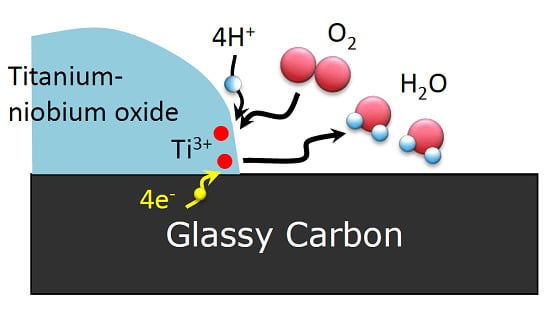
2.1. Characterization of Catalysts
We prepared the titanium-niobium oxide samples with the charged total composition of Ti
0.841Nb
0.126O
2.
Figure 1 shows the X-ray diffraction (XRD) patterns of the titanium-niobium oxide samples prepared at 600, 700, 800, 900, and 1050 °C (a) in air and (b) in Ar containing 4% H
2. The crystalline phase of the catalysts prepared by heat treatment in air at temperatures between 600 and 900 °C was identified as anatase TiO
2 (JCPDS no. 00-021-1272), indicating that the niobium atoms were incorporated into the TiO
2 anatase structure. According to phase diagram of TiO
2–Nb
2O
5 [
12], Nb(V) ions dissolve into TiO
2 rutile structure only below
ca. 10 atomic % in this temperature range. On the other hand, quasi-stable phase, TiO
2 anatase structure, can dissolve more Nb(V) ions. The phase transition from anatase to rutile occurred at temperatures above 900 °C. For samples prepared at higher temperatures, peaks corresponding to the rutile TiO
2 (JCPDS no. 00-021-1276) and TiNb
2O
7 (JCPDS no. 1001270) phases were observed. This is because the Nb(V) ions that cannot dissolve in the TiO
2 rutile structure forms complex oxides TiNb
2O
7 that is solid solution of TiO
2 and Nb
2O
5. Simultaneously, Nb-containing phases such as TiNb
2O
7 appeared at 1050 °C. These results are consistent with previous observations [
13].
Figure 1.
XRD patterns of titanium-niobium oxides prepared at 600, 700, 800, 900, and 1050 °C (a) in air and (b) in Ar containing 4% H2.
Figure 1.
XRD patterns of titanium-niobium oxides prepared at 600, 700, 800, 900, and 1050 °C (a) in air and (b) in Ar containing 4% H2.
The crystalline phase of the samples subjected to heat treatment at 600 and 700 °C in Ar containing 4% H
2 could be indexed to the TiO
2 anatase structure. However, the samples prepared at temperatures above 800 °C under this reductive atmosphere could be indexed to rutile TiO
2 with no Nb
2O
5 peaks. The shift of the XRD peaks to lower angles (
Figure S1) with increasing treatment temperature suggested that the catalysts are substitutional solid solutions in which the niobium ions substitute titanium ions in the rutile TiO
2 lattice. Compared to formation of the rutile phase above 900 °C for the samples heat-treated in air, the rutile was phase formed at 800 °C under reductive atmosphere. Thus, the transformation from the anatase to rutile phase occurred at lower temperature under reductive atmosphere. In addition, the substitutional solid solution (rutile phase) was stable up to 1050 °C under reductive atmosphere. The XRD analysis clearly demonstrated that the TiO
2 rutile-based structure was more stable under reductive atmosphere than in air. This stabilization of the TiO
2 rutile-based structure is not predicted from the viewpoint of thermochemistry. The role of heat-treatment under reductive atmosphere and doped niobium ions must be elucidated.
Figure 2 and
Figure S2 show scanning electron microscopy (SEM) images of the titanium-niobium oxides prepared at 600 °C in air, and 600, 700, 800, 900, and 1050 °C in Ar containing 4% H
2. The SEM images demonstrate that the surface morphology of the titanium-niobium oxides depends on the heat treatment temperature. Very little difference in the surface morphology was observed for the samples prepared by heat treatment at 600 °C under different atmospheres. The particle size of the catalysts prepared at 600 °C was
ca. several tens of nanometers. A significant change in the morphologies of the catalysts was observed with treatment at 800 °C, indicative of particle aggregation above 800 °C. Aggregation became progressive with increasing heat treatment temperatures. Thus, the surface area of the catalysts decreased with temperature, especially above 800 °C.
Figure 2.
SEM images of the titanium-niobium oxides prepared at 600 °C in air, and 600, 700, 800, 900, and 1050 °C in Ar containing 4% H2.
Figure 2.
SEM images of the titanium-niobium oxides prepared at 600 °C in air, and 600, 700, 800, 900, and 1050 °C in Ar containing 4% H2.
Figure 3 shows photographs of the catalysts prepared at 600 °C in air, and 600, 700, 800, 900, and 1050 °C in Ar containing 4% H
2. The powder heat-treated at 600 °C was white, as expected from the wide bandgap of TiO
2 (all samples treated in air were white). On the other hand, the samples heat-treated at 600 °C under reductive atmosphere had a light-blue color and the color deepened with increasing temperature. This color change suggests that there is some difference in the electronic energy levels of the samples prepared under reductive atmosphere relative to those prepared in air. Namely, the difference between the highest occupied and lowest unoccupied electronic energy levels decreases with increasing temperature. This color change suggests the development of a localized energy level of electrons in the bandgap of TiO
2.
Figure 3.
Photographs of the catalysts prepared at 600 °C in air, and 600, 700, 800, 900, and 1050 °C in Ar containing 4% H2.
Figure 3.
Photographs of the catalysts prepared at 600 °C in air, and 600, 700, 800, 900, and 1050 °C in Ar containing 4% H2.
Figure 4a shows the Ti 2p XPS spectra of the catalysts prepared at 800 °C in air and in Ar containing 4% H
2. As anticipated, the Ti 2p XPS spectra revealed that Ti adopted the tetravalent state for the specimen heat-treated in air based on the 2p
3/2 peak (TiO
2; 458.8 eV [
14]). On the other hand, a low valence state,
i.e., Ti
3+ (Ti
2O
3; 456.8 eV [
15]), was observed for the catalyst heat-treated at 800 °C under reductive atmosphere. The ratios of Ti
3+/Ti
4+ calculated from areas of the XPS spectra of the specimens heat-treated at 800 °C in air and in Ar containing 4% H
2 were 5.0% and 10%, respectively. The ratio of the specimen prepared under reductive atmosphere was twice as large as that prepared in air. In addition, the total atomic ratio of Nb/Ti is 0.15 according to the charged total composition of Ti
0.841Nb
0.126O
2. The atomic ratios of Nb/Ti calculated from areas of the XPS spectra of the specimens heat-treated at 800 °C in air and in Ar containing 4% H
2 were 0.43 and 0.23, respectively. Both ratios are larger than the total atomic ratio, suggested that the niobium ions accumulate the surface of the oxide particles. In particular, the Nb/Ti ratio of the specimen heat-treated in air was about three times larger than the total atomic ratio. As mentioned in XRD patterns, because the rutile TiO
2 phase cannot dissolve the Nb(V) ions, the dissolved Nb(V) ions in the anatase TiO
2 phase began to accumulate near the surface of the particles at higher temperature heat treatment.
Figure 4b shows the Ti 2p XPS spectra of the catalysts prepared at 600, 700, 800, 900, and 1050 °C in Ar containing 4% H
2. Low valence states of Ti were observed for the catalyst heat-treated at 600 °C under reductive atmosphere, suggesting that the oxides underwent little reduction at 600 °C in Ar containing 4% H
2 upon treatment for 10 min. Heat-treatment above 700 °C under reductive atmosphere resulted in the formation of low valence state Ti as shown in
Figure 4.
Figure 4.
Ti 2p XPS spectra of the catalysts prepared at 800 °C in air and in Ar containing 4% H2 (a) and prepared at 600, 700, 800, 900, and 1050 °C in Ar containing 4% H2 (b).
Figure 4.
Ti 2p XPS spectra of the catalysts prepared at 800 °C in air and in Ar containing 4% H2 (a) and prepared at 600, 700, 800, 900, and 1050 °C in Ar containing 4% H2 (b).
Figure 5a shows the dependence of the ratios of Ti
3+/Ti
4+ (expressed as
STi(III)/
STi(IV)) calculated from areas of the XPS spectra of the specimens heat-treated under reductive atmosphere on the temperature. The ratio of Ti
3+/Ti
4+ of the specimen prepared at 600 °C is 6.7%. Ti
3+ ions are produced by the substitution of the Nb
5+ ions with Ti
4+ ions of the TiO
2 lattice.
Figure 5b shows the Nb 3d XPS spectra of the catalysts prepared at 600, 700, 800, 900, and 1050 °C in Ar containing 4% H
2. The peak in the Nb 3d spectra shifted to higher binding energy (NbO
2; 205.3 eV [
16], Nb
2O
5; 207.1 eV [
17]) with increasing heat treatment temperatures, in contrast with the Ti 2p peak. Therefore, the Nb 3d XPS spectra revealed that most of Nb ions were highest oxidation state, 5+. Thus, the state of the specimens can be expressed as Ti(IV)
1−2xTi(III)
xNb(V)
xO
2. If all Nb ions substitute Ti
4+ ions of the TiO
2 lattice as Nb(V) ions, the composition is Ti(IV)
0.74Ti(III)
0.13Nb(V)
0.13O
2. Therefore, in that case, the ratio of Ti
3+/Ti
4+ is calculated to be
ca. 18%. The ratio of Ti
3+/Ti
4+ at 600 °C,
ca. 6.7%, was smaller than 18%, indicating that the Nb(V) ions did not sufficiently incorporate into the TiO
2 lattice at 600 °C. As shown in
Figure 5a, the ratio of Ti
3+/Ti
4+ increased with increasing temperature from 600 °C to 700 °C and saturated around 10%. These results deduced that reductive heat-treatment above 700 °C induced the formation of low valence state Ti.
Figure 5.
(a) Dependence of the ratios of Ti3+/Ti4+, STi(III)/STi(IV), calculated from areas of the XPS spectra of the specimens heat-treated under reductive atmosphere on the temperature. (b) Nb 3d XPS spectra of the catalysts prepared at 600, 700, 800, 900, and 1050 °C in Ar containing 4% H2.
Figure 5.
(a) Dependence of the ratios of Ti3+/Ti4+, STi(III)/STi(IV), calculated from areas of the XPS spectra of the specimens heat-treated under reductive atmosphere on the temperature. (b) Nb 3d XPS spectra of the catalysts prepared at 600, 700, 800, 900, and 1050 °C in Ar containing 4% H2.
Figure 6 shows the dependence of the atomic ratio of Nb/Ti calculated from XPS spectra of the specimens prepared under reductive atmosphere on the heat treatment temperature. The atomic ratio of Nb/Ti decreased with increasing temperature above 700 °C and approached the bulk value at 1050 °C. The XRD patterns revealed that the bulk phase transition occurred between 700 and 800 °C under reductive atmosphere. The XPS spectra indicated that the titanium ions near the surface were reduced and the Nb(V) ions near the surface incorporated into the TiO
2 lattice at
ca. 700 °C. Therefore, the phase transition was probably caused by a change in the valence of titanium. We previously demonstrated that tantalum and zirconium oxide-based catalysts had some oxygen vacancies that acted as active sites for the ORR [
6]. In case of the titanium-niobium oxide system, the low valence state of the metal ions does not always indicate the presence of oxygen vacancies. The low valence state of the metal ions can be achieved even in the absence of oxygen vacancies because the highest valence states of titanium and niobium are different. The relationship between the presence of oxygen vacancies and the active sites remains a topic for further study.
Figure 6.
Dependence of the atomic ratio of Nb/Ti calculated from XPS spectra of the specimens prepared under reductive atmosphere on the heat treatment temperature.
Figure 6.
Dependence of the atomic ratio of Nb/Ti calculated from XPS spectra of the specimens prepared under reductive atmosphere on the heat treatment temperature.
It was difficult to evaluate the differences in the electronic state of the catalysts heat-treated under reductive atmosphere at temperatures between 700 and 1050 °C based on the XPS spectra, as shown in
Figure 4b and
Figure 5a. Thus, the ionization potential of the specimens was used as a parameter to evaluate these differences. The ionization potentials of the specimens were measured using a photoelectron spectrometer surface analyzer in order to investigate the differences in the surfaces of the specimens heat-treated in reductive atmosphere at different temperatures.
Figure 7a shows the relationship between the square root of the photoelectric quantum yield and the photon energy (that is, the photoelectron spectra of the specimens heat-treated at 800 °C in air or in Ar containing 4% H
2). The square root of the photoelectric quantum yield increased linearly with an increase in the photon energy applied to each specimen. The slope of the straight line reflects the tendency of the photoelectron emission of the specimens, that is, the density of state of the electrons near the Fermi level. Fewer photoelectrons were emitted in the case of the catalyst prepared in air. The slope of the straight line for the specimen heat-treated in air, where TiO
2 was identified on the sample surface by XPS, was apparently lower than that of the congener prepared under reductive atmosphere. It is remarkable that the slope of this plot was steeper for the specimen prepared in Ar containing 4% H
2. The intersection between the straight line and the background line in the photoelectron spectra provides the threshold energy corresponding to the photoelectric ionization potential. The photoelectric ionization potential corresponds to the highest energy level of the electrons in the materials. The ionization potential is directly affected by the localized electronic levels of the lattice defects and impurities in the metal oxides, such as valence changes due to substitutional metal ions, oxygen vacancies, and donor impurities.
Figure 7b shows the dependence of the ionization potential of the catalysts prepared at 600, 800, and 1050 °C in air, and 600, 700, 800, 900, and 1050 °C in Ar containing 4% H
2 on the heat treatment temperature, θ. The ionization potential of commercial rutile and anatase TiO
2 is 5.8 eV. The ionization potential was the same (
i.e.,
ca. 5.8 eV) for the catalysts prepared at 600, 800, and 1050 °C in air, suggesting that the surface of the catalysts prepared in air had few localized electronic levels from lattice defects and impurities in the metal oxides, similar to commercial TiO
2. On the other hand, the ionization potentials of the catalysts prepared under reductive atmosphere decreased with increasing temperature. The decrease in the ionization potential reflects an increase in the localized electronic levels. In other words, the valence changes due to substitutional metal ions, oxygen vacancies, and donor impurities increase with increasing temperature.
Figure 7.
(a) Relationship between the square root of the photoelectric quantum yield (Y1/2) and the photon energy of the specimens heat-treated at 800 °C in air or in Ar containing 4% H2. (b) Dependence of the ionization potential of the catalysts prepared at 600, 800, and 1050 °C in air, and 600, 700, 800, 900, and 1050 °C in Ar containing 4% H2 on the heat treatment temperature, θ.
Figure 7.
(a) Relationship between the square root of the photoelectric quantum yield (Y1/2) and the photon energy of the specimens heat-treated at 800 °C in air or in Ar containing 4% H2. (b) Dependence of the ionization potential of the catalysts prepared at 600, 800, and 1050 °C in air, and 600, 700, 800, 900, and 1050 °C in Ar containing 4% H2 on the heat treatment temperature, θ.