Synthesis and Characterization of Ag-Ag2O/TiO2@polypyrrole Heterojunction for Enhanced Photocatalytic Degradation of Methylene Blue
Abstract
:1. Introduction
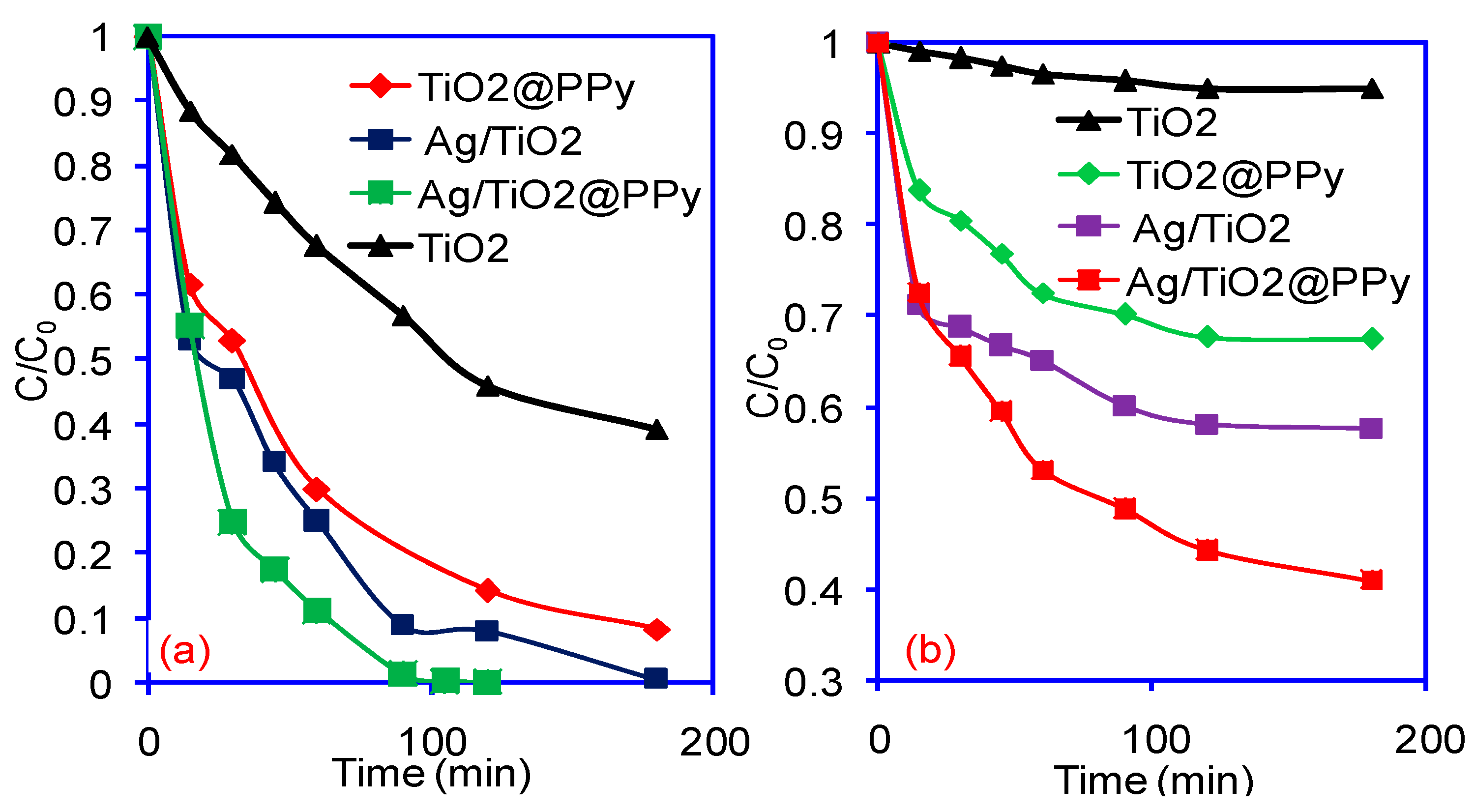
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Ag/TiO2 Nanocomposite
3.2. Synthesis of TiO2@PPy and Ag/TiO2@PPy Nanocomposite
3.3. Characterization
3.4. Photocatalytic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Curti, M.; Bahnemann, D.W.; Mendive, C.B. Mechanisms in Heterogeneous photocatalysis. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Paez, C.A.; Poelman, D.; Pirard, J.P.; Heinrichs, B. Unpredictable photocatalytic ability of H2-reduced rutile-TiO2 xerogel in the degradation of dye-pollutants under UV and visible light irradiation. Appl. Catal. B 2010, 94, 263–271. [Google Scholar] [CrossRef]
- Liua, X.; Zhanga, H.; Liu, C.; Chen, J.; Li, G.; An, T.; Wong, P.K.; Zhao, H. UV and visible light photoelectrocatalytic bactericidal performance of 100% {111} faceted rutile TiO2 photoanode. Catal. Today 2014, 224, 77–82. [Google Scholar] [CrossRef]
- McEvoy, J.G.; Cui, W.; Zhang, Z. Degradative and disinfective properties of carbon-doped anatase-rutile TiO2 mixtures under visible light irradiation. Catal. Today 2013, 207, 191–199. [Google Scholar] [CrossRef]
- Egerton, T.A.; Purnama, H.; Mattinson, J.A. The influence of platinum (II) on TiO2 photocatalyzed dye decolourization by rutile, P25 and PC500. J. Photochem. Photobiol. A 2011, 224, 31–37. [Google Scholar] [CrossRef]
- Dolat, D.; Ohtani, B.; Mozia, S.; Moszynski, D.; Guskos, N.; Bieluna, Z.L.; Morawski, A.W. Preparation, characterization and charge transfer studies of nickel—Modified and nickel, nitrogen co-modified rutile titanium dioxide for photocatalytic application. Chem. Eng. J. 2014, 239, 149–159. [Google Scholar] [CrossRef]
- Dolat, D.; Mozia, S.; Ohtani, B.; Morawski, A.W. Nitrogen, Iron-single modified (N-TiO2, Fe-TiO2) and co-modified (Fe, N-TiO2) rutile titanium dioxide as visible-light active photocatalysts. Chem. Eng. J. 2013, 225, 358–364. [Google Scholar] [CrossRef]
- Ansari, M.O.; Khan, M.M.; Ansari, S.A.; Raju, K.; Lee, J.; Cho, M.H. Enhanced thermal stability under DC electrical conductivity retention and visible light activity of Ag/TiO2@polyaniline nanocomposite film. ACS Appl. Mater. Interfaces 2014, 6, 8124–8133. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wen, J.; Wei, J.; Xiong, R.; Shi, J.; Pan, C. Polypyrrole-decorated Ag-TiO2 nanofibers exhibiting enhanced photocatalytic activity under visible-light illumination. ACS Appl. Mater. Interfaces 2013, 5, 6201–6207. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, S.; Yu, H.; Yu, J.; Liu, S. Ag2O as a new visible-light photocatalyst: Self-stability and high photocatalytic activity. Chem. Eur. J. 2011, 17, 7777–7780. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.J.; Leng, Y.H.; Hou, D.M.; Li, H.D.; Li, L.G.; Li, G.Q.; Liu, H.; Chen, S.W. Phase transformation and enhanced photocatalytic activity of S-Doped Ag2O/TiO2 heterostructured nanobelts. Nanoscale 2014, 6, 4698–4704. [Google Scholar] [CrossRef] [PubMed]
- Duana, F.; Zhanga, Q.; Shi, D.; Chen, M. Enhanced visible light photocatalytic activity of Bi2WO6 via modification with polypyrrole. Appl. Surf. Sci. 2013, 268, 129–135. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Soin, N.; Roy, S.S. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment. RSC Adv. 2014, 4, 3823–3851. [Google Scholar] [CrossRef]
- Deng, F.; Li, Y.; Luo, X.; Yang, L.; Tu, X. Preparation of conductive polypyrrole/TiO2 nanocomposite via surface molecular imprinting technique and its photocatalytic activity under simulated solar light irradiation. Coll. Surf. A 2012, 395, 183–189. [Google Scholar] [CrossRef]
- Haspulat, B.; Gülce, A.; Gülce, H. Efficient photocatalytic decolorization of some textile dyes using Fe ions doped polyaniline film on ito coated glass substrate. J. Hazard. Mater. 2013, 260, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yuan, Y.; Liang, L.; Cheng, Y.; Xu, H.; Shi, G.; Jin, L. Preparation and photoelectrochemical properties of a hybrid electrode composed of polypyrrole encapsulated in highly ordered titanium dioxide nanotube Array. Thin Solid Films 2008, 516, 8663–8667. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, Y.; Mahimwalla, Z.; Johnson, M.B.; Sharma, T.; Brüning, R.; Ghandi, K. Novel synthesis of rutile titanium dioxide–polypyrrole nano composites and their application in hydrogen generation. Synth. Met. 2014, 189, 77–85. [Google Scholar] [CrossRef]
- Gu, S.; Li, B.; Zhao, C.; Xu, Y.; Qian, X.; Chen, G. Preparation and characterization of visible light-driven AgCl/PPy photocatalyst. J. Alloys Comp. 2011, 509, 5677–5682. [Google Scholar] [CrossRef]
- Wang, B.; Li, C.; Pang, J.; Qing, X.; Zhai, J.; Li, Q. Novel Polypyrrole-Sensitized Hollow TiO2/Fly Ash Cenospheres: Synthesis, characterization, and photocatalytic ability under visible light. Appl. Surf. Sci. 2012, 258, 9989–9996. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Dillert, R.D.; Bahnemann, D.W. Enhanced photocatalytic production of molecular hydrogen on TiO2 modified with Pt-polypyrrole nanocomposites. Photochem. Photobiol. Sci. 2009, 8, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.T.; Jia, S.Y.; Wu, Y.; Wu, S.H.; Zhang, T.H.; Han, X. Improved photochemical reactivities of Ag2O/gC3N4 in phenol degradation under UV and visible light. Ind. Eng. Chem. Res. 2014, 53, 17645–17653. [Google Scholar] [CrossRef]
- Kowal, K.; Kopaczynska, K.W.K.M.; Dworniczek, E.; Franiczek, R.; Wawrzynska, M.; Vargova, M.; Zahoran, M.; Rakovsky, E.; Kus, P.; Plesch, G.; et al. In-situ photoexcitation of silver-doped titania nanopowders for activity against bacteria and yeasts. J. Colloid Interface Sci. 2011, 362, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Wang, M.; Jin, L.; Huang, S.; Chen, W.; Xu, M.; He, Q.; Zai, J.; Fang, N.; Qian, X. Highly efficient Ag2O/Bi2O2CO3 p-n heterojunction photocatalysts with improved visible-light responsive activity. ACS Appl. Mater. Interfaces 2014, 6, 11698–11705. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Shen, L.; Wu, M.; Guo, Y.; Meng, Y. A novel chemical synthesis of bowl-shaped polypyrrole particles. Mater. Lett. 2014, 126, 185–188. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoh, K.G.; Ong, Y.K.; Tan, K.L.; Tan, B.T.G. XPS studies of proton modification and some anion exchange processes in polypyrrole. Synth. Met. 1990, 30, 69–80. [Google Scholar] [CrossRef]
- Landureau, E.P.; Nicolau, Y.F.; Delamar, M. XPS study of layer-by-layer deposited polypyrrole thin films. Synth. Met. 1995, 72, 111–119. [Google Scholar] [CrossRef]
- Ansari, M.O.; Yadav, S.K.; Cho, J.W.; Mohammad, F. Thermal stability in terms of DC electrical conductivity retention and the efficacy of mixing technique in the preparation of nanocomposites of graphene/polyaniline over the carbon nanotubes/polyaniline. Compos. Part B 2013, 47, 155–161. [Google Scholar] [CrossRef]
- Pérez-Bustamante, R.; Pérez-Bustamante, F.; Estrada-Guel, I.; Santillán-Rodríguez, C.R.; Matutes-Aquino, J.A.; Herrera-Ramírez, J.M.; Miki-Yoshidaa, M.; Martínez-Sánchez, R. Characterization of Al2024-CNTs composites produced by mechanical alloying. Powder Technol. 2011, 212, 390–396. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, H.; Wang, J.; Liu, D.; Du, G.; Cui, J. Ag2O/TiO2 nanobelts heterostructure with enhanced ultraviolet and visible photocatalytic activity. ACS Appl. Mater. Interfaces 2010, 8, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Xu, L.; Cao, X.; Li, X.; Huang, Y.; Meng, C.; Wang, Z.; Zhu, W. Ag2O/TiO2/V2O5 one-dimensional nanoheterostructures for superior solar light photocatalytic activity. Nanoscale 2014, 6, 6790–6797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bai, R. Surface electric properties of polypyrrole in aqueous solutions. Langmuir 2003, 19, 10703–10709. [Google Scholar] [CrossRef]
- Bhaumik, M.; McCrindle, R.; Maity, A. Efficient removal of Congo red from aqueous solutions by adsorption onto interconnected polypyrrole-polyaniline nanofibres. Chem. Eng. J. 2013, 228, 506–515. [Google Scholar] [CrossRef]
- Li, J.; Feng, J.; Yan, W. Excellent adsorption and desorption characteristics of polypyrrole/TiO2 composite for methylene blue. Appl. Surf. Sci. 2013, 279, 400–408. [Google Scholar] [CrossRef]
- Moziaa, S.; Morawski, A.W.; Toyoda, M.; Tsumura, T. Effect of process parameters on photodegradation of acid yellow 36 in a hybrid photocatalysis-membrane distillation system. Chem. Eng. J. 2009, 150, 152–159. [Google Scholar] [CrossRef]
- Reuterglrdh, L.B.; Iangphasuk, M. Photocatalytic decolourization of reactive azo dye: A comparison between TiO2 and CdS photocatalysis. Chemosphere 1997, 35, 585–596. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, X.; Xu, J.J.; Chen, H.Y. Fe3O4/Polypyrrole/Au nanocomposites with core/shell/shell structure: Synthesis, characterization, and their electrochemical properties. Langmuir 2008, 24, 13748–13752. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Ghosh, C.K.; Mukherjee, S.; Chattopadhyay, K.K. Three dimensional Ag2O/TiO2 type-II (p-n) nanoheterojunctions for superior photocatalytic activity. ACS Appl. Mater. Interfaces 2013, 5, 331–337. [Google Scholar] [CrossRef] [PubMed]






| pH | k (UV) (min−1) | R2 | k (Visible) (min−1) | R2 |
|---|---|---|---|---|
| 3 | 0.000 | 0.834 | 0.000 | 0.488 |
| 5 | 0.014 | 0.987 | 0.002 | 0.987 |
| 7 | 0.024 | 0.929 | 0.003 | 0.955 |
| 9 | 0.044 | 0.968 | 0.003 | 0.947 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; El-Shishtawy, R.M.; Barakat, M.A. Synthesis and Characterization of Ag-Ag2O/TiO2@polypyrrole Heterojunction for Enhanced Photocatalytic Degradation of Methylene Blue. Catalysts 2016, 6, 76. https://doi.org/10.3390/catal6060076
Kumar R, El-Shishtawy RM, Barakat MA. Synthesis and Characterization of Ag-Ag2O/TiO2@polypyrrole Heterojunction for Enhanced Photocatalytic Degradation of Methylene Blue. Catalysts. 2016; 6(6):76. https://doi.org/10.3390/catal6060076
Chicago/Turabian StyleKumar, Rajeev, Reda M. El-Shishtawy, and Mohamed A. Barakat. 2016. "Synthesis and Characterization of Ag-Ag2O/TiO2@polypyrrole Heterojunction for Enhanced Photocatalytic Degradation of Methylene Blue" Catalysts 6, no. 6: 76. https://doi.org/10.3390/catal6060076







