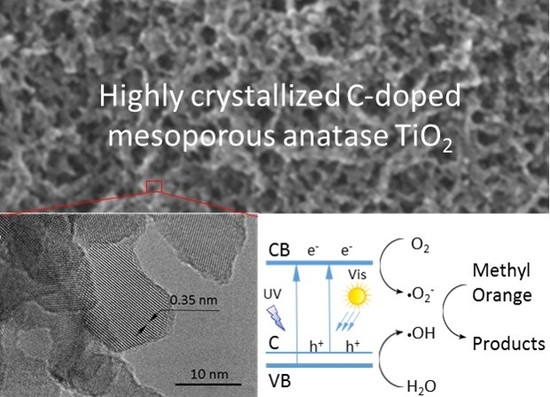
Highly Crystallized C-Doped Mesoporous Anatase TiO2 with Visible Light Photocatalytic Activity
Abstract
:1. Introduction
2. Results
2.1. Morphology and Structure
2.2. Surface Area and Pore Size Distribution
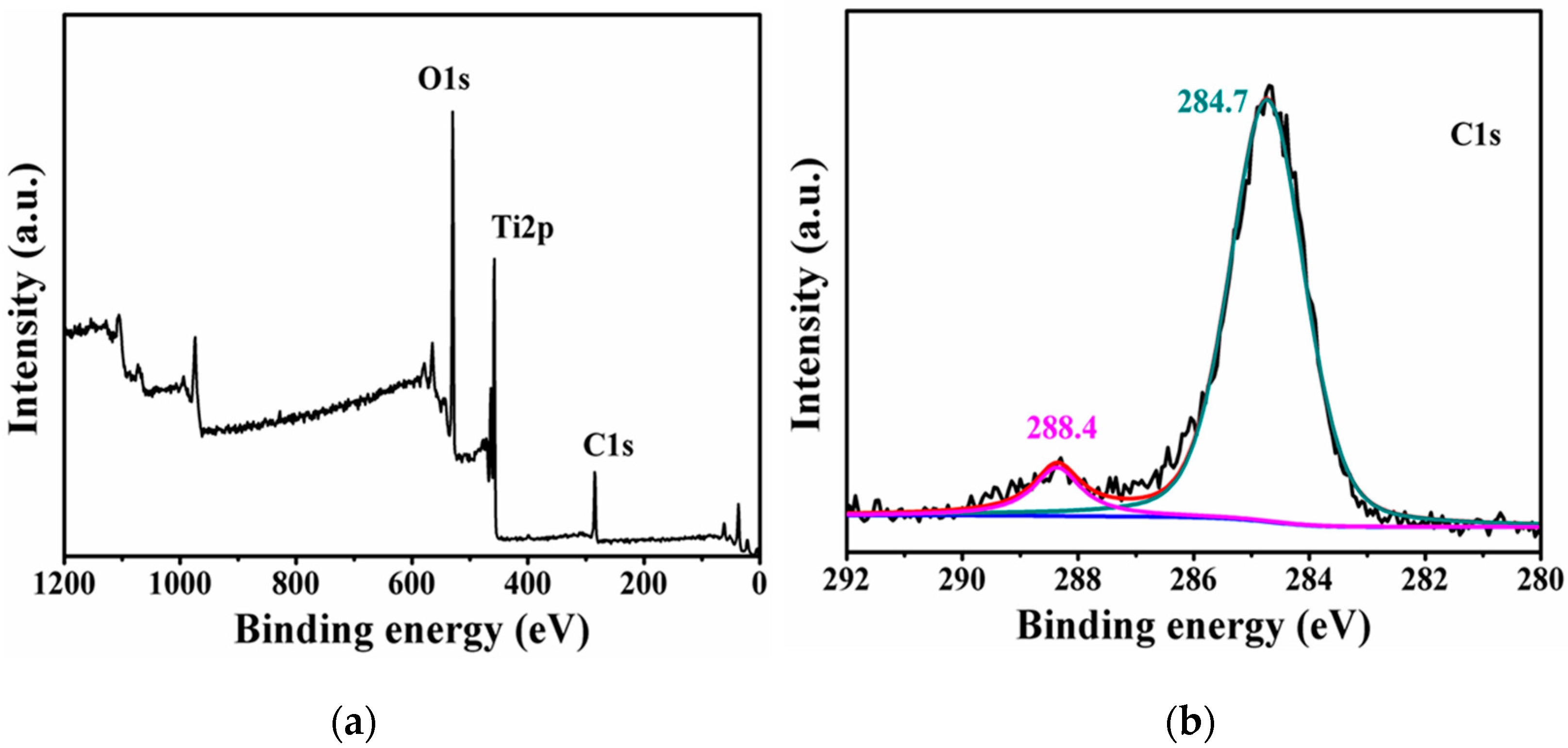
2.3. Carbon Content
2.4. UV–Vis Adsorption
2.5. Visible Light Photcatalytic Properties
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MWCNT | Multi-walled carbon nanotube |
| SEM | Scanning electron microscope |
| HRTEM | High resolution transmission electron microscope |
| XRD | X-Ray Diffractometer |
| XPS | X-Ray Photoelectron spectroscope |
| FWHM | Full width at half maximum |
| IUPAC | International Union of Pure and Applied Chemistry |
| BET | Brunauer–Emmett–Teller |
| BJH | Barrett-Joyner-Halenda |
| PVDF | Polyvinylidene fluoride |
| PTFE | Polytetrafluoroethylene |
| MO IUPAC | Methyl orange |
References
- Tian, J.; Zhao, Z.-H.; Kumar, A.; Boughton, R.I.; Liu, H. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: A review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idrissc, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Chen, X.-B.; Mao, S.-S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.-Y.; Guo, H.; Liu, X.-M.; Wu, S.-L.; Yeung, K.W.K.; Chu, P.-K. Nanostructured TiO2 for energy conversion and storage. RSC Adv. 2013, 3, 24758–24775. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.-Y.; Zhang, J.-L.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal methods: Formation mechanism, structure modification, and photocatalytic applications. Cata. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B., Jr. Efficient photochemical water splitting by a chemically modified N-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.G.; Kavitha, R. Review on modified N-TiO2 for green energy applications under UV/visible light: Selected results and reaction mechanisms. RSC Adv. 2014, 4, 28265–28299. [Google Scholar] [CrossRef]
- Yu, X.-J.; Liu, J.-J.; Yu, Y.-C.; Zuo, S.-L.; Li, B.-S. Preparation and visible light photocatalytic activity of carbon quantum dots/TiO2 nanosheet composites. Carbon 2014, 68, 718–724. [Google Scholar] [CrossRef]
- Li, W.-X.; Yang, J.-Y.; Jiang, Q.-H.; Luo, Y.-B.; Hou, Y.; Zhou, S.-Q.; Zhou, Z.-W. Bi-layer of nanorods and three-dimensional hierarchical structure of TiO2 for high efficiency dye-sensitized solar cells. J. Power Sources 2015, 284, 428–434. [Google Scholar] [CrossRef]
- Liu, J.; Luo, J.; Yang, W.-G.; Wang, Y.-L.; Zhu, L.-Y.; Xu, Y.-Y.; Tang, Y.; Hu, Y.-J.; Wang, C.; Chen, Y.-G.; et al. Synthesis of single-crystalline anatase TiO2 nanorods with high-performance dye-sensitized solar cells. J. Mater. Sci. Technol. 2015, 31, 106–109. [Google Scholar] [CrossRef]
- Wu, H.-J.; Wang, Y.; Ma, Y.; Xiao, T.-X.; Yuan, D.-D.; Zhang, Z.-H. Honeycombed TiO2 nanotube arrays with top-porous/bottom-tubular structures for enhanced photocatalytic activity. Ceram. Int. 2015, 41, 2527–2532. [Google Scholar] [CrossRef]
- Yuan, J.-J.; Li, H.-D.; Wang, Q.-L.; Yu, Q.; Zhang, X.-K.; Yu, H.-J.; Xie, Y.-M. Fabrication, characterization, and photocatalytic activity of double-layer TiO2 nanosheet films. Mater. Lett. 2012, 81, 123–126. [Google Scholar] [CrossRef]
- Chen, F.-T.; Liu, Z.; Liu, Y.; Fang, P.-F.; Dai, Y.-Q. Enhanced adsorption and photocatalytic degradation of high-concentration methylene blue on Ag2O-modified TiO2-based nanosheet. Chem. Eng. J. 2013, 221, 283–291. [Google Scholar] [CrossRef]
- Xiao, N.; Li, Z.-H.; Liu, J.-W.; Gao, Y. A facile template-free method for preparing bi-phase TiO2 nanowire arrays with high photocatalytic activity. Mater. Lett. 2010, 64, 1776–1778. [Google Scholar] [CrossRef]
- Lv, X.-J.; Zhang, H.; Chang, H.-X. Improved photocatalytic activity of highly ordered TiO2 nanowire arrays for methylene blue degradation. Mater. Chem. Phys. 2012, 136, 789–795. [Google Scholar] [CrossRef]
- Hu, A.-M.; Zhang, X.; Oakes, K.D.; Peng, P.; Zhou, Y.-N.; Servos, M.R. Hydrothermal growth of free standing TiO2 nanowire membranes for photocatalytic degradation of pharmaceuticals. J. Hazard. Mater. 2011, 189, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Huo, K.-F.; Gao, B.; Fu, J.-J.; Zhao, L.-Z.; Chu, P.-K. Fabrication, modification, and biomedical applications of anodized TiO2 nanotube arrays. RSC Adv. 2014, 4, 17300–17324. [Google Scholar] [CrossRef]
- Shi, J.-W.; Cui, H.-J.; Chen, J.-W.; Fu, M.-L.; Xu, B.; Luo, H.-Y.; Ye, Z.-L. TiO2/activated carbon fibers photocatalyst: Effects of coating procedures on the microstructure, adhesion property, and photocatalytic ability. J. Colloid Interface Sci. 2012, 388, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kim, B.-H.; Yang, K.S. TiO2 nanoparticles loaded on graphene/carbon composite nanofibers by electrospinning for increased photocatalysis. Carbon 2012, 50, 2472–2481. [Google Scholar] [CrossRef]
- Choi, Y.; Umebayashi, T.; Yoshikawa, M. Fabrication and characterization of C-doped anatase TiO2 photocatalysts. J. Mater. Sci. 2004, 39, 1837–1839. [Google Scholar] [CrossRef]
- Dong, F.; Guo, S.; Wang, H.-Q.; Li, X.-F.; Wu, Z.-B. Enhancement of the visible light photocatalytic activity of C-doped TiO2 Nanomaterials prepared by a green synthetic approach. J. Phys. Chem. C 2011, 115, 13285–13292. [Google Scholar] [CrossRef]
- Shi, J.-W.; Zong, X.; Wu, X.; Cui, H.-J.; Xu, B.; Wang, L.; Fu, M.-L. Carbon-doped titania hollow spheres with tunable hierarchical macroporous channels and enhanced visible light-induced photocatalytic activity. ChemCatChem 2012, 4, 488–491. [Google Scholar] [CrossRef]
- Teng, F.; Zhang, G.-Z.; Wang, Y.-Q.; Gao, C.-T.; Chen, L.-L.; Zhang, P.; Zhang, Z.-X.; Xie, E.-Q. The role of carbon in the photocatalytic reaction of carbon/TiO2 photocatalysts. Appl. Surf. Sci. 2014, 32, 703–709. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Weng, C.-H.; Lin, Y.-H.; Shiesh, C.-C.; Chen, F.-Y. Effect of C content and calcination temperature on the photocatalytic activity of C-doped TiO2 catalyst. Sep. Purif. Technol. 2013, 116, 114–123. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Yu, J.-G.; Dai, G.-P.; Xiang, Q.-J.; Jaroniec, M. Fabrication and enhanced visible-light photocatalytic activity of carbon self-doped TiO2 sheets with exposed {001} facets. J. Mater. Chem. 2011, 21, 1049–1157. [Google Scholar] [CrossRef]
- Ren, W.-J.; Ai, Z.-H.; Jia, F.-L.; Zhang, L.-Z.; Fan, X.-X.; Zhou, Z.-G. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B: Environ. 2007, 69, 138–144. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Wu, Z.-B.; Liu, Y. A simple two-step template approach for preparing carbon-doped mesoporous TiO2 hollow microspheres. J. Phys. Chem. C 2009, 113, 13317–13324. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Ni, D-W.; Yao, Y.; Zhong, Y.-T.; Ma, Y.; Yao, J.-N. High photocatalytic activity of carbon doped TiO2 prepared by fast combustion of organic capping ligands. RSC Adv. 2015, 5, 93635–93643. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.-Y.; Zhang, J.-Y.; Xie, C.; Li, B.-B.; Wang, R.; Li, J.; Niu, C.-M. Carbon nanotube hybrids with MoS2 and WS2 synthesized with control of crystal structure and morphology. Carbon 2015, 85, 168–175. [Google Scholar] [CrossRef]
- Niu, C.-M.; Sichel, E.-K.; Hoch, R.; Moy, D.; Tennent, H. High power electrochemical capacitors based on carbon nanotube electrodes. Appl. Phys. Lett. 1997, 70, 1480–1482. [Google Scholar] [CrossRef]






| Sample | d 1 (nm) | SBET 2 (m2g−1) | V 3 (cm3g−1) | C 4 (%) |
|---|---|---|---|---|
| TiO2-1 | 10.4 | 129.1 | 0.85 | 2.3 |
| TiO2-2 | 11.9 | 102.3 | 0.64 | 2.8 |
| TiO2-3 | 11.9 | 102.9 | 0.66 | 3.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, C.; Yang, S.; Shi, J.; Niu, C. Highly Crystallized C-Doped Mesoporous Anatase TiO2 with Visible Light Photocatalytic Activity. Catalysts 2016, 6, 117. https://doi.org/10.3390/catal6080117
Xie C, Yang S, Shi J, Niu C. Highly Crystallized C-Doped Mesoporous Anatase TiO2 with Visible Light Photocatalytic Activity. Catalysts. 2016; 6(8):117. https://doi.org/10.3390/catal6080117
Chicago/Turabian StyleXie, Chong, Shenghui Yang, Jianwen Shi, and Chunming Niu. 2016. "Highly Crystallized C-Doped Mesoporous Anatase TiO2 with Visible Light Photocatalytic Activity" Catalysts 6, no. 8: 117. https://doi.org/10.3390/catal6080117