]
- Fraile, J.M.; Garcı́a, J.I.; Mayoral, J.A.; Vispe, E. Optimization of cyclohexene epoxidation with dilute hydrogen peroxide and silica-supported titanium catalysts. Appl. Catal. A 2003, 245, 363–376. [Google Scholar] [CrossRef]
- Anand, C.; Srinivasu, P.; Mane, G.P.; Talapaneni, S.N.; Benzigar, M.R.; Vishnu Priya, S.; Al-deyab, S.S.; Sugi, Y.; Vinu, A. Direct synthesis and characterization of highly ordered cobalt substituted KIT-5 with 3D nanocages for cyclohexene epoxidation. Micropor. Mesopor. Mat. 2013, 167, 146–154. [Google Scholar] [CrossRef]
- Jin, F.; Chen, S.-Y.; Jang, L.-Y.; Lee, J.-F.; Cheng, S. New Ti-incorporated MCM-36 as an efficient epoxidation catalyst prepared by pillaring MCM-22 layers with titanosilicate. J. Catal. 2014, 319, 247–257. [Google Scholar] [CrossRef]
- Calvino Casilda, V.; Pérez-Mayoral, E.; Bañares, M.A.; Lozano Diz, E. Real-time Raman monitoring of dry media heterogeneous alkylation of imidazole with acidic and basic catalysts. Chem. Eng. J. 2010, 161, 371–376. [Google Scholar] [CrossRef]
- Mikolajska, E.; Calvino-Casilda, V.; Bañares, M.A. Real-time Raman monitoring of liquid-phase cyclohexene epoxidation over alumina-supported vanadium and phosphorous catalysts. Appl. Catal. A 2012, 421–422, 164–171. [Google Scholar] [CrossRef]
- Schmink, J.R.; Holcomb, J.L.; Leadbeater, N.E. Use of raman spectroscopy as an in situ tool to obtain kinetic data for organic transformations. Chem. Eur. J. 2008, 14, 9943–9950. [Google Scholar] [CrossRef] [PubMed]
- Moreno, T.; Morán López, M.A.; Huerta Illera, I.; Piqueras, C.M.; Sanz Arranz, A.; García Serna, J.; Cocero, M.J. Quantitative Raman determination of hydrogen peroxide using the solvent as internal standard: Online application in the direct synthesis of hydrogen peroxide. Chem. Eng. J. 2011, 166, 1061–1065. [Google Scholar] [CrossRef]
- Mozharov, S.; Nordon, A.; Littlejohn, D.; Wiles, C.; Watts, P.; Dallin, P.; Girkin, J.M. Improved Method for Kinetic Studies in Microreactors Using Flow Manipulation and Noninvasive Raman Spectrometry. J. Am. Chem. Soc. 2011, 133, 3601–3608. [Google Scholar] [CrossRef] [PubMed]
- Calvino-Casilda, V.; Banares, M.A. Recent advances in imaging and monitoring of heterogeneous catalysts with Raman spectroscopy. In Catalysis; The Royal Society of Chemistry: Cambridge, UK, 2012; Volume 24, pp. 1–47. [Google Scholar]
- Bañares, M.A. Operando methodology: Combination of in situ spectroscopy and simultaneous activity measurements under catalytic reaction conditions. Catal. Today 2005, 100, 71–77. [Google Scholar] [CrossRef]
- Salkic, S.; Eckler, L.H.; Nee, M.J. Noninvasive monitoring of photocatalytic degradation of X-ray contrast media using Raman spectrometry. J. Raman Spec. 2013, 44, 1746–1752. [Google Scholar] [CrossRef]
- Gao, X.; Wachs, I.E. Titania–silica as catalysts: molecular structural characteristics and physico-chemical properties. Catal. Today 1999, 51, 233–254. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, D.J.; Chang, S.H.; Ahn, W.S. Catalytic applications of MCM-41 with different pore sizes in selected liquid phase reactions. Appl. Catal. A 2003, 254, 225–237. [Google Scholar] [CrossRef]
- Geobaldo, F.; Bordiga, S.; Zecchina, A.; Giamello, E.; Leofanti, G.; Petrini, G. DRS UV-Vis and EPR spectroscopy of hydroperoxo and superoxo complexes in titanium silicalite. Catal. Lett. 1992, 16, 109–115. [Google Scholar] [CrossRef]
- Laha, S.C.; Kumar, R. Promoter-induced synthesis of MCM-41 type mesoporous materials including Ti- and V-MCM-41 and their catalytic properties in oxidation reactions. Micropor. Mesopor. Mat. 2002, 53, 163–177. [Google Scholar] [CrossRef]
- Arnold, A.B.J.; Niederer, J.P.M.; Nießen, T.E.W.; Hölderich, W.F. The influence of synthesis parameters on the vanadium content and pore size of [V]-MCM-41 materials. Micropor. Mesopor. Mat. 1999, 28, 353–360. [Google Scholar] [CrossRef]
- Lewandowska, A.E.; Banares, M.A.; Tielens, F.; Che, M.; Dzwigaj, S. Different Kinds of Tetrahedral V Species in Vanadium-Containing Zeolites Evidenced by Diffuse Reflectance UV-vis, Raman, and Periodic Density Functional Theory. J. Phys. Chem. C 2010, 114, 19771–19776. [Google Scholar] [CrossRef]
- Peña, M.L.; Dejoz, A.; Fornés, V.; Rey, F.; Vázquez, M.I.; López Nieto, J.M. V-containing MCM-41 and MCM-48 catalysts for the selective oxidation of propane in gas phase. Appl. Catal. A 2001, 209, 155–164. [Google Scholar] [CrossRef]
- Wagner, C.D.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Data for Use in X-ray Photoelectron Spectroscopy; Perkin-Elmer Corp., Physical Electronics Division: Eden Prairie, MN, USA, 1979; pp. 68–71. [Google Scholar]
- Castillo, R.; Koch, B.; Ruiz, P.; Delmon, B. Influence of the Amount of Titania on the Texture and Structure of Titania Supported on Silica. J. Catal. 1996, 161, 524–529. [Google Scholar] [CrossRef]
- Matsuoka, M.; Anpo, M. Local structures, excited states, and photocatalytic reactivities of highly dispersed catalysts constructed within zeolites. J. Photochem. Photobiol. C 2003, 3, 225–252. [Google Scholar] [CrossRef]
- Anpo, M.; Kim, T.-H.; Matsuoka, M. The design of Ti-, V-, Cr-oxide single-site catalysts within zeolite frameworks and their photocatalytic reactivity for the decomposition of undesirable molecules—The role of their excited states and reaction mechanisms. Catal. Today 2009, 142, 114–124. [Google Scholar] [CrossRef]
- Zou, J.-J.; Liu, Y.; Pan, L.; Wang, L.; Zhang, X. Photocatalytic isomerization of norbornadiene to quadricyclane over metal (V, Fe and Cr)-incorporated Ti-MCM-41. Appl. Catal. B 2010, 95, 439–445. [Google Scholar] [CrossRef]
- Davydov, L.; Reddy, E.P.; France, P.; Smirniotis, P.G. Transition-Metal-Substituted Titania-Loaded MCM-41 as Photocatalysts for the Degradation of Aqueous Organics in Visible Light. J. Catal. 2001, 203, 157–167. [Google Scholar] [CrossRef]
- Hung, C.; Bai, H.; Karthik, M. Ordered mesoporous silica particles and Si-MCM-41 for the adsorption of acetone: A comparative study. Sep. Purif. Technol. 2009, 64, 265–272. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, H.-Y.; Nguyen, V.-H.; Wu, J.C.S.; Calvino-Casilda, V.; Bañares, M.A.; Bai, H. Real-Time Raman Monitoring during Photocatalytic Epoxidation of Cyclohexene over V-Ti/MCM-41 Catalysts. Catalysts 2015, 5, 518-533. https://doi.org/10.3390/catal5020518
Chan H-Y, Nguyen V-H, Wu JCS, Calvino-Casilda V, Bañares MA, Bai H. Real-Time Raman Monitoring during Photocatalytic Epoxidation of Cyclohexene over V-Ti/MCM-41 Catalysts. Catalysts. 2015; 5(2):518-533. https://doi.org/10.3390/catal5020518
Chicago/Turabian StyleChan, Hsiang-Yu, Van-Huy Nguyen, Jeffrey C.S. Wu, Vanesa Calvino-Casilda, Miguel A. Bañares, and Hsunling Bai. 2015. "Real-Time Raman Monitoring during Photocatalytic Epoxidation of Cyclohexene over V-Ti/MCM-41 Catalysts" Catalysts 5, no. 2: 518-533. https://doi.org/10.3390/catal5020518