Preparation and Electrocatalytic Characteristics of PdW/C Catalyst for Ethanol Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. TEM
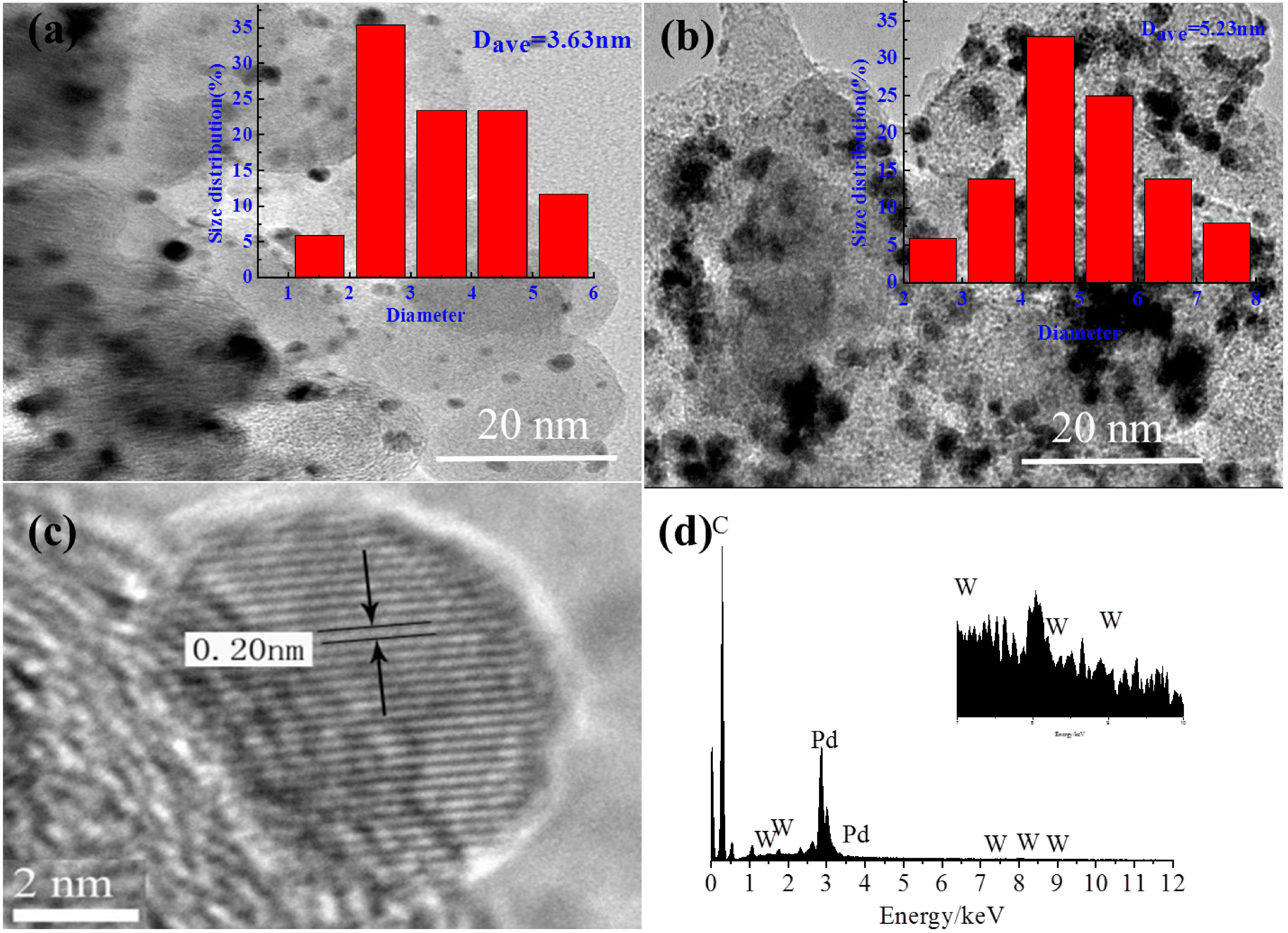
2.2. XRD

| Catalysts | Pd Metal Loading Detected by ICP-AES | Diameter Calculated Form XRD/nm | Diameter Measured by TEM/nm | EASA/m2·g−1 |
|---|---|---|---|---|
| PdW/C | 10.6% | 3.9 | 3.6 | 144.1 |
| Pd/C | 19.3% | 5.74 | 5.32 | 71.2 |
2.3. Electrochemical Measurements
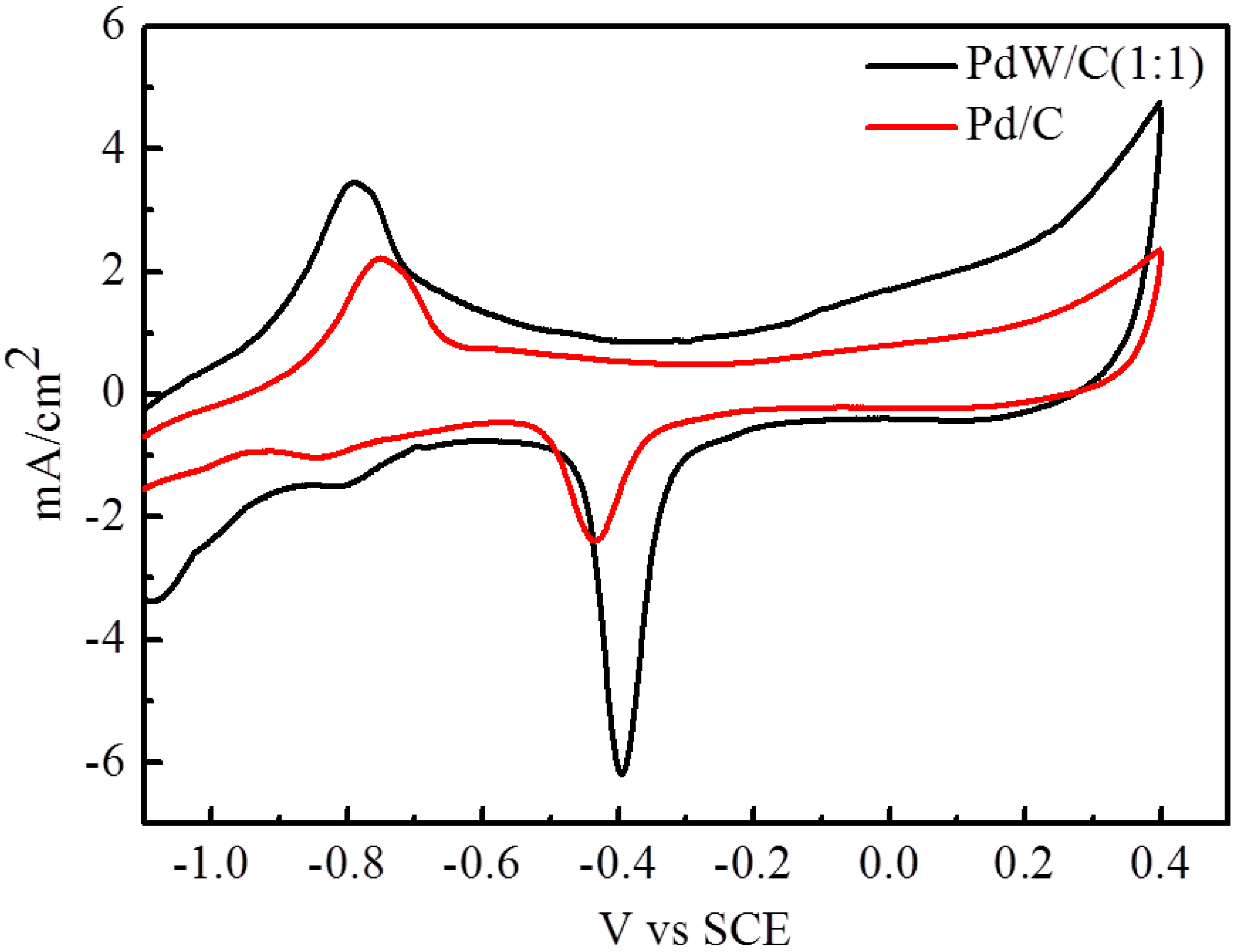
| Catalysts | Eonset/V | Ep/V | ip/mA·cm−2 | i (after 3600 s)/mA·cm−2 |
|---|---|---|---|---|
| PdW/C (1:1) | −0.71 | −0.27 | 62.29 | 6.94 |
| PdW/C (1:2) | −0.63 | −0.32 | 14.02 | 0.42 |
| PdW/C (2:1) | −0.68 | −0.29 | 37.39 | 0.19 |
| PdW/C (4:1) | −0.70 | −0.40 | 15.37 | 0.03 |
| Pd/C | −0.64 | −0.25 | 39.58 | 1.58 |
| Pd/C (JM) | −0.65 | −0.17 | 48.70 | 2.29 |


3. Experimental Section
3.1. Materials
3.2. Catalyst Preparation
3.3. Characterization
3.4. Electrochemical Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cai, Z.-X.; Liu, C.-C.; Wu, G.-H.; Chen, X.-M.; Chen, X. Green synthesis of Pt-on-Pd bimetallic nanodendrites on graphene via in situ reduction, and their enhanced electrocatalytic activity for methanol oxidation. Electrochim. Acta 2014, 127, 377–383. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, Q.J.; Fan, J.C.; Zhou, Y.; Wang, L.L. A Review of Graphene Supported Electrocatalysts for Direct Methanol Fuel Cells. Adv. Mater. Res. 2015, 1070–1072, 492–496. [Google Scholar] [CrossRef]
- Li, Q.X.; Liu, M.S.; Xu, Q.J.; Mao, H.M. Preparation and Electrocatalytic Characteristics Research of Pd/C Catalyst for Direct Ethanol Fuel Cell. J. Chem. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jeon, S. Highly Active Graphene-Supported NixPd100−x Binary Alloyed Catalysts for Electro-Oxidation of Ethanol in an Alkaline Media. ACS Catal. 2014, 4, 1830–1837. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, F.-F.; Yang, Y.-Y.; Cai, W.-B. Carbon supported Pd–Ni–P nanoalloy as an efficient catalyst for ethanol electro-oxidation in alkaline media. J. Power Sources 2013, 243, 369–373. [Google Scholar] [CrossRef]
- Liang, Z.X.; Zhao, T.S.; Xu, J.B.; Zhu, L.D. Mechanism study of the ethanol oxidation reaction on palladium in alkaline media. Electrochim. Acta 2009, 54, 2203–2208. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, J.; Cai, W.; Liang, L.; Xing, W.; Liu, C. Single passive direct methanol fuel cell supplied with pure methanol. J. Power Sources 2011, 196, 2750–2753. [Google Scholar] [CrossRef]
- Wang, Y.; He, Q.; Guo, J.; Wei, H.; Ding, K.; Lin, H.; Bhana, S.; Huang, X.; Luo, Z.; Shen, T.D.; et al. Carboxyl Multiwalled Carbon-Nanotube-Stabilized Palladium Nanocatalysts toward Improved Methanol Oxidation Reaction. ChemElectroChem 2015, 2, 559–570. [Google Scholar] [CrossRef]
- Lim, E.J.; Choi, S.M.; Seo, M.H.; Kim, Y.; Lee, S.; Kim, W.B. Highly dispersed Ag nanoparticles on nanosheets of reduced graphene oxide for oxygen reduction reaction in alkaline media. Electrochem. Commun. 2013, 28, 100–103. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Rego, R.; Fernandes, L.S.; Tavares, P.B. Evaluation of the catalytic activity of Pd–Ag alloys on ethanol oxidation and oxygen reduction reactions in alkaline medium. J. Power Sources 2011, 196, 6092–6098. [Google Scholar] [CrossRef]
- Guo, S.; Sun, S. FePt nanoparticles assembled on graphene as enhanced catalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 2492–2495. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Momma, T.; Osaka, T. Cell performance of Pd–Sn catalyst in passive direct methanol alkaline fuel cell using anion exchange membrane. J. Power Sources 2009, 189, 999–1002. [Google Scholar] [CrossRef]
- Mao, H.; Wang, L.; Zhu, P.; Xu, Q.; Li, Q. Carbon-supported PdSn–SnO2 catalyst for ethanol electro-oxidation in alkaline media. Int. J. Hydrogen Energy 2014, 39, 17583–17588. [Google Scholar] [CrossRef]
- Ding, K.; Wang, Y.; Yang, H.; Zheng, C.; Cao, Y.; Wei, H.; Wang, Y.; Guo, Z. Electrocatalytic activity of multi-walled carbon nanotubes-supported PtxPdy catalysts prepared by a pyrolysis process toward ethanol oxidation reaction. Electrochim. Acta 2013, 100, 147–156. [Google Scholar] [CrossRef]
- Jiang, K.; Cai, W.-B. Carbon supported Pd–Pt–Cu nanocatalysts for formic acid electrooxidation: Synthetic screening and componential functions. App. Catal. 2014, 147, 185–192. [Google Scholar] [CrossRef]
- Sieben, J.M.; Comignani, V.; Alvarez, A.E.; Duarte, M.M.E. Synthesis and characterization of Cu core Pt–Ru shell nanoparticles for the electro-oxidation of alcohols. Int. J. Hydrogen Energy 2014, 39, 8667–8674. [Google Scholar] [CrossRef]
- Ding, L.X.; Wang, A.L.; Li, G.R.; Liu, Z.Q.; Zhao, W.X.; Su, C.Y.; Tong, Y.X. Porous Pt–Ni–P composite nanotube arrays: Highly electroactive and durable catalysts for methanol electrooxidation. J. Am. Chem. Soc. 2012, 134, 5730–5733. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Yin, J.; Liu, M.; Dong, Q.; Su, Y. Synthesis and electrocatalytic alcohol oxidation performance of Pd–Co bimetallic nanoparticles supported on graphene. Int. J. Hydrogen Energy 2014, 39, 1325–1335. [Google Scholar] [CrossRef]
- Kim, Y.; Noh, Y.; Lim, E.J.; Lee, S.; Choi, S.M.; Kim, W.B. Star-shaped Pd@Pt core-shell catalysts supported on reduced graphene oxide with superior electrocatalytic performance. J. Mater. Chem. A 2014, 2, 6976. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, G.; Shu, H.; Oyama, M.; Liu, X.; He, Y. Synthesis of Pt–Pd bimetallic nanoparticles anchored on graphene for highly active methanol electro-oxidation. J. Power Sources 2014, 262, 279–285. [Google Scholar] [CrossRef]
- Datta, J.; Dutta, A.; Mukherjee, S. The Beneficial Role of the Cometals Pd and Au in the Carbon-Supported PtPdAu Catalyst Toward Promoting Ethanol Oxidation Kinetics in Alkaline Fuel Cells: Temperature Effect and Reaction Mechanism. J. Phys. Chem. C 2011, 115, 15324–15334. [Google Scholar] [CrossRef]
- Feng, L.; Yan, L.; Cui, Z.; Liu, C.; Xing, W. High activity of Pd–WO3/C catalyst as anodic catalyst for direct formic acid fuel cell. J. Power Sources 2011, 196, 2469–2474. [Google Scholar] [CrossRef]
- Wang, Z.-B.; Zuo, P.-J.; Yin, G.-P. Effect of W on activity of Pt–Ru/C catalyst for methanol electrooxidation in acidic medium. J. Alloy. Compd. 2009, 479, 395–400. [Google Scholar] [CrossRef]
- Mellinger, Z.J.; Kelly, T.G.; Chen, J.G. Pd-Modified Tungsten Carbide for Methanol Electro-oxidation: From Surface Science Studies to Electrochemical Evaluation. ACS Catal. 2012, 2, 751–758. [Google Scholar] [CrossRef]
- Moon, J.-S.; Lee, Y.-W.; Han, S.-B.; Park, K.-W. Pd nanoparticles on mesoporous tungsten carbide as a non-Pt electrocatalyst for methanol electrooxidation reaction in alkaline solution. Int. J. Hydrogen Energy 2014, 39, 7798–7804. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, Y.; Gao, X.; Wang, X.; Chen, W. Strongly coupled Pd nanotetrahedron/tungsten oxide nanosheet hybrids with enhanced catalytic activity and stability as oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2014, 136, 11687–11697. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhao, Y.; Chen, D.; Wang, X.; Peng, X.; Tian, J. Synthesis of Pd nanoparticles supported on PDDA functionalized graphene for ethanol electro-oxidation. Int. J. Hydrogen Energy 2015, 40, 322–329. [Google Scholar] [CrossRef]
- Hong, W.; Fang, Y.; Wang, J.; Wang, E. One-step and rapid synthesis of porous Pd nanoparticles with superior catalytic activity toward ethanol/formic acid electrooxidation. J. Power Sources 2014, 248, 553–559. [Google Scholar] [CrossRef]
- Wang, Y.; He, Q.; Ding, K.; Wei, H.; Guo, J.; Wang, Q.; O’Connor, R.; Huang, X.; Luo, Z.; Shen, T.D.; et al. Multiwalled Carbon Nanotubes Composited with Palladium Nanocatalysts for Highly Efficient Ethanol Oxidation. J. Electrochem. Soc. 2015, 162, F755–F763. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Zhang, Z.; Xin, L.; Sun, K.; Li, W. Pd–Ni electrocatalysts for efficient ethanol oxidation reaction in alkaline electrolyte. Int. J. Hydrogen Energy 2011, 36, 12686–12697. [Google Scholar] [CrossRef]
- Maghsodi, A.; Milani Hoseini, M.R.; Dehghani Mobarakeh, M.; Kheirmand, M.; Samiee, L.; Shoghi, F.; Kameli, M. Exploration of bimetallic Pt-Pd/C nanoparticles as an electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2011, 257, 6353–6357. [Google Scholar] [CrossRef]
- Shi, J.; Ci, P.; Wang, F.; Peng, H.; Yang, P.; Wang, L.; Wang, Q.; Chu, P.K. Pd/Ni/Si-microchannel-plate-based amperometric sensor for ethanol detection. Electrochim. Acta 2011, 56, 4197–4202. [Google Scholar] [CrossRef]
- Yi, Q.; Niu, F.; Sun, L. Fabrication of novel porous Pd particles and their electroactivity towards ethanol oxidation in alkaline media. Fuel 2011, 90, 2617–2623. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Liu, M.; Li, Q.; Xu, Q. Preparation and Electrocatalytic Characteristics of PdW/C Catalyst for Ethanol Oxidation. Catalysts 2015, 5, 1068-1078. https://doi.org/10.3390/catal5031068
Liu Q, Liu M, Li Q, Xu Q. Preparation and Electrocatalytic Characteristics of PdW/C Catalyst for Ethanol Oxidation. Catalysts. 2015; 5(3):1068-1078. https://doi.org/10.3390/catal5031068
Chicago/Turabian StyleLiu, Qi, Mingshuang Liu, Qiaoxia Li, and Qunjie Xu. 2015. "Preparation and Electrocatalytic Characteristics of PdW/C Catalyst for Ethanol Oxidation" Catalysts 5, no. 3: 1068-1078. https://doi.org/10.3390/catal5031068





