Chitosan Aerogel Catalyzed Asymmetric Aldol Reaction in Water: Highly Enantioselective Construction of 3-Substituted-3-hydroxy-2-oxindoles
Abstract
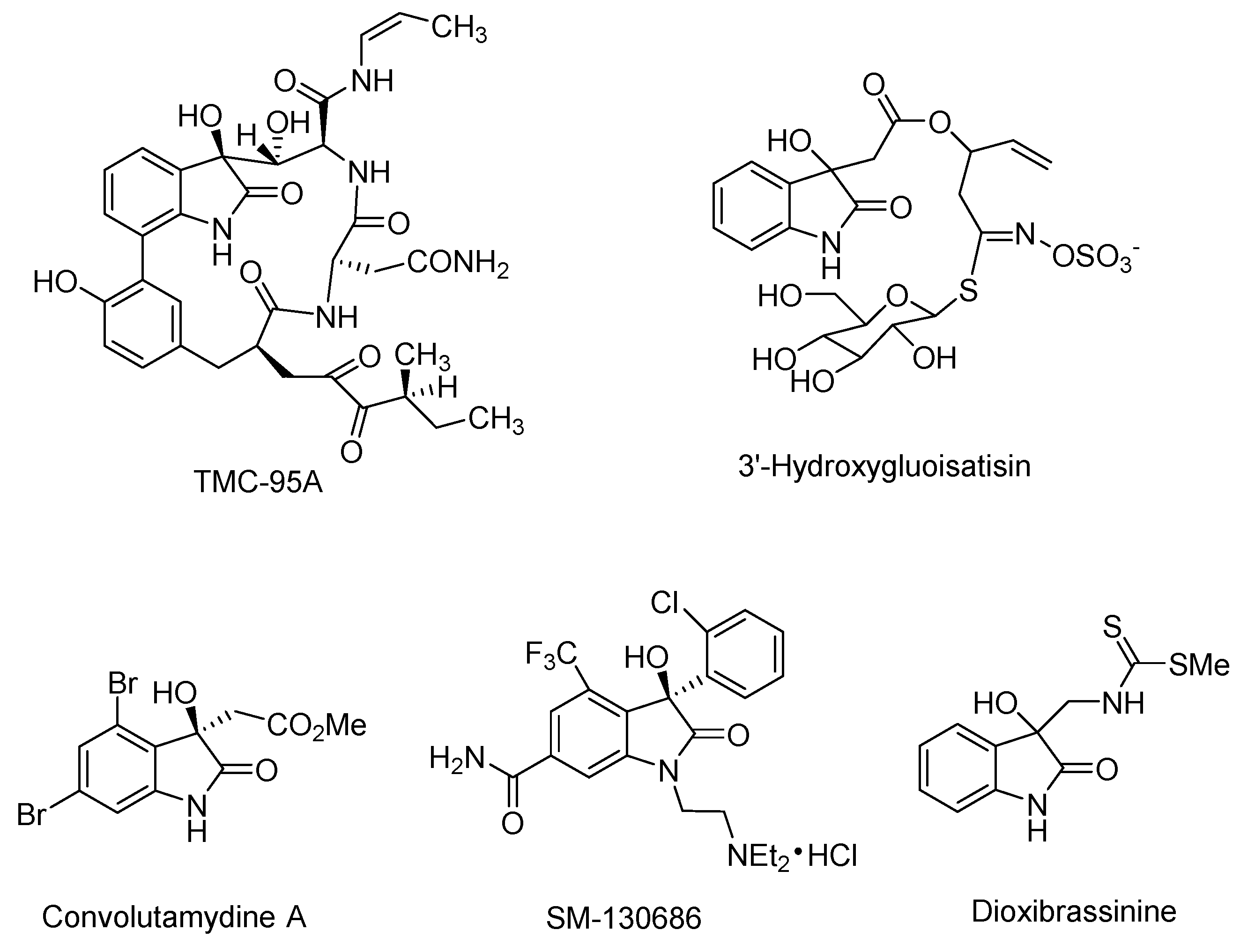
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. General Procedure for the Asymmetric Aldol Reaction of Isatins with Ketones
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rasmussen, H.B.; Macleod, J.K. Total synthesis of donaxaridine. J. Nat. Prod. 1997, 60, 1152–1154. [Google Scholar] [CrossRef]
- Hibino, S.; Choshi, T. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat. Prod. Rep. 2001, 18, 66–87. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Q.; Sattler, I.; Thiericke, R.; Grabley, S.; Feng, X.Z. Maremycins C and D, new diketopiperazines, and maremycins E and F, novel polycyclic spiro-indole metabolites isolated from Streptomyces sp. Eur.J. Org. Chem. 2001, 261–267. [Google Scholar] [CrossRef]
- Hewawasam, P.; Erway, M.; Moon, S.L.; Knipe, J.; Weiner, H.; Boissard, C.G.; Post-Munson, D.J.; Gao, Q.; Huang, S.; Gribkoff, V.K.; et al. Synthesis and structure−activity relationships of 3-aryloxindoles: A new class of calcium-dependent, large conductance potassium (Maxi-K) channel openers with neuroprotective properties. J. Med. Chem. 2002, 45, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Rao, P.B.; Hao, J.; Reddy, M.V.; Rassias, G.; Huang, X. The second total synthesis of diazonamide A. Angew. Chem. Int. Ed. 2003, 42, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Morita, H.; Shiro, M.; Kobayashi, J. Celogentin K, a new cyclic peptide from the seeds of Celosia argentea and X-ray structure of moroidin. Tetrahedron 2004, 60, 2489–2495. [Google Scholar] [CrossRef]
- Chen, W.B.; Du, X.L.; Cun, L.F.; Zhang, X.M.; Yuan, W.C. Highly enantioselective aldol reaction of acetaldehyde and isatins only with 4-hydroxydiarylprolinol as catalyst: Concise stereoselective synthesis of (R)-convolutamydines B and E, (−)-donaxaridine and (R)-chimonamidine. Tetrahedron 2010, 66, 1441–1446. [Google Scholar] [CrossRef]
- Niu, R.; Xiao, J.; Liang, T.; Li, X.W. Facile synthesis of azaarene-substituted 3-hydroxy-2-oxindoles via brønsted acid catalyzed sp3 C–H functionalization. Org. Lett. 2012, 14, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Koguchi, Y.; Kohno, J.; Nishio, M.; Takahashi, K.; Okuda, T.; Ohnuki, T.; Komatsubara, S. TMC-95A, B, C, and D, novel proteasome inhibitors produced by Apiospora montagnei Sacc. TC 1093 taxonomy, production, isolation, and biological activities. J. Antibiot. 2000, 53, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Kohno, J.; Koguchi, Y.; Nishio, M.; Nakao, K.; Kuroda, M.; Shimizu, R.; Ohnuki, T.; Komatsubara, S. Structures of TMC-95A−D: Novel proteasome inhibitors from Apiospora montagnei Sacc. TC 1093. J. Org. Chem. 2000, 65, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Monde, K.; Sasaki, K.; Shirata, A.; Takasugi, M. Brassicanal C and two dioxindoles from cabbage. Phytochemistry 1991, 30, 2915–2917. [Google Scholar] [CrossRef]
- Suchý, M.; Kutschy, P.; Monde, K.; Goto, H.; Harada, N.; Takasugi, M.; Dzurilla, M.; Balentova, E. Synthesis, absolute configuration, and enantiomeric enrichment of a cruciferous oxindolephytoalexin, (S)-(−)-spirobrassinin, and its oxazoline analog. J.Org. Chem. 2001, 66, 3940–3947. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, T.; Hume, W.E.; Umezome, T.; Okazaki, K.; Ueki, Y.; Kumagai, K.; Hourai, S.; Nagamine, J.; Seki, H.; Taiji, M.; et al. Oxindole derivatives as orally active potent growth hormone secretagogues. J. Med. Chem. 2001, 44, 4641–4649. [Google Scholar] [CrossRef] [PubMed]
- Fréchard, A.; Fabre, N.; Péan, C.; Montaut, S.; Fauvel, M.T.; Rollin, P.; Fourasté, I. Novel indole-type glucosinolates from woad (Isatis tinctoria L.). Tetrahedron Lett. 2001, 42, 9015–9017. [Google Scholar] [CrossRef]
- Kamano, Y.; Zhang, H.P.; Ichihara, Y.; Kizu, H.; Komiyama, K.; Pettit, G.R. Convolutamydine A, a novel bioactive hydroxyoxindole alkaloid from marine bryozoan Amathia convolute. Tetrahedron Lett. 1995, 36, 2783–2784. [Google Scholar] [CrossRef]
- Garden, S.J.; Silva, R.B.; Pinto, A.C. A versatile synthetic methodology for the synthesis of tryptophols. Tetrahedron 2002, 58, 8399–8412. [Google Scholar] [CrossRef]
- Chen, J.R.; Liu, X.P.; Zhu, X.Y.; Li, L.; Qiao, Y.F.; Zhang, J.M.; Xiao, W.J. Organocatalytic asymmetric aldol reaction of ketones with isatins: Straightforward stereoselective synthesis of 3-alkyl-3-hydroxyindolin-2-ones. Tetrahedron 2007, 63, 10437–10444. [Google Scholar] [CrossRef]
- Chen, W.B.; Liao, Y.H.; Du, X.L.; Zhang, X.M.; Yuan, W.C. Catalyst-free aldol condensation of ketones and isatins under mild reaction conditions in DMF with molecular sieves 4 Å as additive. Green Chem. 2009, 11, 1465–1476. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, W.; Lee, R.; Han, Z.; Yang, W.; Tan, D.; Huang, K.W.; Jiang, Z. Direct asymmetric vinylogousaldol reaction of allyl ketones with isatins: divergent synthesis of 3-hydroxy-2-oxindole derivatives. Angew. Chem. Int. Ed. 2013, 52, 6666–6670. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.; Ramesh, P.; Kumar, G.S.; Swetha, A.; Nanubolu, J.B.; Meshram, H.M. A Ru(III)–catalyzed α-cross-coupling aldol type addition reaction of activated olefins with isatins. RSC Adv. 2016, 6, 1705–1709. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, Y.; Huang, T.; Liu, X.; Lin, L.; Feng, X.M. Copper/Guanidine-catalyzed asymmetric alkynylation of isatins. Angew. Chem. Int. Ed. 2016, 55, 5286–5289. [Google Scholar] [CrossRef] [PubMed]
- Luppi, G.; Cozzi, P.G.; Monari, M.; Kaptein, B.; Broxterman, Q.B.; Tomasini, C. Dipeptide-catalyzed asymmetric aldol condensation of acetone with (N-alkylated) isatins. J. Org. Chem. 2005, 70, 7418–7421. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Hara, N.; Nakashima, H.; Kubo, K.; Shibata, N.; Toru, T. First enantioselective synthesis of (R)-convolutamydine B and E with N-(heteroarenesulfonyl) prolinamides. Chem. Eur. J. 2009, 15, 6790–6793. [Google Scholar]
- Guo, Q.; Bhanushali, M.; Zhao, C. Quinidine thiourea-catalyzed aldol reaction of unactivated ketones: Highly enantioselective synthesis of 3-alkyl-3-hydroxyindolin-2-ones. Angew. Chem. Int. Ed. 2010, 49, 9460–9464. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Xiang, Z.W.; Shen, Z.; Wu, Q.; Lin, X.F. Enzymatic enantioselective aldol reactions of isatin derivatives with cyclic ketones under solvent-free conditions. Biochimie 2014, 101, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ju, Y.; Yang, T.; Li, Z.; Wei, A.; Sang, Z.; Liu, J.; Lou, Y. Natural amino acid salt catalyzed aldol reactions of isatins with ketones: Highly enantioselective construction of 3-alkyl-3-hydroxyindolin-2-ones. Tetrahedron Asymmetry 2015, 26, 943–947. [Google Scholar] [CrossRef]
- Kaplan, D.L. Biopolymers from Renewable Resources; Springer: Berlin, Germany, 1998. [Google Scholar]
- Guibal, E. Heterogeneous catalysis on chitosan-based materials: A review. Prog. Polym. Sci. 2005, 30, 71–109. [Google Scholar]
- Roberts, G.A.F.; Taylo, K.E. Chitosan gels, 3. The formation of gels by reaction of chitosan with glutaraldehyde. Makromol. Chem. 1989, 190, 951–960. [Google Scholar] [CrossRef]
- Wei, Y.C.; Hudson, S.M.; Mayer, J.M.; Kaplan, D.L.J. The crosslinking of chitosan fibers. Polym. Sci. Part A 1992, 30, 2187–2193. [Google Scholar] [CrossRef]
- Zeng, X.; Ruckenstein, E. Trypsin purification by p-aminobenzamidine immobilized on macroporous chitosan membranes. Ind. Eng. Chem. Res. 1998, 37, 159–165. [Google Scholar] [CrossRef]
- Quignard, F.; Valentin, R.; Renzo, F.D. Aerogel materials from marine polysaccharides. New J. Chem. 2008, 32, 1300–1310. [Google Scholar] [CrossRef]
- Quignard, F.; Choplin, A.; Domard, A. Chitosan: A natural polymeric support of catalysts for the synthesis of fine chemicals. Langmuir 2000, 16, 9106–9108. [Google Scholar] [CrossRef]
- Sun, W.; Xia, C.G.; Wang, H.W. Efficient heterogeneous catalysts for the cyclopropanation of olefins. New J. Chem. 2002, 26, 755–758. [Google Scholar] [CrossRef]
- Hardy, J.J.E.; Hubert, S.; Macquarrie, D.J.; Wilson, A.J. Chitosan-based heterogeneous catalysts for Suzuki and Heck reactions. Green Chem. 2004, 6, 53–56. [Google Scholar] [CrossRef]
- Chtchigrovsky, M.; Primo, A.; Gonzalez, P.; Molvinger, K.; Robitzer, M.; Quignard, F.; Taran, F. Functionalized chitosan as a green, recyclable, biopolymer-supported catalyst for the [3 + 2] Huisgen cycloaddition. Angew. Chem. Int. Ed. 2009, 48, 5916–5920. [Google Scholar] [CrossRef] [PubMed]
- Macquarrie, D.J.; Hardy, J.J.E. Applications of functionalized chitosan in catalysis. Ind. Eng. Chem. Res. 2005, 44, 8499–8520. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, W.; Zou, J.; Yi, L.; Li, R.; Cui, Y. Aldol reaction catalyzed by a hydrophilic catalyst in aqueous micelle as an enzyme mimic system. Chirality 2009, 21, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Rajgopal, K.; Maheswari, C.U.; Kantam, M.L. Chitosan hydrogel: A green and recyclable biopolymer catalyst for aldol and Knoevenagel reactions. New J. Chem. 2006, 30, 1549–1552. [Google Scholar] [CrossRef]
- Gioia, C.; Ricci, A.; Bernardi, L.; Bourahla, K.; Tanchoux, N.; Robitzer, M.; Quignard, F. Chitosan aerogel beads as a heterogeneous organocatalyst for the asymmetric aldol reaction in the presence of water: An assessment of the effect of additives. Eur. J. Org. Chem. 2013, 588–594. [Google Scholar] [CrossRef]
- Zhao, W.; Qu, C.; Yang, L.; Cui, Y. Chitosan-supported cinchonine as an efficient organocatalyst for direct asymmetric aldol reaction in water. Chin. J. Catal. 2015, 36, 367–371. [Google Scholar] [CrossRef]
- Ricci, A.; Bernardi, L.; Gioia, C.; Vierucci, S.; Robitzer, M.; Quignard, F. Chitosan aerogel: A recyclable, heterogeneous organocatalyst for the asymmetric direct aldol reaction in water. Chem. Commun. 2010, 46, 6288–6290. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, Y.; Yasunaga, K.; Ishimaru, K. Asymmetric aldol reaction using a very simple primary amine catalyst: divergent stereoselectivity by using 2,6-difluorophenyl moiety. Tetrahedron 2014, 70, 2816–2821. [Google Scholar] [CrossRef]
| Entry | Solvent | Time (h) | Yield (%) b | syn:anti c | ee (%) d |
|---|---|---|---|---|---|
| 1 | EtOAc | 3 | 92 | 71:29 | 11 (16) |
| 2 | MeCN | 2 | 90 | 72:28 | 21 (20) |
| 3 | Et2O | 3 | 94 | 52:48 | 7 (4) |
| 4 | CHCl3 | 2 | 92 | 65:35 | 31 (30) |
| 5 | PhMe | 3 | 95 | 52:48 | 35 (22) |
| 6 | DCM | 2 | 97 | 65:35 | 34 (10) |
| 7 | Hexane | 1.5 | 93 | 65:35 | 40 (20) |
| 8 | H2O | 2 | 90 | 73:27 | 37 (57) |
| 9 | EtOH | 2 | 80 | 70:30 | 17 (20) |
| 10 | MeOH | 2 | 88 | 67:33 | 29 (20) |
| 11 | Dioxane | 7 | 70 | 61:39 | 20 (13) |
| 12 | i-PrOH | 6 | 70 | 57:43 | 18 (5) |
| Entry | Hydroxyacetone (Equivalents) | Time (h) | Yield (%) b | syn:anti c | ee (%) d |
|---|---|---|---|---|---|
| 1 | 5 | 2.5 | 88 | 71:29 | 33 (43) |
| 2 | 10 | 2 | 96 | 72:28 | 40 (51) |
| 3 | 20 | 2 | 99 | 57:43 | 44 (59) |
| 4 | 40 | 1.5 | 99 | 53:47 | 31 (30) |
| Entry | Additive | Time (h) | Yield (%) b | syn:anti c | ee (%) d |
|---|---|---|---|---|---|
| 1 | sulfamic acid | 2.5 | 98 | 56:44 | 2 (1) |
| 2 | formic acid | 4 | 97 | 68:32 | 38 (47) |
| 3 | p-toluenesulfonic acid | 2.5 | 93 | 60:40 | 11 (20) |
| 4 | 4 Å molecular sieves(10 mg) e | 2.5 | 90 | 54:46 | 11 (26) |
| 5 | 4 Å molecular sieves(20 mg) e | 2.5 | 93 | 61:39 | 10 (12) |
| 6 | 4 Å molecular sieves(30 mg) e | 4.5 | 96 | 72:28 | 31 (56) |
| 7 | acetic acid glacial | 2.5 | 94 | 59:41 | 17 (22) |
| 8 | l-proline | 2.5 | 95 | 64:36 | 24 (29) |
| 9 | benzoic acid | 2.5 | 97 | 57:43 | 16 (18) |
| 10 | stearic acid | 5 | 94 | 51:49 | 11 (4) |
| 11 | 3-nitrobenzoic acid | 4 | 92 | 61:39 | 29 (25) |
| 12 | 2,4-dinitrophenol | 4 | 94 | 69:31 | 47 (60) |
| 13 | polyethylene glycol | 6 | 87 | 65:35 | 34 (41) |
| 14 | oxalic acid | 2.5 | 82 | 58:42 | 46 (50) |
| 15 | 1,1′-bi-2-naphthol | 3 | 98 | 68:32 | 63 (62) |
| 16 | 2,5-dihydroxybenzoic acid | 2.5 | 96 | 69:31 | 66 (72) |
| 17 f | 2,5-dihydroxybenzoic acid | 48 | 90 | 53:47 | 75 (80) |
 | |||
|---|---|---|---|
 |  |  |  |
 |  |  |  |
 |  |  |  |
 |  |  |  |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Liu, J.; Ma, L.; Ouyang, L. Chitosan Aerogel Catalyzed Asymmetric Aldol Reaction in Water: Highly Enantioselective Construction of 3-Substituted-3-hydroxy-2-oxindoles. Catalysts 2016, 6, 186. https://doi.org/10.3390/catal6120186
Dong H, Liu J, Ma L, Ouyang L. Chitosan Aerogel Catalyzed Asymmetric Aldol Reaction in Water: Highly Enantioselective Construction of 3-Substituted-3-hydroxy-2-oxindoles. Catalysts. 2016; 6(12):186. https://doi.org/10.3390/catal6120186
Chicago/Turabian StyleDong, Hui, Jie Liu, Lifang Ma, and Liang Ouyang. 2016. "Chitosan Aerogel Catalyzed Asymmetric Aldol Reaction in Water: Highly Enantioselective Construction of 3-Substituted-3-hydroxy-2-oxindoles" Catalysts 6, no. 12: 186. https://doi.org/10.3390/catal6120186










