Micro/Mesoporous Zeolitic Composites: Recent Developments in Synthesis and Catalytic Applications
Abstract
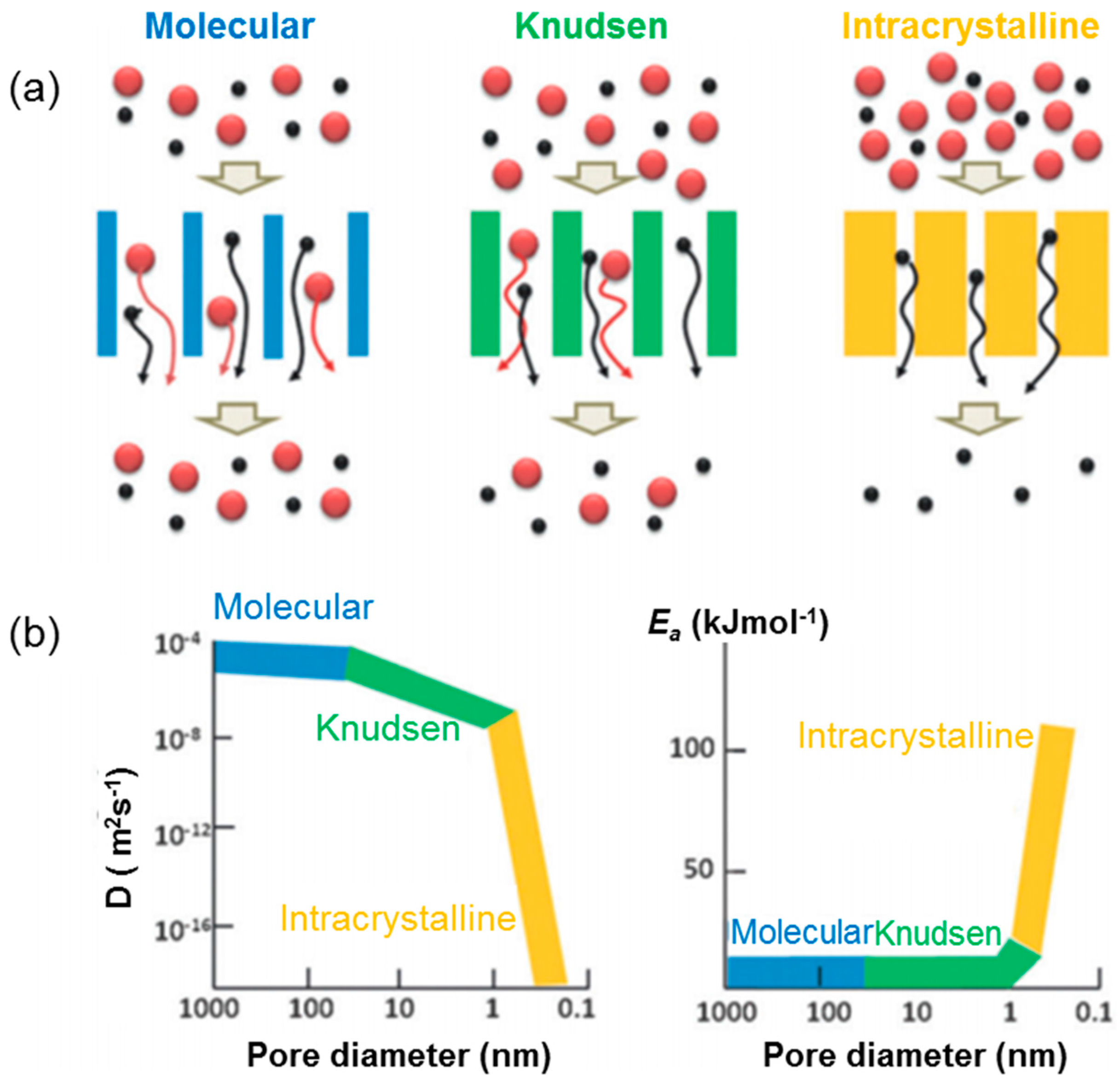
:1. Introduction
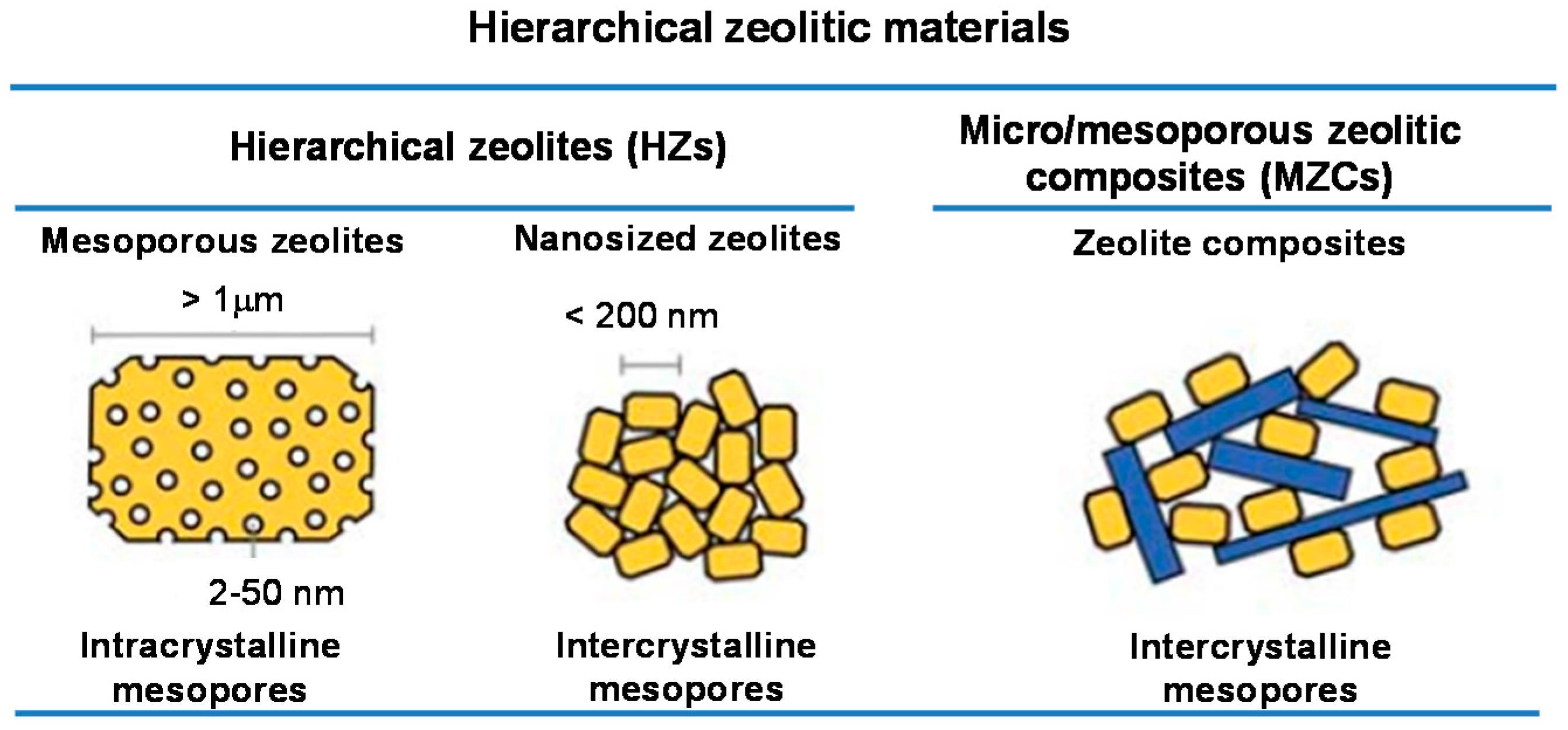
2. Synthesis Strategies of Micro/Mesoporous Zeolitic Composites
2.1. Direct-Synthesis Using Zeolite Precursors
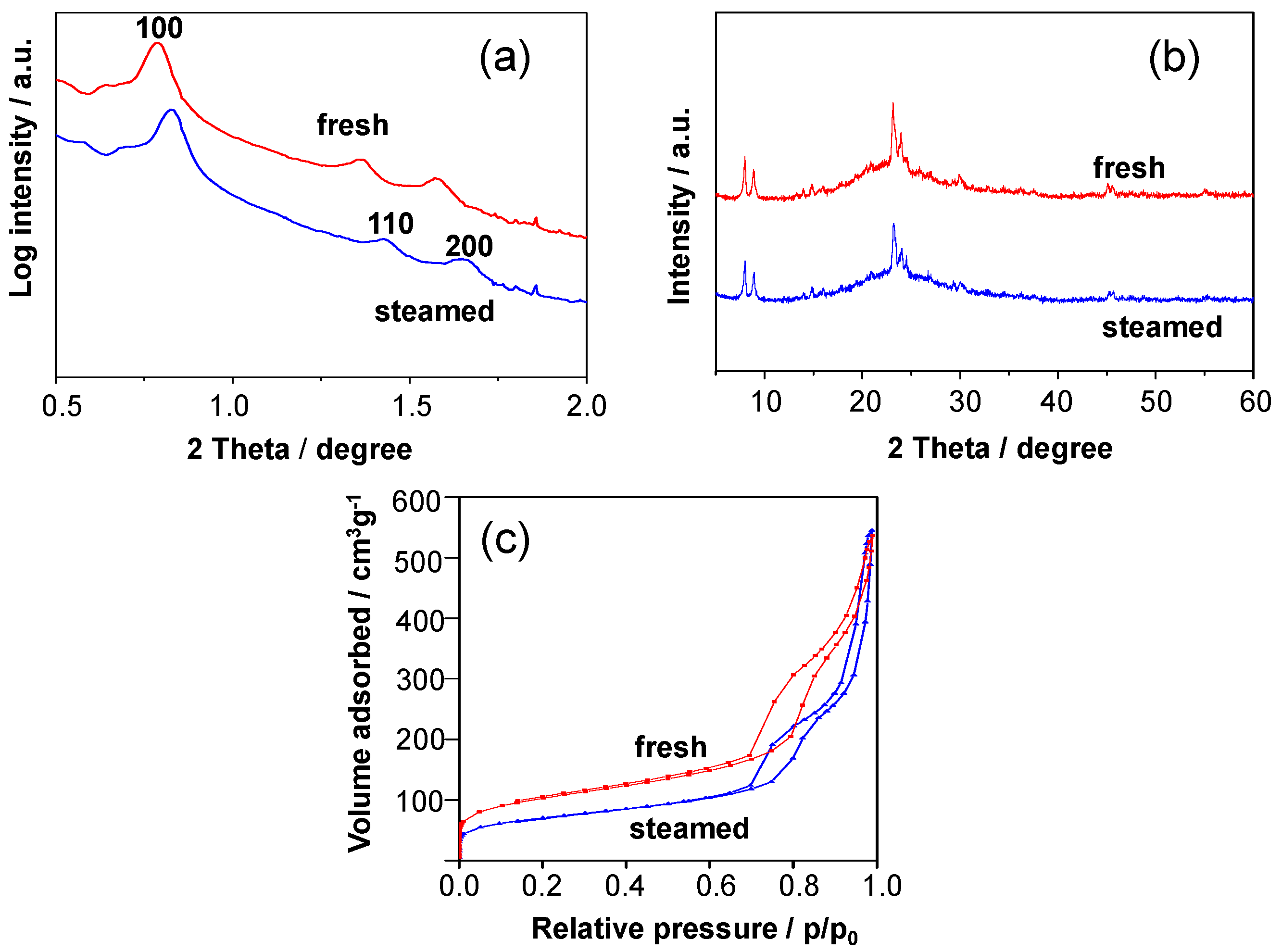
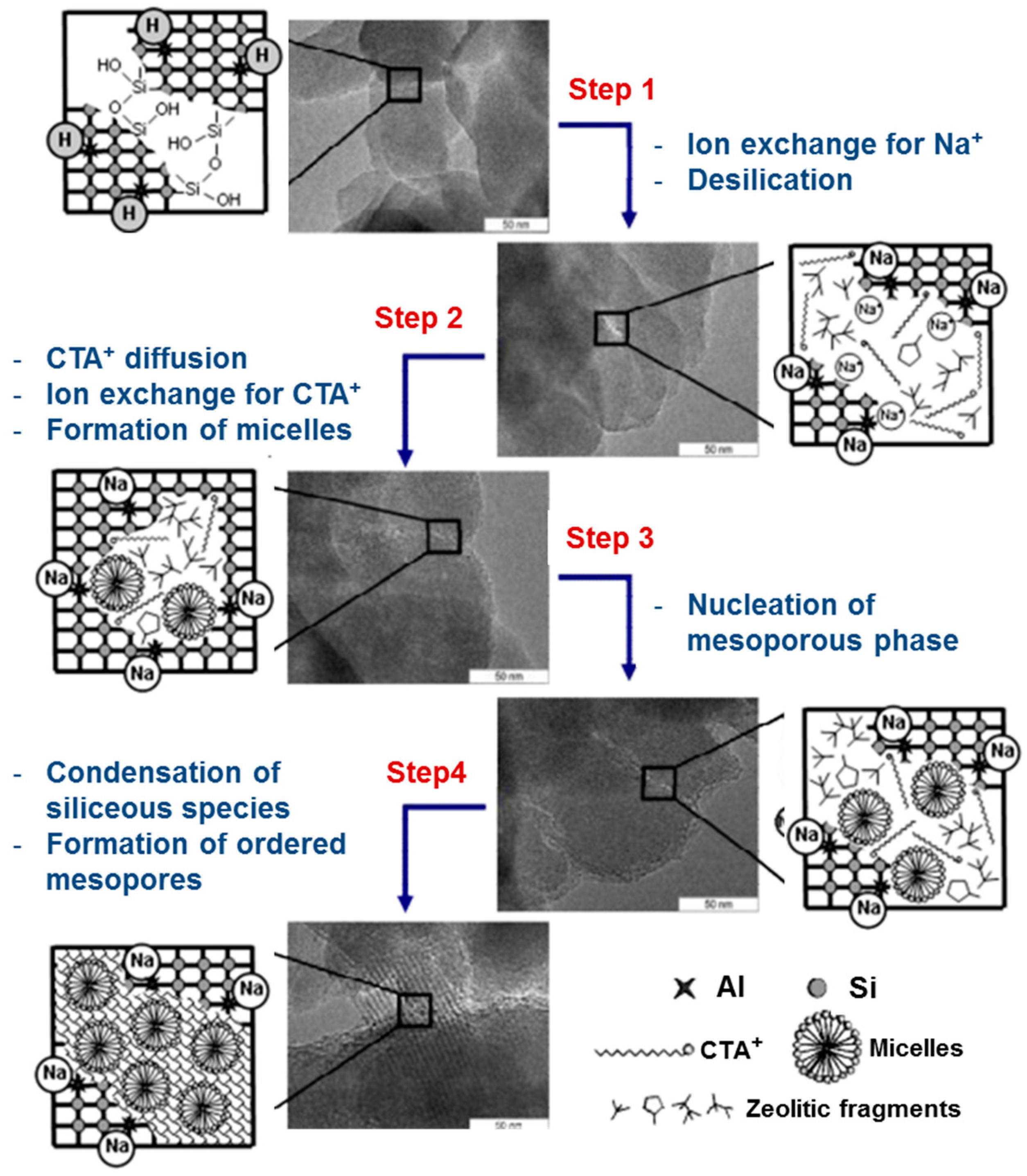
2.2. Recrystallization of Zeolite Crystals
2.3. Zeolitization of Preformed Mesoporous Materials
3. Catalytic Applications of Micro/Mesoporous Zeolitic Composites
3.1. Catalytic Cracking
3.2. Alkylation of Aromatics
3.3. Methanol-to-Hydrocarbons
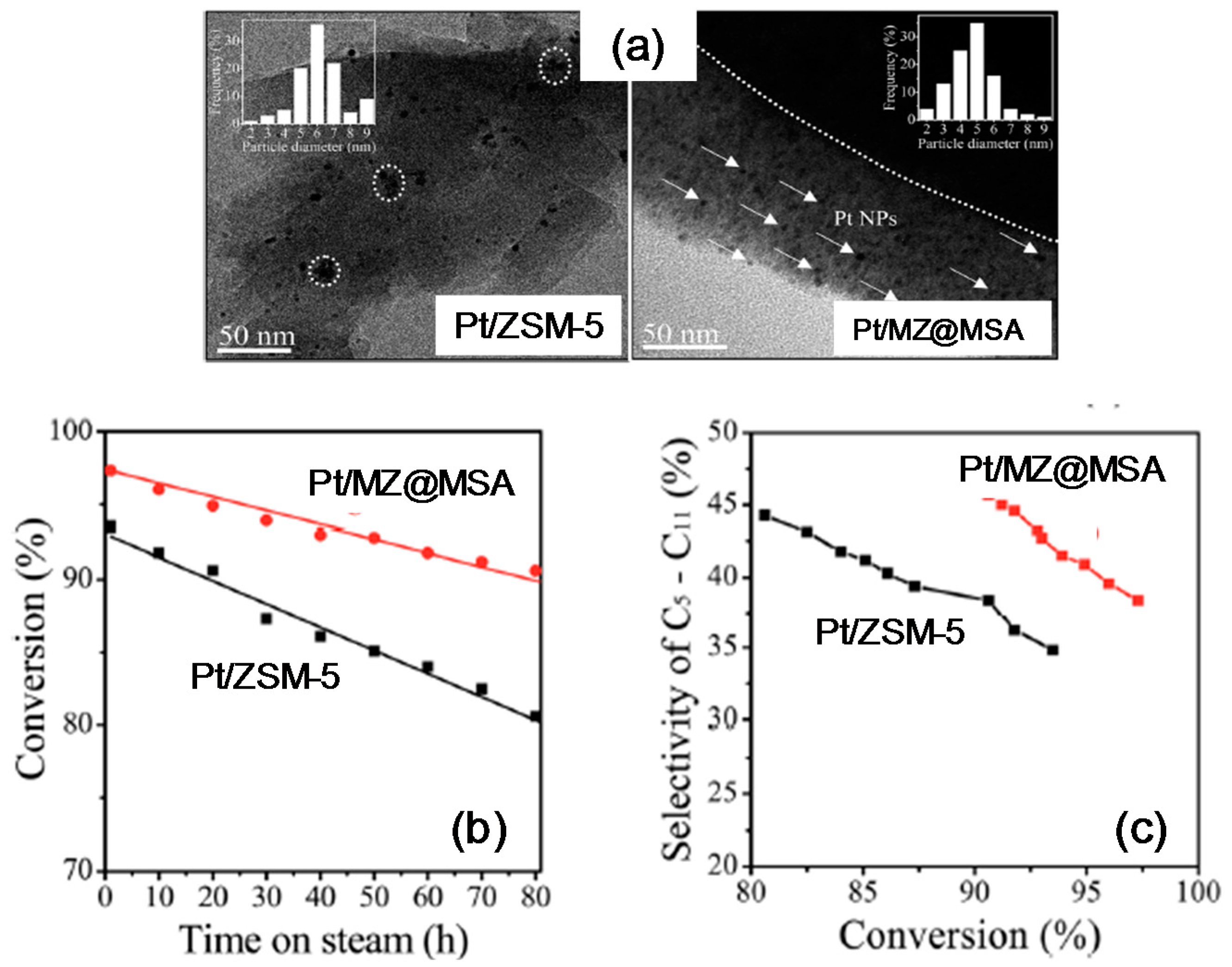
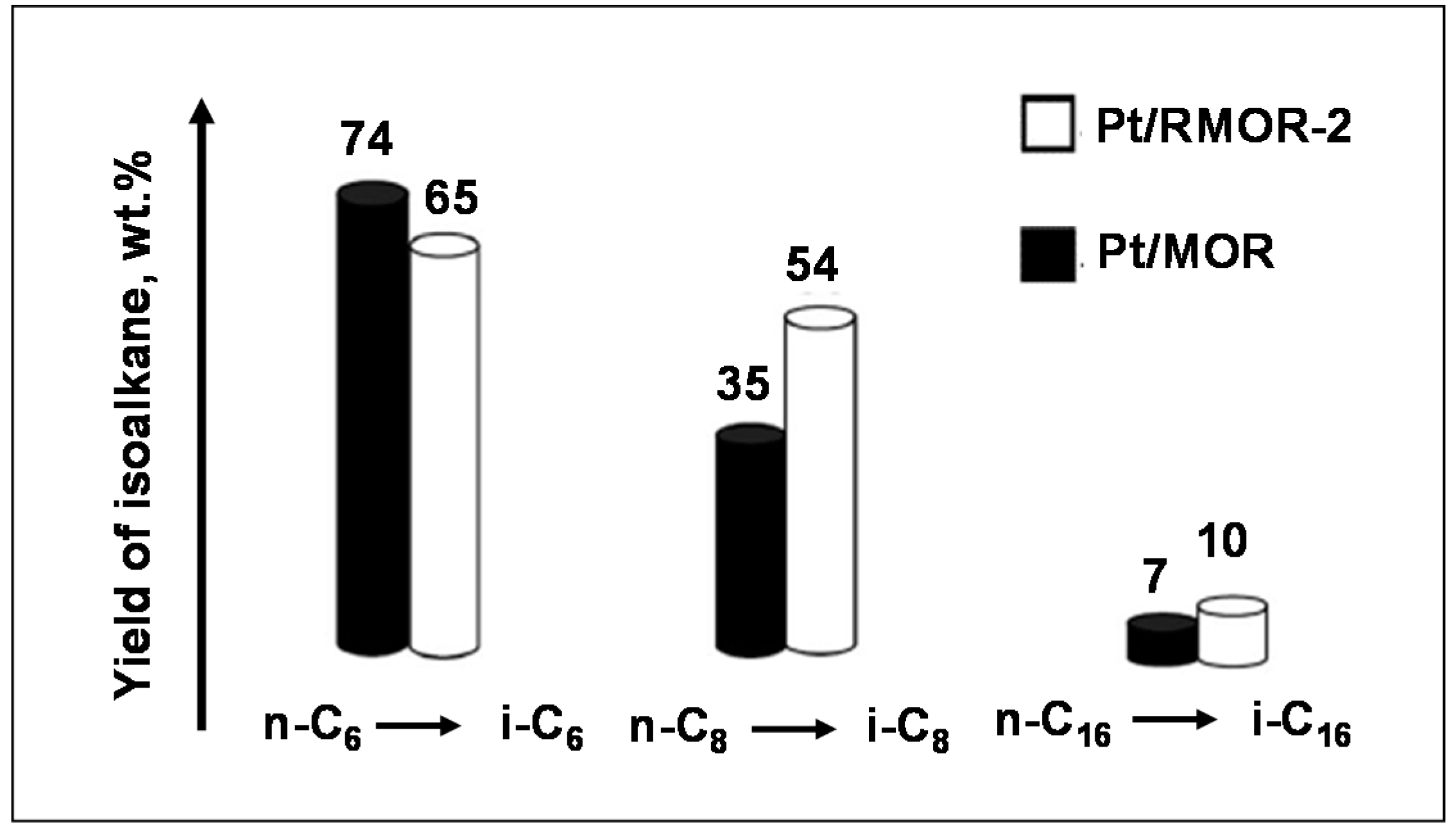
3.4. Hydroconversion
4. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McCusker, L.B.; Baerlocher, C. Zeolite structures. In Introduction to Zeolite Science and Practice, 2nd ed.; Jacobs, P.A., Flanigen, E.M., Jansen, J.C., van Bekkum, H., Eds.; Elsevier Science Publisher: Amsterdam, The Netherlands, 2001; Volume 137, pp. 37–67. [Google Scholar]
- International Zeolite Association Database, 2006. Available online: http://www.iza-structure.org/database (accessed on 20 October 2016).
- Corma, A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003, 216, 298–312. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef] [PubMed]
- Parlett, C.M.A.; Wilson, K.; Lee, A.F. Hierarchical porous materials: Catalytic applications. Chem. Soc. Rev. 2013, 42, 3876–3893. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Valla, J.; Garcia-Martinez, J. Realizing the commercial potential of hierarchical zeolites: New opportunities in catalytic cracking. ChemCatChem 2014, 6, 46–66. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Yang, W.; Tang, Y.; Xie, Z. Recent advances of pore system construction in zeolite-catalyzed chemical industry processes. Chem. Soc. Rev. 2015, 44, 8877–8903. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Machoke, A.G.; Schwieger, W. Catalytic test reactions for the evaluation of hierarchical zeolites. Chem. Soc. Rev. 2016, 45, 3313–3330. [Google Scholar] [CrossRef] [PubMed]
- Chal, R.; Gérardin, C.; Bulut, M.; van Donk, S. Overview and industrial assessment of synthesis strategies towards zeolites with mesopores. ChemCatChem 2011, 3, 67–81. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef] [PubMed]
- Malgras, V.; Ji, Q.; Kamachi, Y.; Mori, T.; Shieh, F.K.; Wu, K.C.W.; Ariga, K.; Yamauchi, Y. Templated synthesis for nanoarchitectured porous materials. Bull. Chem. Soc. Jpn. 2015, 88, 1171–1200. [Google Scholar] [CrossRef]
- Wu, K.C.W.; Yamauchi, Y. Controlling physical features of mesoporous silica nanoparticles (MSNs) for emerging applications. J. Mater. Chem. 2012, 22, 1251–1256. [Google Scholar] [CrossRef]
- Čejka, J.; Mintova, S. Perspectives of Micro/mesoporous composites in catalysis. Catal. Rev. Sci. Eng. 2007, 49, 457–509. [Google Scholar] [CrossRef]
- Liu, Y.; Pinnavaia, T.J. Aluminosilicate mesostructures with improved acidity and hydrothermal stability. J. Mater. Chem. 2002, 12, 3179–3190. [Google Scholar]
- Vu, X.H.; Steinfeldt, N.; Armbruster, U.; Martin, A. Improved hydrothermal stability and acidic properties of ordered mesoporous SBA-15 analogs assembled from nanosized ZSM-5 precursors. Microporous Mesoporous Mater. 2012, 164, 120–126. [Google Scholar] [CrossRef]
- Schwieger, W.; Machoke, A.G.; Weissenberger, T.; Inayat, A.; Selvam, T.; Klumpp, M.; Inayat, A. Hierarchy concepts: Classification and preparation strategies for zeolite containing materials with hierarchical porosity. Chem. Soc. Rev. 2016, 45, 3353–3376. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Orozco, S.; Inayat, A.; Schwab, A.; Selvam, T.; Schwieger, W. Zeolitic materials with hierarchical porous structures. Adv. Mater. 2011, 23, 2602–2615. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Bein, T. Mesoporosity—A new dimension for zeolites. Chem. Soc. Rev. 2013, 42, 3689–3707. [Google Scholar]
- Verboekend, D.; Nuttens, N.; Locus, R.; Van Aelst, J.; Verolme, P.; Groen, J.C.; Pérez-Ramírez, J.; Selsa, B.F. Synthesis, characterisation, and catalytic evaluation of hierarchical faujasite zeolites: Milestones, challenges, and future directions. Chem. Soc. Rev. 2016, 45, 3331–3352. [Google Scholar] [CrossRef] [PubMed]
- Valtchev, V.; Majano, G.; Mintova, S.; Pérez-Ramírez, J. Tailored crystalline microporous materials by post-synthesis modification. Chem. Soc. Rev. 2013, 42, 263–290. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.P.; Escola, J.M.; Pizarro, P. Synthesis strategies in the search for hierarchical zeolites. Chem. Soc. Rev. 2013, 42, 4004–4035. [Google Scholar] [PubMed]
- Egeblad, K.; Christensen, C.H.; Kustova, M.; Christensen, C.H. Templating Mesoporous Zeolites. Chem. Mater. 2008, 20, 946–960. [Google Scholar] [CrossRef]
- Na, K.; Choi, M.; Ryoo, R. Recent advances in the synthesis of hierarchically nanoporous zeolites. Microporous Mesoporous Mater. 2013, 166, 3–19. [Google Scholar] [CrossRef]
- Zhang, K.; Ostraat, M.L. Innovations in hierarchical zeolite synthesis. Catal. Today 2016, 264, 3–15. [Google Scholar] [CrossRef]
- Yan, Y.; Guo, X.; Zhang, Y.; Tang, Y. Future of nano-/hierarchical zeolites in catalysis: Gaseous phase or liquid phase system. Catal. Sci. Technol. 2015, 5, 772–785. [Google Scholar] [CrossRef]
- Hua, Z.L.; Zhou, J.; Shi, J.L. Recent advances in hierarchically structured zeolites: Synthesis and material performances. Chem. Commun. 2011, 47, 10536–10547. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Li, X.Y.; Rooke, J.C.; Zhang, Y.H.; Yang, X.Y.; Tang, Y.; Xiao, F.S.; Su, B.L. Hierarchically structured zeolites: Synthesis, mass transport properties and applications. J. Mater. Chem. 2012, 22, 17381–17403. [Google Scholar] [CrossRef]
- Ivanova, I.I.; Knyazeva, E.E. Micro-mesoporous materials obtained by zeolite recrystallization: Synthesis, characterization and catalytic applications. Chem. Soc. Rev. 2013, 42, 3671–3688. [Google Scholar] [CrossRef] [PubMed]
- Prasomsri, T.; Jiao, W.; Weng, S.Z.; Martinez, J.G. Mesostructured zeolites: Bridging the gap between zeolites and MCM-41. Chem. Commun. 2015, 51, 8900–8911. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, W.; Pinnavaia, T.J. Steam-stable aluminosilicate mesostructures assembled from zeolite type Y seeds. J. Am. Chem. Soc. 2000, 122, 8791–8792. [Google Scholar] [CrossRef]
- Waller, P.; Shan, Z.; Marchese, L.; Tartaglione, G.; Zhou, W.; Jansen, J.C.; Maschmeyer, T. Zeolite nanocrystals inside mesoporous TUD-1: A high-performance catalytic composite. Chem. Eur. J. 2004, 10, 4970–4976. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xiong, G.; Liu, L.; Wang, L. Investigation on the effect of zeolite precursor on the formation process of MCM-41 containing zeolite Y building units. Spectrochim. Acta Part. A: Mol. Biomol. Spectrosc. 2013, 107, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Mokaya, R. On the synthesis and characterization of ZSM-5/MCM-48 aluminosilicate composite materials. J. Mater. Chem. 2004, 14, 863–870. [Google Scholar] [CrossRef]
- Xia, Y.; Mokaya, R. Are mesoporous silicas and aluminosilicas assembled from zeolite seeds inherently hydrothermally stable? Comparative evaluation of MCM-48 materials assembled from zeolite seeds. J. Mater. Chem. 2004, 14, 3427–3435. [Google Scholar] [CrossRef]
- Huo, Q.; Gong, Y.; Dou, T.; Zhao, Z.; Pan, H.; Deng, F. Novel micro- and mesoporous composite molecular sieve assembled by zeolite L nanocrystal and its performance for the hydrodesulfurization (HDS) of fluidized catalytic cracking (FCC) gasoline. Energy Fuels 2010, 24, 3764–3771. [Google Scholar] [CrossRef]
- Du, P.; Zheng, P.; Song, S.; Wang, X.; Zhang, M.; Chi, K.; Xu, C.; Duan, A.; Zhao, Z. Synthesis of a novel micro/mesoporous composite material Beta-FDU-12 and its hydro-upgrading performance for FCC gasoline. RSC Adv. 2016, 6, 1018–1026. [Google Scholar] [CrossRef]
- Gao, A.; Duan, A.; Zhang, X.; Chi, K.; Zhao, Z.; Li, J.; Qin, Y.; Wang, X.; Xu, C. Self-assembly of monodispersed hierarchically porous Beta-SBA-15 with different morphologies and their hydro-upgrading performances of FCC gasoline. J. Mater. Chem. A 2015, 3, 16501–16512. [Google Scholar] [CrossRef]
- Di, Z.; Yang, C.; Jiao, X.; Li, J.; Wu, J.; Zhang, D. A ZSM-5/MCM-48 based catalyst for methanol to gasoline conversion. Fuel 2013, 104, 878–881. [Google Scholar] [CrossRef]
- Ooi, Y.S.; Zakaria, R.; Mohamed, A.R.; Bhatia, S. Synthesis of composite material MCM-41/Beta and its catalytic performance in waste used palm oil cracking. Appl. Catal. A: Gen. 2004, 274, 15–23. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, H.; Lu, Y.; Yao, Y.; Lu, S. Two-step hydrothermal synthesis of beta-MCM-41 composite molecular sieves as supports of bifunctional catalysts for hydroisomerization of n-heptane. J. Porous Mater. 2016. [Google Scholar] [CrossRef]
- Vu, X.H.; Bentrup, U.; Hunger, M.; Kraehnert, R.; Armbruster, U.; Martin, A. Direct synthesis of nanosized-ZSM-5/SBA-15 analog composites from preformed ZSM-5 precursors for improved catalytic performance as cracking catalyst. J. Mater. Sci. 2014, 49, 5676–5689. [Google Scholar] [CrossRef]
- Xue, B.; Xu, J.; Xu, C.; Wu, R.; Li, Y.; Zhang, K. A novel, shape-selective H-MCM-22/MCM-41 composite catalyst: Synthesis, characterization and catalytic performance. Catal. Commun. 2010, 12, 95–99. [Google Scholar] [CrossRef]
- Qian, X.; Du, J.; Li, B.; Si, M.; Yang, Y.; Hu, Y.; Niu, G.; Zhang, Y.; Xu, H.; Tu, B.; et al. Controllable fabrication of uniform core-shell structured zeolite@SBA-15 composites. Chem. Sci. 2011, 2, 2006–2016. [Google Scholar] [CrossRef]
- Lima, S.; Antunes, M.M.; Fernandes, A.; Pillinger, M.; Ribeiro, M.F.; Valente, A.A. Catalytic cyclodehydration of xylose to furfural in the presence of zeolite H-Beta and a micro/mesoporous Beta/TUD-1 composite material. Appl. Catal. A: Gen. 2010, 388, 141–148. [Google Scholar] [CrossRef]
- Prokešová, P.; Žilková, N.; Mintova, S.; Bein, T.; Čejka, J. Catalytic activity of micro/mesoporous composites in toluene alkylation with propylene. Appl. Catal. A: Gen. 2005, 281, 85–91. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, D.; Li, Q. Synthesis, characterization and influence parameters on the overgrowth of micro/mesoporous Y-zeolite-MCM-41 composite material under acidic conditions. Microporous Mesoporous Mater. 2011, 142, 503–510. [Google Scholar] [CrossRef]
- Enterría, M.; Suárez-García, F.; Martínez-Alonso, A.; Tascón, J.M.D. Preparation of hierarchical micro-mesoporous aluminosilicate composites by simple Y zeolite/MCM-48 silica assembly. J. Alloys Compd. 2014, 583, 60–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Li, Y. Synthesis and characteristics of Y-zeolite/MCM-48 biporous molecular sieve. Appl. Catal. A: Gen. 2008, 345, 73–79. [Google Scholar]
- Wei, L.; Zhang, H.; Dong, Y.; Song, W.; Liu, X.; Zhao, Z. Synthesis and characterization of MCM-49/MCM-41 composite molecular sieve: An effective adsorbent for chromate from water. RSC Adv. 2016, 6, 71375–71383. [Google Scholar] [CrossRef]
- Goto, Y.; Fukushima, Y.; Ratu, P.; Imada, Y.; Kubota, Y.; Sugi, Y.; Ogura, M.; Matsukata, M. Mesoporous material from zeolite. J. Porous Mater. 2002, 9, 43–48. [Google Scholar] [CrossRef]
- Ivanova, I.I.; Kuznetsov, A.S.; Knyazeva, E.E.; Fajula, F.; Thibault-Starzyk, F.; Fernandez, C.; Gilson, J.P. Design of hierarchically structured catalysts by mordenites recrystallization: Application in naphthalene alkylation. Catal. Today 2011, 168, 133–139. [Google Scholar] [CrossRef]
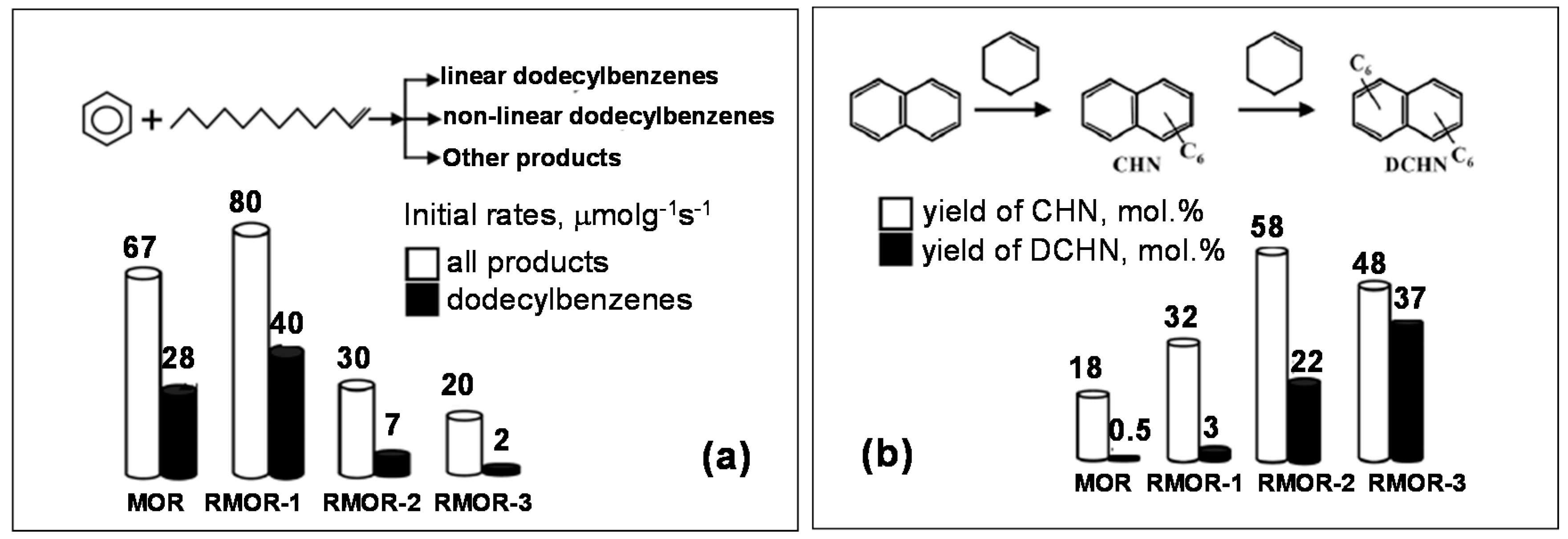
- Ponomareva, O.A.; Timoshin, C.E.; Monakhova, Y.V.; Knyazeva, E.E.; Yuschenko, V.V.; Ivanova, I.I. Benzene Alkylation with Dodecene_1 over Micro–Mesoporous Molecular Sieves. Pet. Chem. 2010, 50, 427–436. [Google Scholar] [CrossRef]
- Jiao, W.Q.; Ding, J.; Shi, Z.B.; Liang, X.M.; Wang, Y.M.; Zhang, Y.H.; Tang, Y.; He, M.Y. Preparation of Y zeolite composites with adjustable, highly dispersed intra-crystal mesoporosity: Effect of lactic acid treatment in CTAB-assisted two-step approach. Microporous Mesoporous Mater. 2016, 228, 237–247. [Google Scholar] [CrossRef]
- Liu, X.; Yang, T.; Bai, P.; Han, L. Y/MCM-41 composites assembled from nanocrystals. Microporous Mesoporous Mater. 2011, 2, 2006–2016. [Google Scholar] [CrossRef]
- Sang, Y.; Liu, H.; He, S.; Li, H.; Jiao, Q.; Wu, Q.; Sun, K. Catalytic performance of hierarchical H-ZSM-5/MCM-41 for methanol dehydration to dimethyl ether. J. Energy Chem. 2013, 22, 769–777. [Google Scholar] [CrossRef]
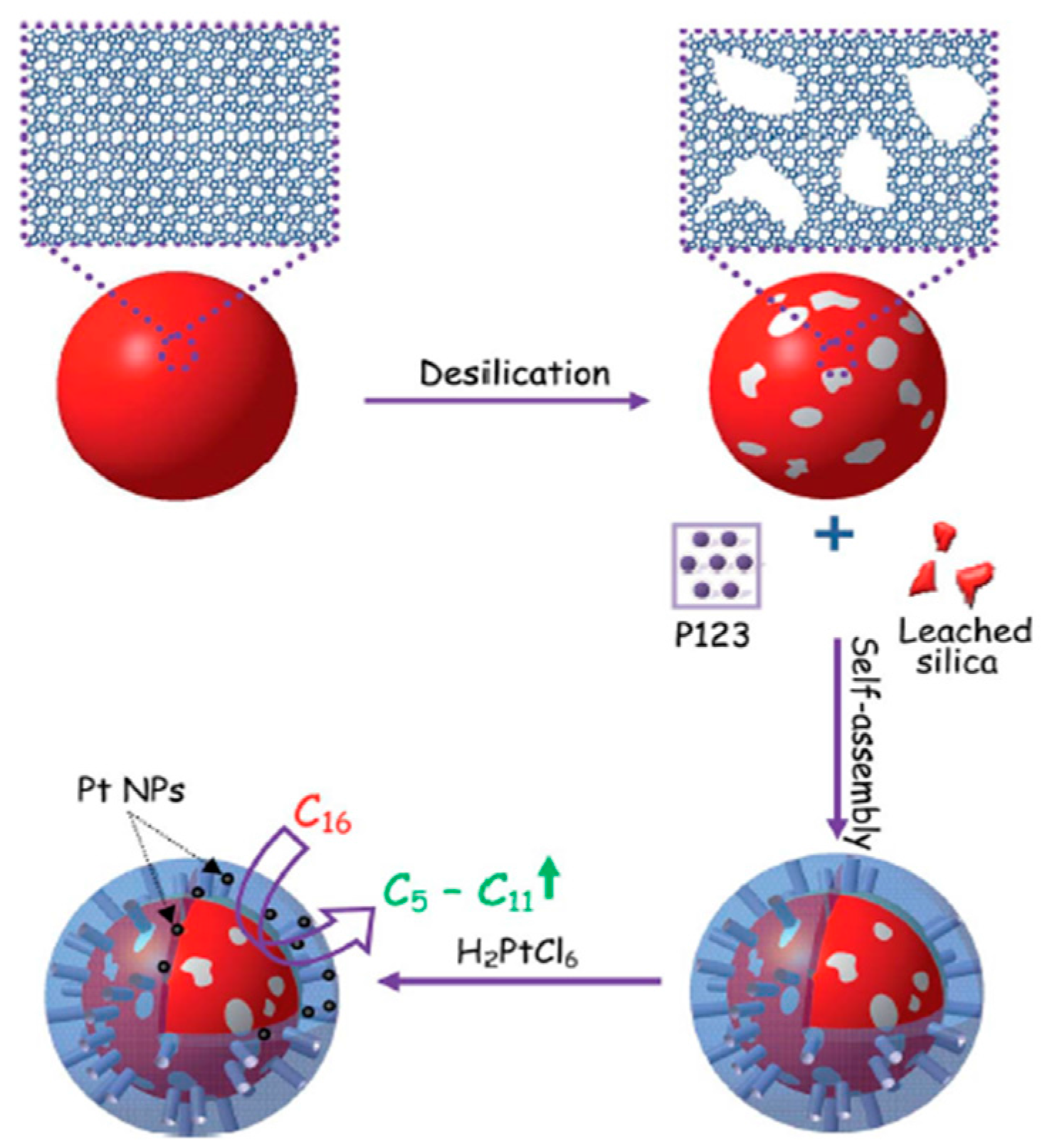
- Peng, P.; Wang, Y.; Zhang, Z.; Qiao, K.; Liu, X.; Yan, Z.; Subhan, F.; Komarneni, S. ZSM-5-based mesostructures by combined alkali dissolution and re-assembly: Process controlling and scale-up. Chem. Eng. J. 2016, 302, 323–333. [Google Scholar] [CrossRef]
- Boukoussa, B.; Aouad, N.; Hamacha, R.; Bengueddach, A. Key factor affecting the structural and textural properties of ZSM-5/MCM-41 composite. J. Phys. Chem. Solids 2015, 78, 78–83. [Google Scholar] [CrossRef]
- Ivanova, I.I.; Kasyanov, I.A.; Maerle, A.A.; Zaikovskii, V.I. Mechanistic study of zeolites recrystallization into micro-mesoporous materials. Microporous Mesoporous Mater. 2014, 189, 163–172. [Google Scholar] [CrossRef]
- Liu, S.; Ren, J.; Zhang, H.; Lv, E.; Yang, Y.; Li, Y.W. Synthesis, characterization and isomerization performance of micro/mesoporous materials based on H-ZSM-22 zeolite. J. Catal. 2016, 335, 11–23. [Google Scholar] [CrossRef]
- Ponomareva, O.A.; Mal’tseva, A.A.; Maerle, A.A.; Rodionova, L.I.; Pavlov, V.S.; Dobryakova, I.V.; Belova, M.V.; Ivanova, I.I. Production of isobutylene from acetone over micro-mesoporous catalysts. Pet. Chem. 2016, 56, 253–258. [Google Scholar] [CrossRef]
- Verboekend, D.; Milina, M.; Mitchell, S.; Pérez-Ramírez, J. Hierarchical zeolites by desilication: Occurrence and catalytic impact of recrystallization and restructuring. Cryst. Growth Des. 2013, 13, 5025–5035. [Google Scholar] [CrossRef]
- Diao, Z.; Wang, L.; Zhang, X.; Liu, G. Catalytic cracking of supercritical n-dodecane over meso-HZSM-5@Al-MCM-41 zeolites. Chem. Eng. Sci. 2015, 135, 452–460. [Google Scholar] [CrossRef]
- Yoo, W.C.; Zhang, X.; Tsapatsis, M.; Stein, A. Synthesis of mesoporous ZSM-5 zeolites through desilication and re-assembly processes. Microporous Mesoporous Mater. 2012, 149, 147–157. [Google Scholar] [CrossRef]
- Li, G.; Diao, Z.; Na, J.; Wang, L. Exploring suitable ZSM-5/MCM-41 zeolites for catalytic cracking of n-dodecane: Effect of initial particle size and Si/Al ratio. Chin. J. Chem. Eng. 2015, 23, 1655–1661. [Google Scholar] [CrossRef]
- Gao, N.; Xie, S.; Liu, S.; An, J.; Zhu, X.; Hu, L.; Wei, H.; Li, X.; Xu, L. Catalytic degradation of LDPE and PP over MCM-49 based micro–mesoporous composites. Catal. Lett. 2014, 144, 1296–1304. [Google Scholar] [CrossRef]
- Peng, P.; Wang, Y.; Rood, M.J.; Zhang, Z.; Subhan, F.; Yan, Z.; Qin, L.; Zhang, Z.; Zhang, Z.; Gao, X. Effects of dissolution alkalinity and self-assembly on ZSM-5-based micro-/mesoporous composites: A study of the relationship between porosity, acidity, and catalytic performance. Cryst. Eng. Comm. 2015, 17, 3820–3828. [Google Scholar] [CrossRef]
- Kasyanov, I.A.; Maerle, A.A.; Ivanova, I.I.; Zaikovskii, V.I. Towards understanding of the mechanism of stepwise zeolite recrystallization into micro/mesoporous materials. J. Mater. Chem. A 2014, 2, 16978–16988. [Google Scholar] [CrossRef]
- Wang, D.; Xu, L.; Wu, P. Hierarchical, core-shell meso-ZSM-5@mesoporous aluminosilicate-supported Pt nanoparticles for bifunctional hydrocracking. J. Mater. Chem. A 2014, 2, 15535–15545. [Google Scholar] [CrossRef]
- Ren, B.; Bai, S.; Sun, J.; Zhang, F.; Fan, M. Controllable synthesis of obvious core–shell structured Y/Beta composite zeolite by a stepwise-induced method. RSC Adv. 2014, 4, 22755–22758. [Google Scholar] [CrossRef]
- Odedairo, T.; Balasamy, R.J.; Al-Khattaf, S. Aromatic transformations over aluminosilicate micro/mesoporous composite materials. Catal. Sci. Technol. 2012, 2, 1275–1286. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Wang, X.; Zhang, Y.; Dou, T.; Xie, K. Synthesis, characterization, and catalytic properties of a hydrothermally stable Beta/MCM-41 composite from well-crystallized zeolite Beta. J. Porous Mater. 2008, 15, 133–138. [Google Scholar] [CrossRef]
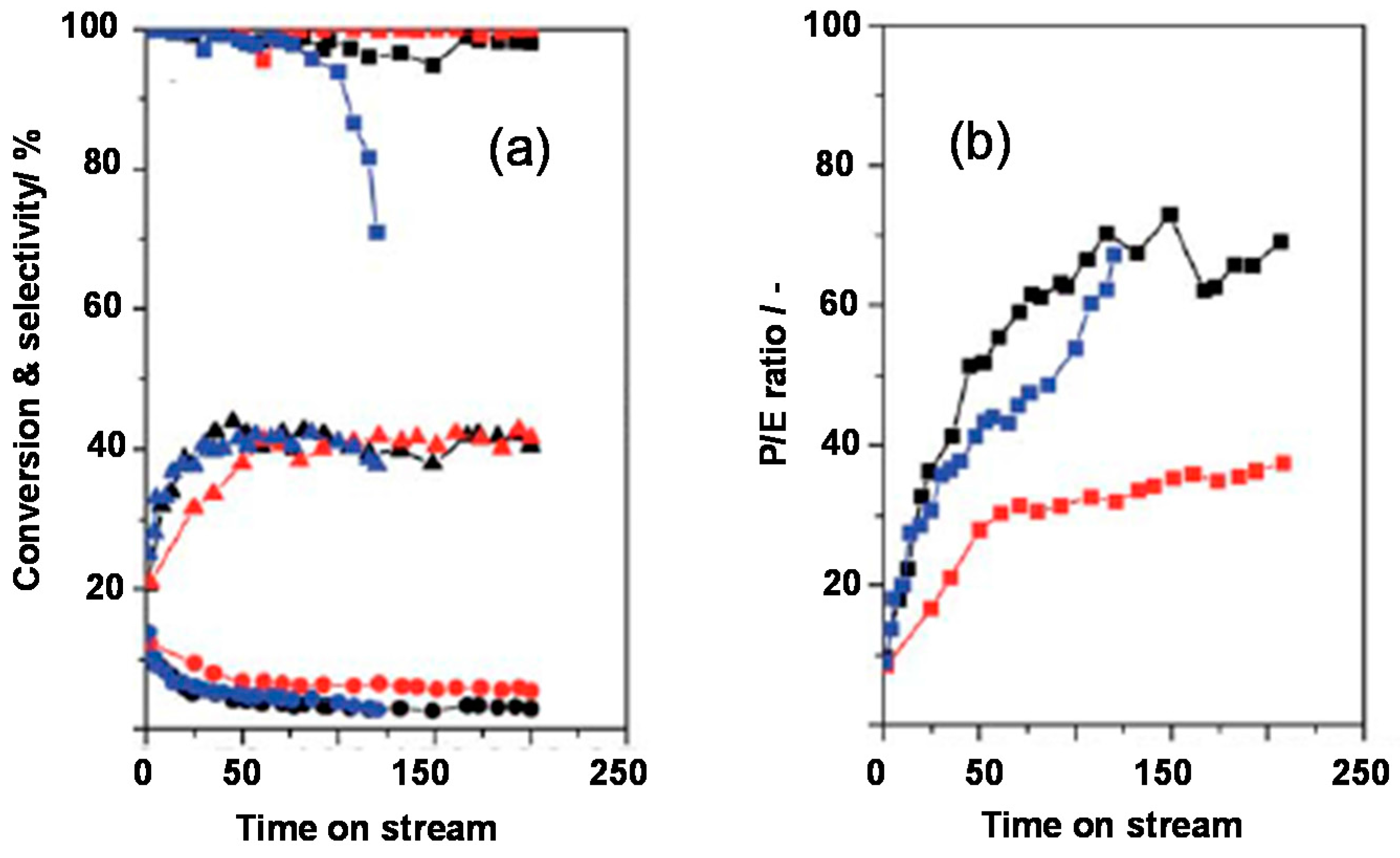
- Konnov, S.V.; Ivanova, I.I.; Ponomareva, O.A.; Zaikovskii, V.I. Hydroisomerization of n-alkanes over Pt-modified micro/mesoporous materials obtained by mordenite recrystallization. Microporous Mesoporous Mater. 2012, 164, 222–231. [Google Scholar] [CrossRef]
- Kloetstra, K.R.; van Bekkum, H.; Jansen, J.C. Mesoporous material containing framework tectosilicate by pore-wall recrystallization. Chem. Commun. 1997, 22, 2281–2282. [Google Scholar] [CrossRef]
- Verhoef, M.J.; Kooyman, P.J.; van der Waal, J.C.; Rigutto, M.S.; Peters, J.A.; van Bekkum, H. Partial Transformation of MCM-41 material into zeolites: Formation of nanosized MFI type crystallites. Chem. Mater. 2001, 13, 683–687. [Google Scholar] [CrossRef]
- Chen, H.L.; Zhang, K.; Wang, Y.M. Steam-assisted crystallization of TPA+-exchanged MCM-41 type mesoporous materials with thick pore walls. Mater. Res. Bull. 2012, 47, 1774–1782. [Google Scholar] [CrossRef]
- Huang, L.; Guo, W.; Deng, P.; Xue, Z.; Li, Q. Investigation of Synthesizing MCM-41/ZSM-5 Composites. J. Phys. Chem. B 2000, 104, 2817–2823. [Google Scholar] [CrossRef]
- Campos, A.A.; Dimitrov, L.; da Silva, C.R.; Wallau, M.; Urquieta-González, E.A. Recrystallisation of mesoporous SBA-15 into microporous ZSM-5. Microporous Mesoporous Mater. 2006, 95, 92–103. [Google Scholar] [CrossRef]
- On, D.T.; Kaliaguine, S. Large-pore mesoporous materials with semicrystalline zeolitic frameworks. Angew. Chem. Int. Ed. 2001, 40, 3248–3251. [Google Scholar]
- Wang, J.; Groen, J.C.; Yue, W.; Zhou, W.; Coppens, M.O. Single-template synthesis of zeolite ZSM-5 composites with tunable mesoporosity. J. Mater. Chem. 2008, 18, 468–474. [Google Scholar] [CrossRef]
- Wang, J.; Yue, W.; Zhou, W.; Coppens, M.O. TUD-C: A tunable, hierarchically structured mesoporous zeolite composite. Microporous Mesoporous Mater. 2009, 120, 19–28. [Google Scholar] [CrossRef]
- Zhou, J.; Hua, Z.; Zhao, J.; Gao, Z.; Zeng, S.; Shi, J. A micro/mesoporous aluminosilicate: Key factors affecting framework crystallization during steam-assisted synthesis and its catalytic property. J. Mater. Chem. 2010, 20, 6764–6771. [Google Scholar] [CrossRef]
- Ogura, M.; Inoue, K.; Yamaguchi, T. A mechanistic study on the synthesis of MCM-22 from SBA-15 by dry gel conversion to form a micro- and mesoporous composite. Catal. Today 2011, 168, 118–123. [Google Scholar] [CrossRef]
- Koekkoek, A.J.J.; Degirmenci, V.; Hensen, E.J.M. Dry gel conversion of organosilane templated mesoporous silica: From amorphous to crystalline catalysts for benzene oxidation. J. Mater. Chem. 2011, 21, 9279–9289. [Google Scholar] [CrossRef]
- Pashkova, V.; Włoch, E.; Mikołajczyk, A.; Łaniecki, M.; Sulikowski, B.; Derewinski, M. Composite SBA-15/MFI type materials: Preparation, characterization and catalytic performance. Catal. Lett. 2009, 128, 64–71. [Google Scholar] [CrossRef]
- Vu, X.H.; Nguyen, S.; Dang, T.T.; Phan, B.M.Q.; Nguyen, D.A.; Armbruster, U.; Martin, A. Catalytic cracking of triglyceride-rich biomass toward lower olefins over a nano-ZSM-5/SBA-15 analog composite. Catalysts 2015, 5, 1692–1703. [Google Scholar] [CrossRef]
- Bagshaw, S.A.; Baxter, N.I.; Brew, D.R.M.; Hosie, C.F.; Yuntong, N.; Jaenicke, S.; Khuan, C.G. Highly ordered mesoporous MSU-SBEA/zeolite Beta composite material. J. Mater. Chem. 2006, 16, 2235–2244. [Google Scholar] [CrossRef]
- Morales-Pacheco, P.; Domínguez, J.M.; Bucio, L.; Alvarez, F.; Sedran, U.; Falco, M. Synthesis of FAU(Y)- and MFI(ZSM5)-nanosized crystallites for catalytic cracking of 1,3,5-triisopropylbenzene. Catal. Today 2011, 166, 25–38. [Google Scholar]
- Frunz, L.; Prins, R.; Pirngruber, G.D. ZSM-5 precursors assembled to a mesoporous structure and its subsequent transformation into a zeolitic phase-from low to high catalytic activity. Microporous Mesoporous Mater. 2006, 88, 152–162. [Google Scholar] [CrossRef]
- Aghakhani, M.S.; Khodadadi, A.A.; Najafi, Sh.; Mortazavi, Y. Enhanced triisopropylbenzene cracking and suppressed coking on tailored composite of Y-zeolite/amorphous silica-alumina catalyst. J. Ind. Eng. Chem. 2014, 20, 3037–3045. [Google Scholar] [CrossRef]
- Coriolano, A.C.F.; Silva, C.G.C.; Costa, M.J.F.; Pergher, S.B.C.; Caldeira, V.P.S.; Araujo, A.S. Development of HZSM-5/AlMCM-41 hybrid micro-mesoporous material and application for pyrolysis of vacuum gasoil. Microporous Mesoporous Mater. 2013, 72, 206–212. [Google Scholar] [CrossRef]
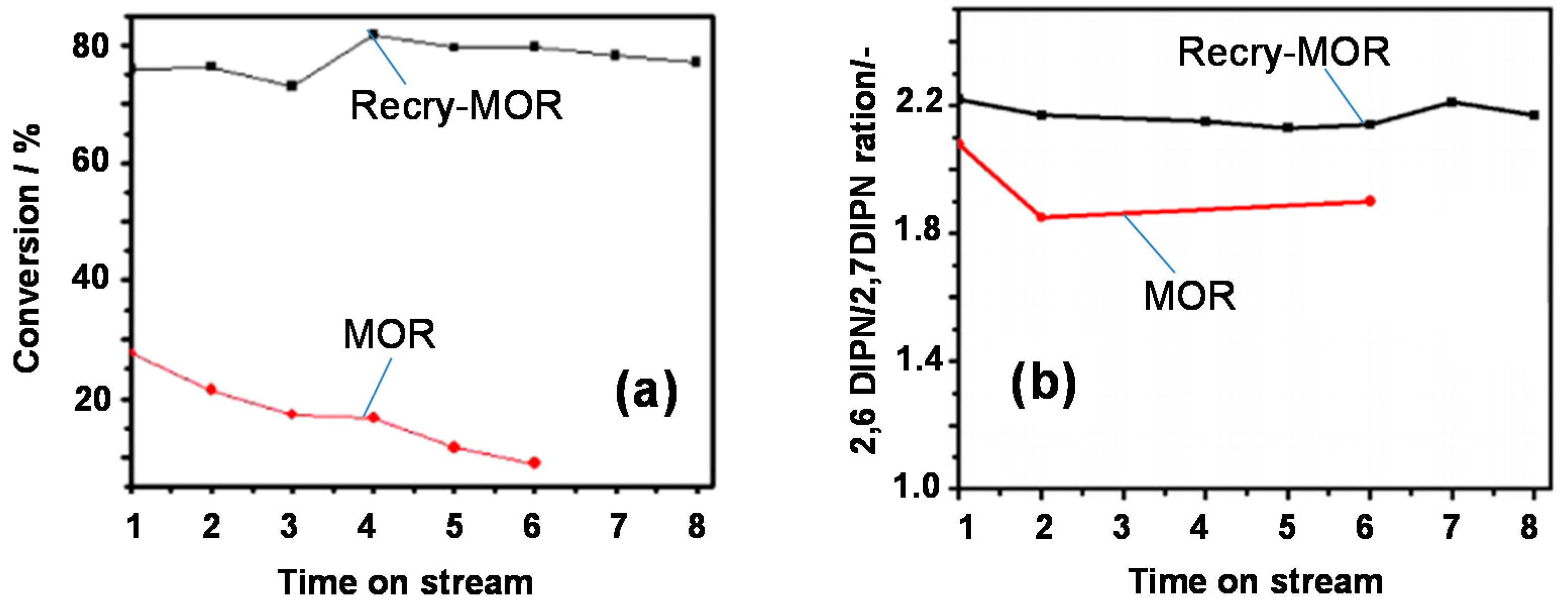
- Banu, M.; Lee, Y.H.; Magesh, G.; Nam, C.-M.; Lee, J.S. MOR/SBA-15 composite catalysts with interconnected meso/micropores for improved activity and stability in isopropylation of naphthalene. ChemCatChem 2015, 7, 2354–2360. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, W.Y.; Park, H.; Choi, Y.H.; Lee, J.S. Highly active and coke-tolerant hierarchical mordenite catalysts synthesized by recrystallization for the isopropylation of naphthalene. ChemCatChem 2016, 8, 2996–3001. [Google Scholar] [CrossRef]
- Song, K.; Guan, J.; Wu, S.; Yang, Y.; Liu, B.; Kan, Q. Alkylation of phenol with tert-butanol catalyzed by mesoporous material with enhanced acidity synthesized from zeolite MCM-22. Catal. Lett. 2008, 126, 333–340. [Google Scholar] [CrossRef]
- Wu, H.; Duan, A.; Zhao, Z.; Li, T.; Prins, R.; Zhou, X. Synthesis of NiMo hydrodesulfurization catalyst supported on a composite of nano-sized ZSM-5 zeolite enwrapped with mesoporous KIT-6 material and its high isomerization selectivity. J. Catal. 2014, 317, 303–317. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, A.; Zhao, Z.; Xu, C. Synthesis, characterization, and catalytic performance of NiMo catalysts supported on hierarchically porous Beta-KIT-6 material in the hydrodesulfurization of dibenzothiophene. J. Catal. 2010, 274, 273–286. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, X.; Zhang, Z.; Ling, F.; Sun, W. Synthesis, characterization and catalytic properties of Y-β zeolite composites. Pet. Sci. 2011, 8, 224–228. [Google Scholar] [CrossRef]
- Zhang, X.W.; Guo, Q.; Qin, B.; Zhang, Z.; Ling, F.; Sun, W.; Li, R. Structural features of binary microporous zeolite composite Y-beta and its hydrocracking performance. Catal. Today 2010, 149, 212–217. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Y.; Zhang, K.; Wang, J. BETA zeolite made from mesoporous material and its hydrocracking performance. Catal. Today 2006, 116, 2–5. [Google Scholar] [CrossRef]
- Busse, O.; Räuchle, K.; Reschetilowski, W. Hydrocracking of ethyl laurate on bifunctional micro-/mesoporous zeolite catalysts. ChemSusChem 2010, 3, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Konnov, S.V.; Sushkevich, V.L.; Monachova, Y.V.; Yushcenko, V.V.; Ponomareva, O.A.; Ivanova, I.I. Hydroisomerization of n-Octane over Pt-containing micro/mesoporous molecular sieves. Stud. Surf. Sci. Catal. 2008, 174, 1167–1170. [Google Scholar]
- Liu, P.; Yao, Y.; Wang, J. Using beta-MCM41 composite molecular sieves as supports of bifunctional catalysts for the hydroisomerization of n-heptane. React. Kinet. Mech. Catal. 2010, 101, 465–475. [Google Scholar] [CrossRef]
- Paixão, V.; Santos, C.; Nunes, R.; Silva, J.M.; Pires, J.; Carvalho, A.P.; Martins, A. n-Hexane hydroisomerization over composite catalysts based on beta zeolite and mesoporous materials. Catal. Lett. 2009, 129, 331–335. [Google Scholar] [CrossRef]


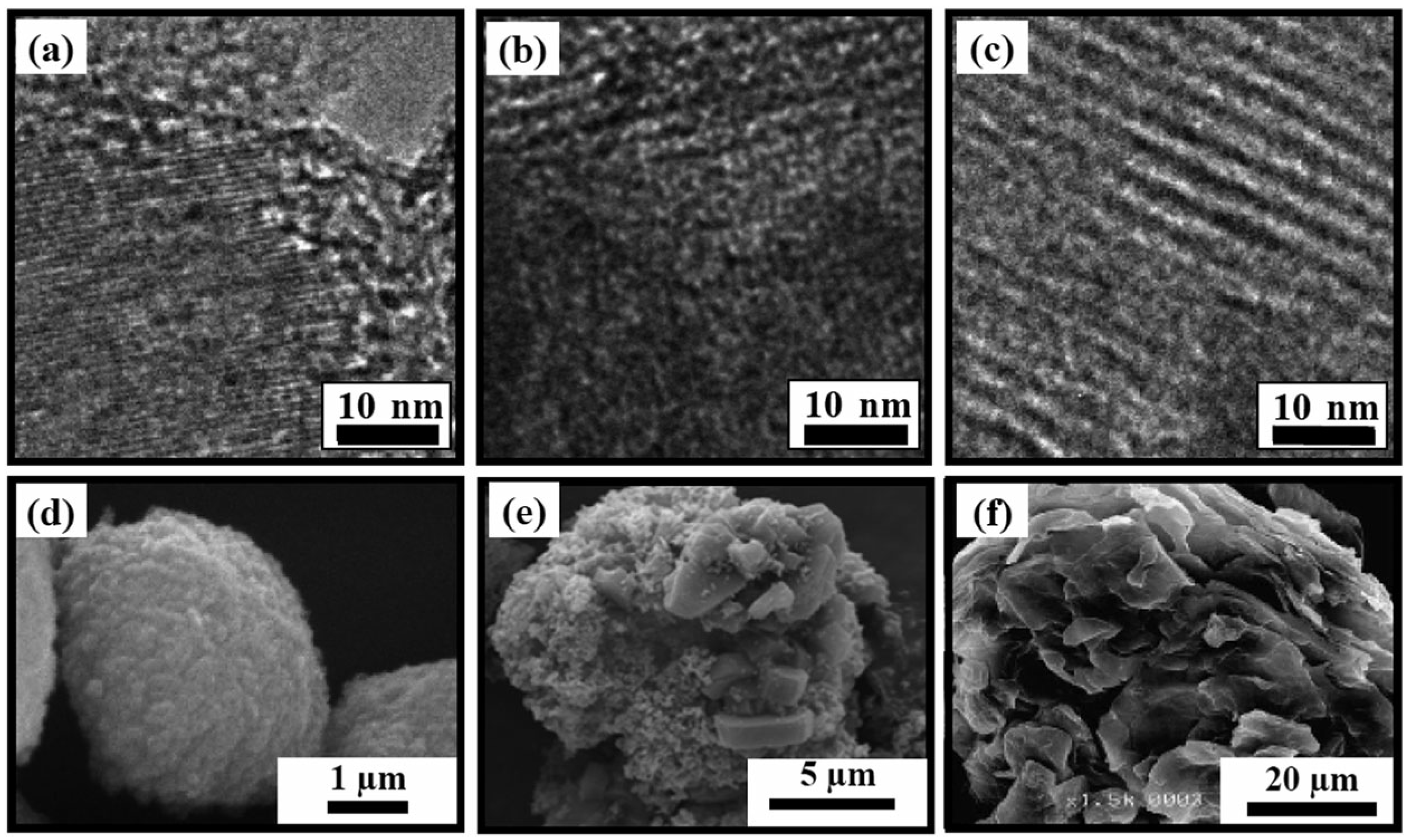
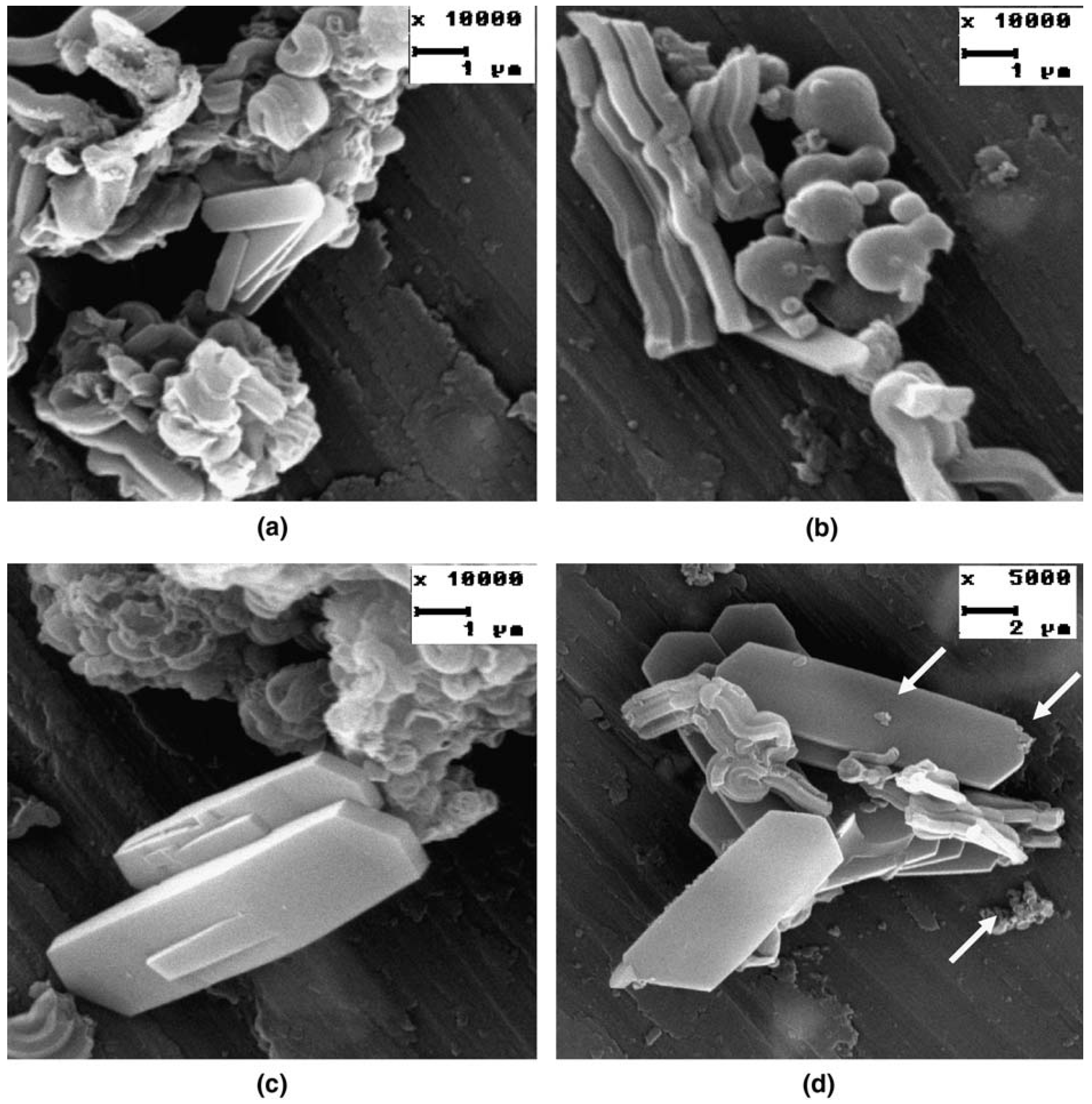
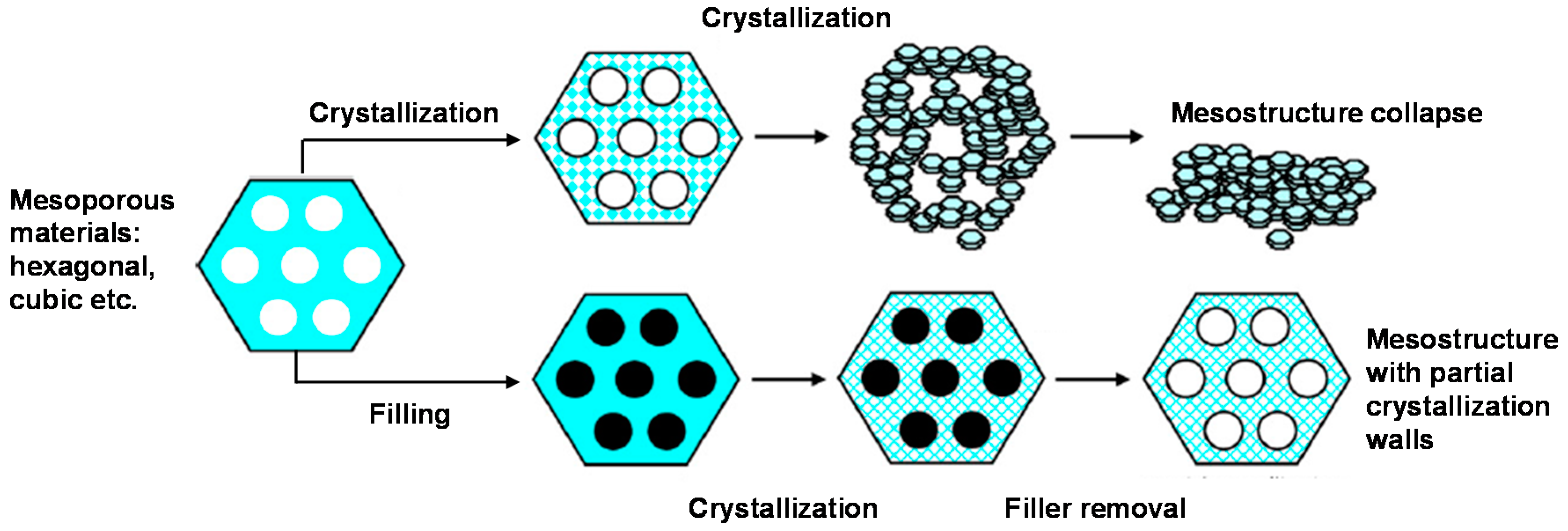
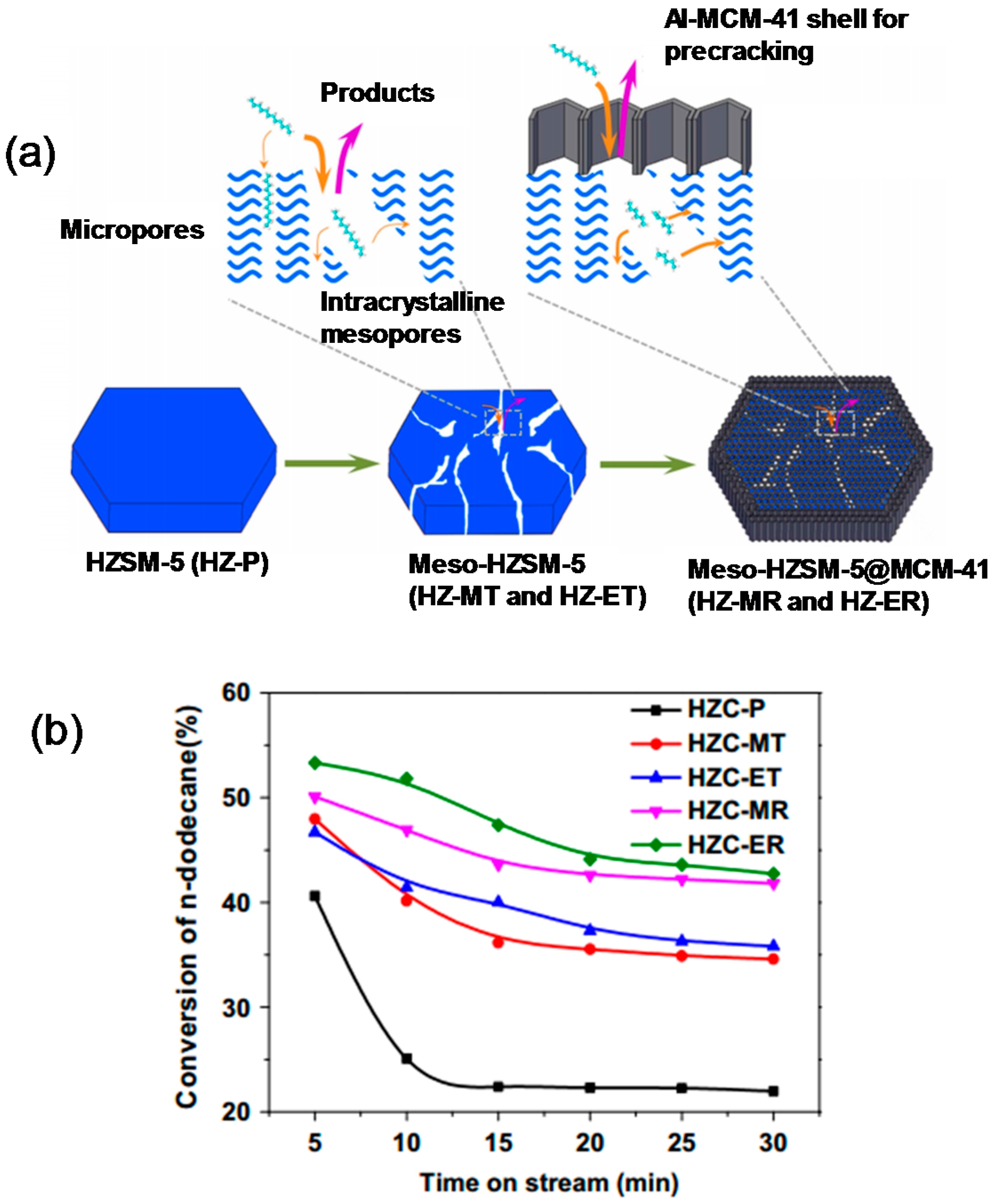

| Compound | ZSC-24 | HZSM-5 | Al-SBA-15 |
|---|---|---|---|
| Methane | 1.7 | 0.9 | 5.1 |
| Ethane | 2.3 | 1.7 | 6.0 |
| Ethene | 6.5 | 16.9 | 9.0 |
| Propane | 2.4 | 14.2 | 5.3 |
| Propene | 41.9 | 32.9 | 30.4 |
| i-Butane | 0.6 | 4.8 | 0.5 |
| n-Butane | 1.5 | 5.6 | 4.1 |
| t-2-Butene | 10.9 | 5.6 | 11.0 |
| 1-Butene | 8.7 | 4.4 | 8.9 |
| i-Butene | 15.9 | 8.9 | 11.6 |
| c-2-Butene | 7.9 | 4.1 | 8.2 |
| Selectivity to C2–C4 olefins (%) a | 93.2 | 73.5 | 83.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, X.H.; Armbruster, U.; Martin, A. Micro/Mesoporous Zeolitic Composites: Recent Developments in Synthesis and Catalytic Applications. Catalysts 2016, 6, 183. https://doi.org/10.3390/catal6120183
Vu XH, Armbruster U, Martin A. Micro/Mesoporous Zeolitic Composites: Recent Developments in Synthesis and Catalytic Applications. Catalysts. 2016; 6(12):183. https://doi.org/10.3390/catal6120183
Chicago/Turabian StyleVu, Xuan Hoan, Udo Armbruster, and Andreas Martin. 2016. "Micro/Mesoporous Zeolitic Composites: Recent Developments in Synthesis and Catalytic Applications" Catalysts 6, no. 12: 183. https://doi.org/10.3390/catal6120183
APA StyleVu, X. H., Armbruster, U., & Martin, A. (2016). Micro/Mesoporous Zeolitic Composites: Recent Developments in Synthesis and Catalytic Applications. Catalysts, 6(12), 183. https://doi.org/10.3390/catal6120183






