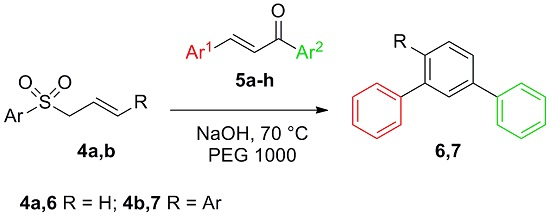
Solventless Synthesis of Quaterphenyls and Terphenyls from Chalcones and Allylsulfones under Phase Transfer Catalysis Conditions
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of Sulfones 4 and Chalcones 5
3.3. General Procedure for the Synthesis of meta-Terphenyls 6a–c under PTC Conditions
3.4. General Procedure for the Solventless Synthesis of Quaterphenyls 8 under PTC Conditions
3.5. Desulfonation of the meta-Terphenyl (6a) and Sulphonyl-meta-terphenyl (8a) Mixture
Author Contributions
Conflicts of Interest
References and Note
- Cho, C.H.; Kim, I.S.; Park, K. Preparation of unsymmetrical terphenyls via the nickel-catalyzed cross-coupling of alkyl biphenylsulfonates with aryl Grignard reagents. Tetrahedron 2004, 60, 4589–4599. [Google Scholar] [CrossRef]
- Miguez, J.M.A.; Adrio, L.A.; Sousa-Pedrares, A.; Vila, J.M.; Hii, K.K. A practical and general synthesis of unsymmetrical terphenyls. J. Org. Chem. 2007, 72, 7771–7774. [Google Scholar] [CrossRef] [PubMed]
- Todd, M.H.; Balasubramanian, S.; Abell, C. Studies on the synthesis, characterisation and reactivity of aromatic diboronic acids. Tetrahedron Lett. 1997, 38, 6781–6784. [Google Scholar] [CrossRef]
- Shimizu, H.; Manabe, K. Negishi coupling strategy of a repetitive two-step method for oligoarene synthesis. Tetrahedron Lett. 2006, 47, 5927–5931. [Google Scholar] [CrossRef]
- Taylor, R.H.; Felpin, F.X. Suzuki–Miyaura reactions of arenediazonium salts catalyzed by Pd(0)/C. One-pot chemoselective double cross-coupling reactions. Org. Lett. 2007, 9, 2911–2914. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Yamamoto, Y. Recent advances in the transition-metal-catalyzed regioselective approaches to polysubstituted benzene derivatives. Chem. Rev. 2000, 100, 2901–2916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liang, F.; Cheng, X.; Liu, Q. A new route to multifunctionalized p-terphenyls and heteroaryl analogues via [5C + 1C(N)] annulation strategy. J. Org. Chem. 2009, 74, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Ballini, R.; Palmieri, A.; Bartoni, L. Nitroalkanes as new, ideal precursors for the synthesis of benzene derivatives. Chem. Commun. 2008. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K. Natural terphenyls: Developments since 1877. Chem. Rev. 2006, 106, 2209–2223. [Google Scholar] [CrossRef] [PubMed]
- Kays, D.L. Recent developments in transition metal diaryl chemistry. Dalton Trans. 2011, 40, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Wang, C.; Han, Y.; Zhu, J.; Kuttner, J.; Hilt, G.; Gottfried, J.M. Surface-assisted formation, assembly, and dynamics of planar organometallic macrocycles and zigzag shaped polymer chains with C–Cu–C bonds. ACS Nano 2014, 8, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.A.; Chou, H.H.; Shih, C.H.; Wu, F.I.; Cheng, C.H.; Huang, H.L.; Chao, T.C.; Tseng, M.R. Synthesis and physical properties of meta-terphenyloxadiazole derivatives and their application as electron transporting materials for blue phosphorescent and fluorescent devices. J. Mater. Chem. 2012, 22, 17792–17799. [Google Scholar] [CrossRef]
- Taylor, P.C.; Wall, M.D.; Woodward, P.R. Synthesis of dendritic oligo(aryl sulfone)s as supports for synthesis. Tetrahedron 2005, 61, 12314–12322. [Google Scholar] [CrossRef]
- Sasabe, H.; Seino, Y.; Kimura, M.; Kido, J. A m-terphenyl-modifed sulfone derivative as a host material for high-efficiency blue and green phosphorescent OLEDs. Chem. Mater. 2012, 24, 1404–1406. [Google Scholar] [CrossRef]
- Gopi, E.; Namboothiri, I.N. One-pot regioselective synthesis of meta-terphenyls via [3 + 3] annulation of nitroallylic acetates with alkylidenemalononitriles. J. Org. Chem. 2014, 79, 7468–7476. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Chan, C.K.; Lin, S.Y.; Wu, M.H. One-pot synthesis of multifunctionalized m-terphenyls. Tetrahedron 2013, 69, 9616–9624. [Google Scholar] [CrossRef]
- Chang, M.Y.; Chan, C.K.; Lin, S.Y.; Wu, M.H. One-pot synthesis of multisubstituted quaterphenyls and cyclopropanes. Tetrahedron 2013, 69, 10036–10044. [Google Scholar] [CrossRef]
- El-Awa, A.; Noshi, M.N.; Mollat du Jourdin, X.; Fuchs, P.L. Evolving organic synthesis fostered by the pluripotent phenylsulfone moiety. Chem. Rev. 2009, 109, 2315–2349. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M. Chemical chameleons. organosulfones as synthetic building blocks. Bull. Chem. Soc. Jpn. 1988, 61, 107–124. [Google Scholar] [CrossRef]
- Simpkins, N.S. Sulphones in Organic Synthesis; Pergamon: Oxford, UK, 1993. [Google Scholar]
- Sulphones and Sulphoxides; Patai, S.; Rappoport, Z.Z.; Stirling, C. (Eds.) Wiley: Chichester, UK, 1988.
- Dehmlow, E.V.; Dehmlow, S.S. Phase Transfer Catalysis, 3rd ed.; Wiley-VCH: Weinheim, Germany, 1993. [Google Scholar]
- Starks, C.M.; Liotta, C.L.; Halpern, M. Phase-Transfer Catalysis; Chapman & Hall: New York, NY, USA, 1994. [Google Scholar]
- Handbook of Phase-Transfer Catalysis; Sasson, Y.; Neumann, R. (Eds.) Blackie Academic & Professional: London, UK, 1997.
- Phase-Transfer Catalysis; Halpern, M.E. (Ed.) ACS Symposium Series 659; American Chemical Society: Washington, DC, USA, 1997.
- Albanese, D. Phase-Transfer Catalysis. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: New York, NY, USA, 2008; pp. 1–22. [Google Scholar] [CrossRef]
- Albanese, D.; Foschi, F.; Penso, M. Sustainable oxidations under phase-transfer catalysis conditions. Org. Proc. Res. Dev. 2016, 20, 129–139. [Google Scholar] [CrossRef]
- Albanese, D.; Landini, D.; Penso, M. Synthesis of 2-amino acids via selective mono-N-alkylation of trichloroacetamide by 2-bromo carboxylic esters under solid-liquid phase-transfer catalysis conditions. J. Org. Chem. 1992, 57, 1603–1605. [Google Scholar] [CrossRef]
- Dramatically reduced cost of PEG related to crown ether discouraged us to test crown ethers as alternative phase transfer catalysts.
- Fukuda, N.; Ikemoto, T. Imide-catalyzed oxidation system: Sulfides to sulfoxides and sulfones. J. Org. Chem. 2010, 75, 4629–4631. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.J.; Craig, D.; East, S.P. Application of 5-endo-trig cyclisation in the total synthesis of (+)-preussin. Arkivoc 2007, xii, 67–90. [Google Scholar]
- Kohler, E.P.; Chadwell, H.M. Benzalacetophenone. Org. Synth. 1922, 2, 1. [Google Scholar]
- Stroba, A.; Schaeffer, F.; Hindie, V.; Lopez-Garcia, L.; Adrian, I.; Fröhner, W.; Hartmann, R.W.; Biondi, R.M.; Engel, M. 3,5-Diphenylpent-2-enoic acids as allosteric activators of the protein kinase PDK1: structure-activity relationship and thermodynamic characterization of binding as paradigms for PIF-binding pocket-targeting compounds PDB code of 2Z with PDK1:3HRF. J. Med. Chem. 2009, 52, 4683–4693. [Google Scholar] [CrossRef] [PubMed]
- Gottumukkala, A.L.; Teichert, J.F.; Heijnen, D.; Eisink, N.; van Dijk, S.; Ferrer, C.; van den Hoogenband, A.; Minnaard, A.J. Pd-Diimine: A highly selective catalyst system for the base-free oxidative Heck reaction. J. Org. Chem. 2011, 76, 3498–3501. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.R.; Athar, F.; Azam, A. Bis-pyrazolines: Synthesis, characterization and antiamoebic activity as inhibitors of growth of Entamoeba hystolytica. Eur. J. Med. Chem. 2009, 44, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, X.F. Ruthenium-catalyzed conjugate hydrogenation of α,β-enones by in situ generated dihydrogen from paraformaldehyde and water. Eur. J. Org. Chem. 2015, 331–335. [Google Scholar] [CrossRef]
- Jin, H.; Geng, Y.; Yu, Z.; Tao, K.; Hou, T. Lead optimization and anti-plant pathogenic fungi activities of daphneolone analogues from Stellera chamaejasme L. Pestic. Biochem. Physiol. 2009, 93, 133–137. [Google Scholar] [CrossRef]
- Schmink, J.R.; Holcomb, J.L.; Leadbeater, N.E. Testing the validity of microwave-interfaced, in situ Raman spectroscopy as a tool for kinetic studies. Org. Lett. 2009, 11, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Bradsher, C.K.; Swerlick, I. Reactions in the m-terphenyl series. J. Am. Chem. Soc. 1950, 72, 4189–4192. [Google Scholar] [CrossRef]
- Beaumard, F.; Dauban, P.; Dodd, R.H. One-pot double Suzuki–Miyaura couplings: Rapid access to nonsymmetrical tri(hetero)aryl derivatives. Org. Lett. 2009, 11, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Morimoto, I.; Mitsudo, K.; Tanaka, H. RhCl3/amine-catalyzed [2 + 2 + 2] cyclization of alkynes. Tetrahedron 2008, 64, 5800–5807. [Google Scholar] [CrossRef] [Green Version]

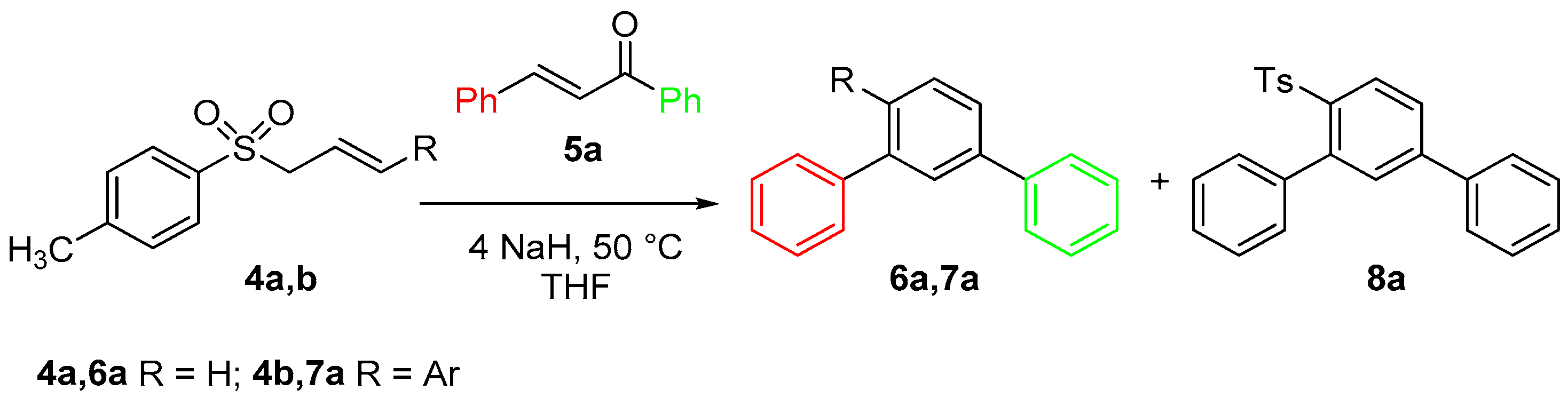
| Entry | Base (equiv) | Catalyst (equiv) | Chalcone | Solvent | t (h) | 6 (%) | 8 (%) |
|---|---|---|---|---|---|---|---|
| 1 | NaOH (1) | TEBA (0.1) | 5a | Toluene | 3 | 13 | 23 |
| 2 | NaOH (4) | TEBA (0.1) | 5a | Toluene | 3 | 20 | 28 |
| 3 | NaOH (4) | TEBA (0.1) | 5a | CH3CN | 3 | 14 | 17 |
| 4 b | NaOH (4) | TEBA (0.1) | 5a | CH3CN | 3 | 5 | 29 |
| 5 c | NaOH (4) | TEBA (0.1) | 5a | CH3CN | 3 | 38 | 36 |
| 6 | NaOH (4) | TEBA (0.1) | 5a | DMSO | 1 | 31 | 8 |
| 7 | NaOH (4) | TEBA (0.1) | 5b | DMSO | 3 | 23 | 6 |
| 8 | NaOH (4) | TEBA (0.1) | 5c | DMSO | 3 | 39 | 2 |
| 9 | (C3H7)4NOH 1M (1) | 5a | Toluene | 3 | 31 | 8 | |
| 10 | 50% NaOH (4.1) | Bu4N+HSO4− (0.1) | 5a | Toluene | 21 | 11 | 26 |
| 11 | NaOH (4) | PEG 1000 (0.4) | 5a | – | 1 | 22 | – |
| Entry | Base (equiv) | Catalyst (equiv) | Chalcone | Solvent | t (h) | 7a (%) | 9a (%) |
|---|---|---|---|---|---|---|---|
| 1 | NaOH (4) | Aliquat (0.1) | 5a | DMSO | 1 | 65 | – |
| 2 b | NaOH (4) | Aliquat (0.1) | 5a | DMSO | 1 | 60 | 5 |
| 3 | NaOH (1.1) | Aliquat (0.1) | 5a | DMSO | 3 | 48 | 9 |
| 4 | NaOH (4) | – | 5a | DMSO | 1 | 42 | 24 |
| 5 | NaOH (4) | PEG 1000 (0.4) | 5a | – | 1 | 66 | – |
| 6 | NaOH (4) | PEG 1000 (1.2) | 5a | – | 1 | 58 | – |
| 7 | NaOH (4) | PEG 1000 (0.1) | 5a | – | 1 | 41 | – |
| Entry | Calchone 5 | Quaterphenyl 7 | Yield (%) |
|---|---|---|---|
| 1 |  |  | 71 |
| 2 |  |  | 69 |
| 3 |  |  | 70 |
| 4 b |  |  | 19 |
| 5 c | 5e | 7e | 15 |
| 6 |  |  | 63 |
| 7 |  |  | 51 |
| 8 |  |  | 63 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albanese, D.C.M.; Brunialti, S.; Destro, D. Solventless Synthesis of Quaterphenyls and Terphenyls from Chalcones and Allylsulfones under Phase Transfer Catalysis Conditions. Catalysts 2016, 6, 142. https://doi.org/10.3390/catal6090142
Albanese DCM, Brunialti S, Destro D. Solventless Synthesis of Quaterphenyls and Terphenyls from Chalcones and Allylsulfones under Phase Transfer Catalysis Conditions. Catalysts. 2016; 6(9):142. https://doi.org/10.3390/catal6090142
Chicago/Turabian StyleAlbanese, Domenico C. M., Selene Brunialti, and Dario Destro. 2016. "Solventless Synthesis of Quaterphenyls and Terphenyls from Chalcones and Allylsulfones under Phase Transfer Catalysis Conditions" Catalysts 6, no. 9: 142. https://doi.org/10.3390/catal6090142