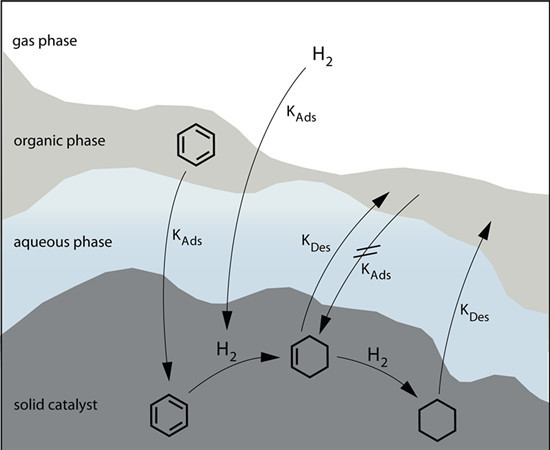
Performance of Ru/La2O3–ZnO Catalyst for the Selective Hydrogenation of Benzene to Cyclohexene
Abstract
:1. Introduction


2. Results and Discussion
| Catalyst (wt. % Ru/Support) | t (h) | STY (gcyclohexene·gRu−1·h−1) | Ru/BEN (1000 mol/mol) | BEN/Add (mol/mol) | Add/Ru (mol/mol) |
|---|---|---|---|---|---|
| 2Ru/Al2O3 [8] | 3.47 | 82 | 0.14 | - | - |
| 5Ru/Al2O3 [13] | 1.5 | 395 | 0.18 | 1371 | 4.14 |
| 7.4Zn–Ru [14] | 1 | 334 | 1.48 | 155 | 4.43 |
| 9RuB–2.5Zn/ZrO2·xH2O [9] | 0.92 | 253 | 1.59 | - | - |
| 4Ru/Ga2O3–ZnO [15] | 2 | 131 | 1.18 | 14 | 62.67 |
| 2Ru/La2O3–ZnO [15] | 2.16 | 224 | 0.59 | 14 | 125.34 |
| 8Ru–Zn/ZrO2 [16] | 0.38 | 656 | 1.41 | 40 | 17.58 |
| 18.4Ru0.08La18.86B/ZrO2 [17] | 1 | 94 | 4.64 | 312 | 0.69 |
| Ru/SiO2 [18] | 0.66 | 605 | 0.85 | 9 | 125.02 |
| 3.8Ru1.2CoB/Al2O3 [10] | 0.5 | 1048 | 0.45 | - | - |
| 12Ru–La/SBA-15 [19] | 0.57 | 387 | 2.12 | 13 | 35.51 |
| 3Ru/ZnO–ZrOx(OH)y [11] | 3.08 | 196 | 0.76 | - | - |
| 8Ru–B/ZrO2–T [20] | 0.25 | 1083 | 1.41 | 8 | 88.41 |
| 5Ru/La2O3 [7] | 3.5 | 74 | 0.44 | 9872 | 0.23 |
| 10Mn–Ru [21] | 0.25 | 157 | 11.45 | 9 | 9.51 |
| Ru + CeO2 [22] | 0.33 | 127 | 11.34 | 9 | 9.61 |
| 2.8Zn–Ru [23] | 0.25 | 171 | 12.24 | 5 | 15.84 |
| Ce–Ru(0,19) [24] | 0.25 | 165 | 11.34 | 5 | 17.11 |
| 2.5Ru–Zn/HAP [25] | 0.83 | 366 | 0.88 | 8 | 151.11 |
| 2Ru/La2O3–ZnO | 3.5 | 97 | 0.53 | 12,488 | 0.15 |
| 1Ru/La2O3 [26] | 1.15 | 187 | 0.53 | 833 | 2.27 |
| 7.4Ru/B–ZrO2 [27] | 0.5 | 598 | 1.30 | 81 | 9.5 |
| 8.8Ru–La/ZrO2–MCM41 [28] | 0.25 | 968 | 1.55 | 12 | 51.57 |
| 1.95Ru–B/TNS [12] | 0.25 | 19,173 | 0.09 | - | - |
2.1. Batch Reaction


2.2. Batch vs. Continuous Process

2.3. Continuous Process


| Line | Catalyst | mcatalyst (g) | mNaDCA (mg) | Flow Breaker |
|---|---|---|---|---|
| (a) | 2Ru/La2O3–ZnOIWR | 3 | 8 | no |
| (b) | 2Ru/La2O3IPR | 0.5 | 20 | no |
| (c) | 2Ru/La2O3IPR | 0.5 | 20 | yes |
| (d) | 2Ru/La2O3–ZnOIWR | 1.5 | 4 | no |
| (e) | 2Ru/La2O3–ZnOIWR | 1.5 | 4 | yes |

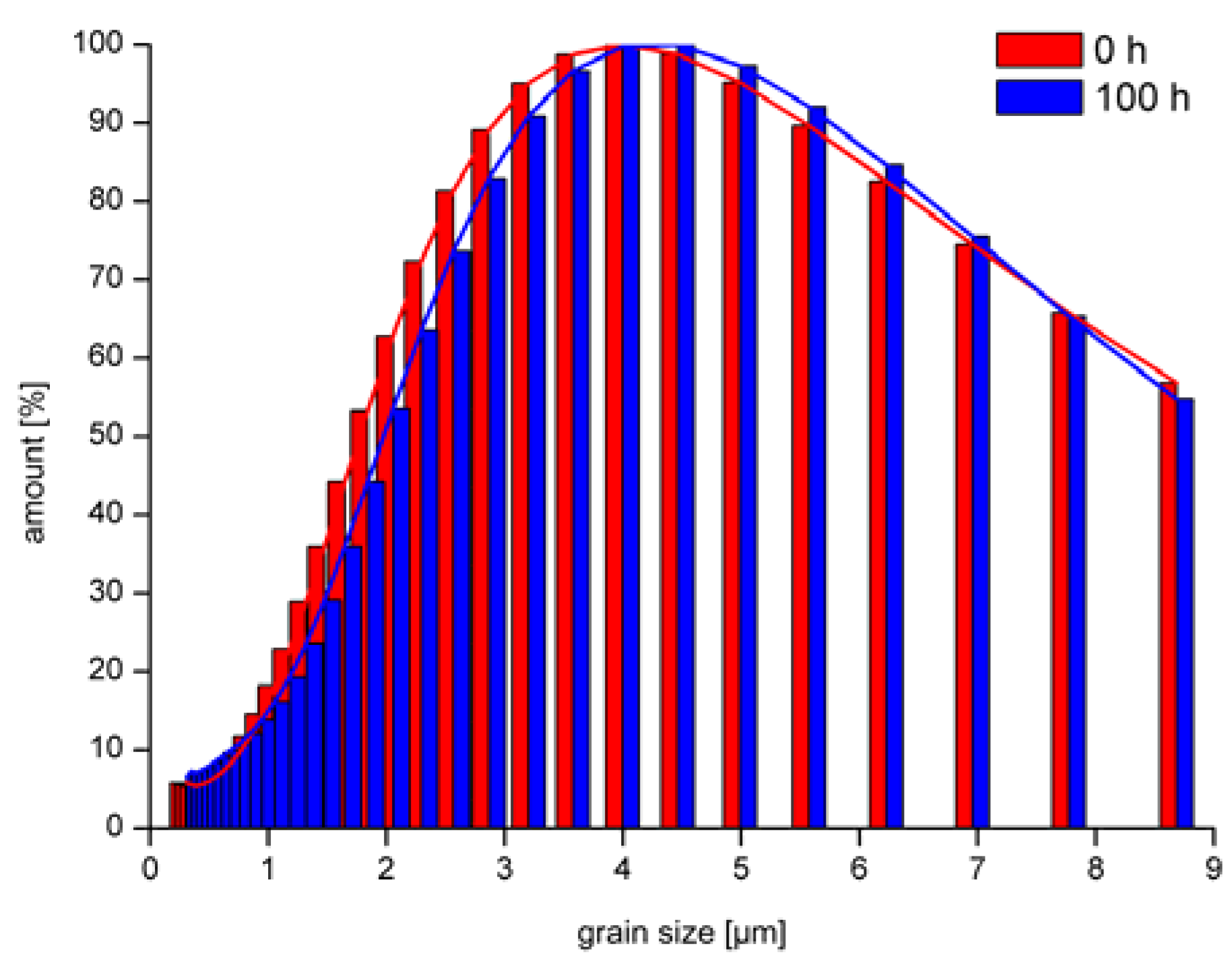
| Stress (h) | Mean Grain Size (µm) | wt. % Ru |
|---|---|---|
| 0 | 3.8 | 2.2 |
| 100 | 5.0 | 2.06 |
2.4. Characterization


3. Experimental Section
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Testing
3.3.1. Batch
3.3.2. Continuous
3.4. Characterization
4. Conclusions
Author Contributions
Conflicts of Interest
Nomenclature
| Add | additive |
| BEN | benzene |
| CHE | cyclohexene |
| ICP-OES | inductively coupled plasma optical emission spectrometry |
| m(cyclohexene)Y,max | mass of cyclohexene at maximum yield |
| m(Ru) | mass of ruthenium |
| NaDCA | sodium dicyanamide |
| S | selectivity |
| STY | Space-time yield |
| t | time |
| tY,max | time at maximum yield of cyclohexene |
| TEM | transmission electron spectroscopy |
| X | conversion |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| Y | yield |
References
- Ronchin, L.; Toniolo, L. Selective hydrogenation of benzene to cyclohexene catalyzed by Ru supported catalysts: Influence of the alkali promoters on kinetics, selectivity and yield. Catal. Today 2001, 66, 363–369. [Google Scholar] [CrossRef]
- De Almeida, M.P.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Lauterbach, T.; Rominger, F.; Hashmi, A.S.K.; Pombeiro, A.J.L.; Figueiredo, J.L. Homogeneous and heterogenised new gold c-scorpionate complexes as catalysts for cyclohexane oxidation. Catal. Sci. Technol. 2013, 3, 3056–3069. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Martins, L.M.D.R.S.; Avalos-Borja, M.; Buijnsters, J.G.; Pombeiro, A.J.L.; Figueiredo, J.L. Gold nanoparticles supported on carbon materials for cyclohexane oxidation with hydrogen peroxide. Appl. Catal. A 2013, 467, 279–290. [Google Scholar] [CrossRef]
- Shan, X.; Cheng, Z.; Li, Y. Solvent effects on hydration of cyclohexene over H-ZSM-5 catalyst. J. Chem. Eng. Data 2011, 56, 4310–4316. [Google Scholar] [CrossRef]
- Shan, X.; Cheng, Z.; Yuan, P. Reaction kinetics and mechanism for hydration of cyclohexene over ion-exchange resin and H-ZSM-5. Chem. Eng. J. 2011, 175, 423–432. [Google Scholar] [CrossRef]
- Schwab, F.; Lucas, M.; Claus, P. Ruthenium-catalyzed selective hydrogenation of benzene to cyclohexene in the presence of an ionic liquid. Angew. Chem. Int. Ed. 2011, 50, 10453–10456. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.; Lucas, M.; Claus, P. Simple selective hydrogenation of benzene to cyclohexene in the presence of sodium dicyanamide. Green Chem. 2013, 15, 646–649. [Google Scholar] [CrossRef]
- Milone, C.; Neri, G.; Donato, A.; Musolino, M.G.; Mercadante, L. Selective hydrogenation of benzene to cyclohexene on Ru/γ-Al2O3. J. Catal. 1996, 159, 253–258. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, S.; Liu, B.; Deng, J.-F. Benzene-selective hydrogenation to cyclohexene over supported ruthenium boride catalysts prepared by a novel method. New J. Chem. 1999, 23, 1057–1057. [Google Scholar] [CrossRef]
- Fan, G.-Y.; Jiang, W.-D.; Wang, J.-B.; Li, R.-X.; Chen, H.; Li, X.-J. Selective hydrogenation of benzene to cyclohexene over RuCoB/γ-Al2O3 without additive. Catal. Commun. 2008, 10, 98–102. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, T.; Han, B.; Liang, S.; Wang, W.; Wu, T.; Yang, G. Highly selective benzene hydrogenation to cyclohexene over supported Ru catalyst without additives. Green Chem. 2011, 13, 1106–1109. [Google Scholar] [CrossRef]
- Liu, J.; He, S.; Li, C.; Wang, F.; Wei, M.; Evans, D.G.; Duan, X. Confined synthesis of ultrafine Ru–B amorphous alloy and its catalytic behavior toward selective hydrogenation of benzene. J. Mater. Chem. A 2014, 2, 7570–7577. [Google Scholar] [CrossRef]
- Suryawanshi, P.T.; Mahajani, V.V. Liquid-phase hydrogenation of benzene to cyclohexene using ruthenium-based heterogeneous catalyst. J. Chem. Technol. Biotechnol. 1997, 69, 154–160. [Google Scholar] [CrossRef]
- Nagahara, H.; Ono, M.; Konishi, M.; Fukuoka, Y. Partial hydrogenation of benzene to cyclohexene. Appl. Surf. Sci. 1997, 121–122, 448–451. [Google Scholar] [CrossRef]
- Hu, S.-C.; Chen, Y.-W. Liquid phase hydrogenation of benzene to cyclohexene on ruthenium catalysts supported on zinc oxide-based binary oxides. J. Chem. Technol. Biotechnol. 2001, 76, 954–958. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Xie, S.; Qiao, M.; Li, H.; Fan, K. Partial hydrogenation of benzene to cyclohexene on a Ru–Zn/m-ZrO2 nanocomposite catalyst. Appl. Catal. A 2004, 272, 29–36. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Z.; Wang, Z.; Zhao, S.; Wu, Y. A novel amorphous alloy Ru–La–B/ZrO2 catalyst with high activity and selectivity for benzene selective hydrogenation. Appl. Catal. A 2006, 313, 49–57. [Google Scholar] [CrossRef]
- Ning, J.; Xu, J.; Liu, J.; Lu, F. Selective hydrogenation of benzene to cyclohexene over colloidal ruthenium catalyst stabilized by silica. Catal. Lett. 2006, 109, 175–180. [Google Scholar] [CrossRef]
- Liu, J.-L.; Zhu, Y.; Liu, J.; Pei, Y.; Li, Z.H.; Li, H.; Li, H.-X.; Qiao, M.-H.; Fan, K.-N. Discrimination of the roles of CdSO4 and ZnSO4 in liquid phase hydrogenation of benzene to cyclohexene. J. Catal. 2009, 268, 100–105. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, J.; Tan, X.; Pei, Y.; Qiao, M.; Fan, K.; Zong, B. Effect of support acidity on liquid-phase hydrogenation of benzene to cyclohexene over Ru–B/ZrO2 catalysts. Ind. Eng. Chem. Res. 2012, 51, 12205–12213. [Google Scholar]
- Sun, H.-J.; Pan, Y.-J.; Jiang, H.-B.; Li, S.-H.; Zhang, Y.-X.; Liu, S.-C.; Liu, Z.-Y. Effect of transition metals (Cr, Mn, Fe, Co, Ni, Cu and Zn) on the hydrogenation properties of benzene over Ru-based catalyst. Appl. Catal. A 2013, 464–465, 1–9. [Google Scholar] [CrossRef]
- Sun, H.; Chen, L.; Li, S.; Jiang, H.; Zhang, Y.; Ren, B.; Liu, Z.; Liu, S. Selective hydrogenation of benzene to cyclohexene over monometallic ruthenium catalysts in the presence of CeO2 and ZnSO4 as co-modifiers. J. Rare Earths 2013, 31, 1023–1028. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, H.; Li, S.; Dong, Y.; Wang, H.; Pan, Y.; Liu, S.; Tang, M.; Liu, Z. Effect of alcohols as additives on the performance of a nano-sized Ru–Zn(2.8%) catalyst for selective hydrogenation of benzene to cyclohexene. Chem. Eng. J. 2013, 218, 415–424. [Google Scholar] [CrossRef]
- Sun, H.; Pan, Y.; Li, S.; Zhang, Y.; Dong, Y.; Liu, S.; Liu, Z. Selective hydrogenation of benzene to cyclohexene over ce-promoted Ru catalysts. J. Energy Chem. 2013, 22, 710–716. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, T.; Jiang, T.; Wang, W.; Liu, H.; Fan, H.; Zhang, Z.; Han, B. Ru–Zn supported on hydroxyapatite as an effective catalyst for partial hydrogenation of benzene. Green Chem. 2013, 15, 152–159. [Google Scholar] [CrossRef]
- Schwab, F. Eine Grüne Route der Selektiven Hydrierung von Benzol zu Cyclohexen. Ph.D. Thesis, Technische Universität, Darmstadt, Germany, August 2014. [Google Scholar]
- Zhou, G.; Pei, Y.; Jiang, Z.; Fan, K.; Qiao, M.; Sun, B.; Zong, B. Doping effects of B in ZrO2 on structural and catalytic properties of Ru/B–ZrO2 catalysts for benzene partial hydrogenation. J. Catal. 2014, 311, 393–403. [Google Scholar] [CrossRef]
- Liao, H.; Ouyang, D.; Zhang, J.; Xiao, Y.; Liu, P.; Hao, F.; You, K.; Luo, H.A. Benzene hydrogenation over oxide-modified MCM-41 supported ruthenium-lanthanum catalyst: The influence of zirconia crystal form and surface hydrophilicity. Chem. Eng. J. 2014, 243, 207–216. [Google Scholar] [CrossRef]
- Sun, H.; Guo, W.; Zhou, X.; Chen, Z.; Liu, Z.; Liu, S. Progress in Ru-based amorphous alloy catalysts for selective hydrogenation of benzene to cyclohexene. Chin. J. Catal. 2011, 32, 1–16. [Google Scholar] [CrossRef]
- Musser, M.T. Cyclohexanol and cyclohexanone. In Ullmanns Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Spod, H.; Lucas, M.; Claus, P. Continuously conducted selective hydrogenation of benzene to cyclohexene in a four-phase system. Chem. Eng. Technol. 2015, 38, 1340–1342. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spod, H.; Lucas, M.; Claus, P. Performance of Ru/La2O3–ZnO Catalyst for the Selective Hydrogenation of Benzene to Cyclohexene. Catalysts 2015, 5, 1756-1769. https://doi.org/10.3390/catal5041756
Spod H, Lucas M, Claus P. Performance of Ru/La2O3–ZnO Catalyst for the Selective Hydrogenation of Benzene to Cyclohexene. Catalysts. 2015; 5(4):1756-1769. https://doi.org/10.3390/catal5041756
Chicago/Turabian StyleSpod, Hendrik, Martin Lucas, and Peter Claus. 2015. "Performance of Ru/La2O3–ZnO Catalyst for the Selective Hydrogenation of Benzene to Cyclohexene" Catalysts 5, no. 4: 1756-1769. https://doi.org/10.3390/catal5041756