Total Oxidation of Naphthalene with Zirconia-Supported Cobalt, Copper and Nickel Catalysts
Abstract
:1. Introduction
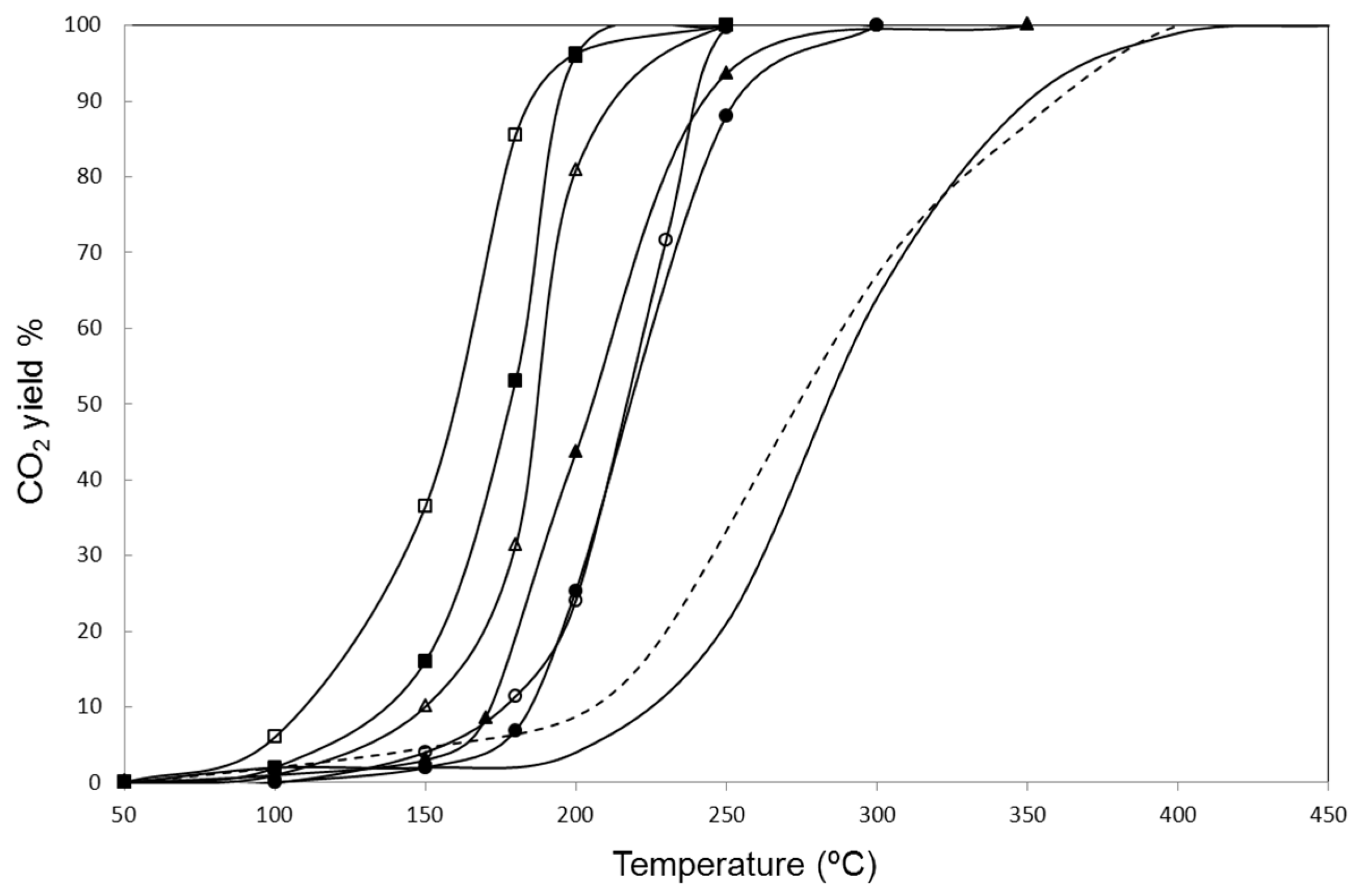
2. Results and Discussion
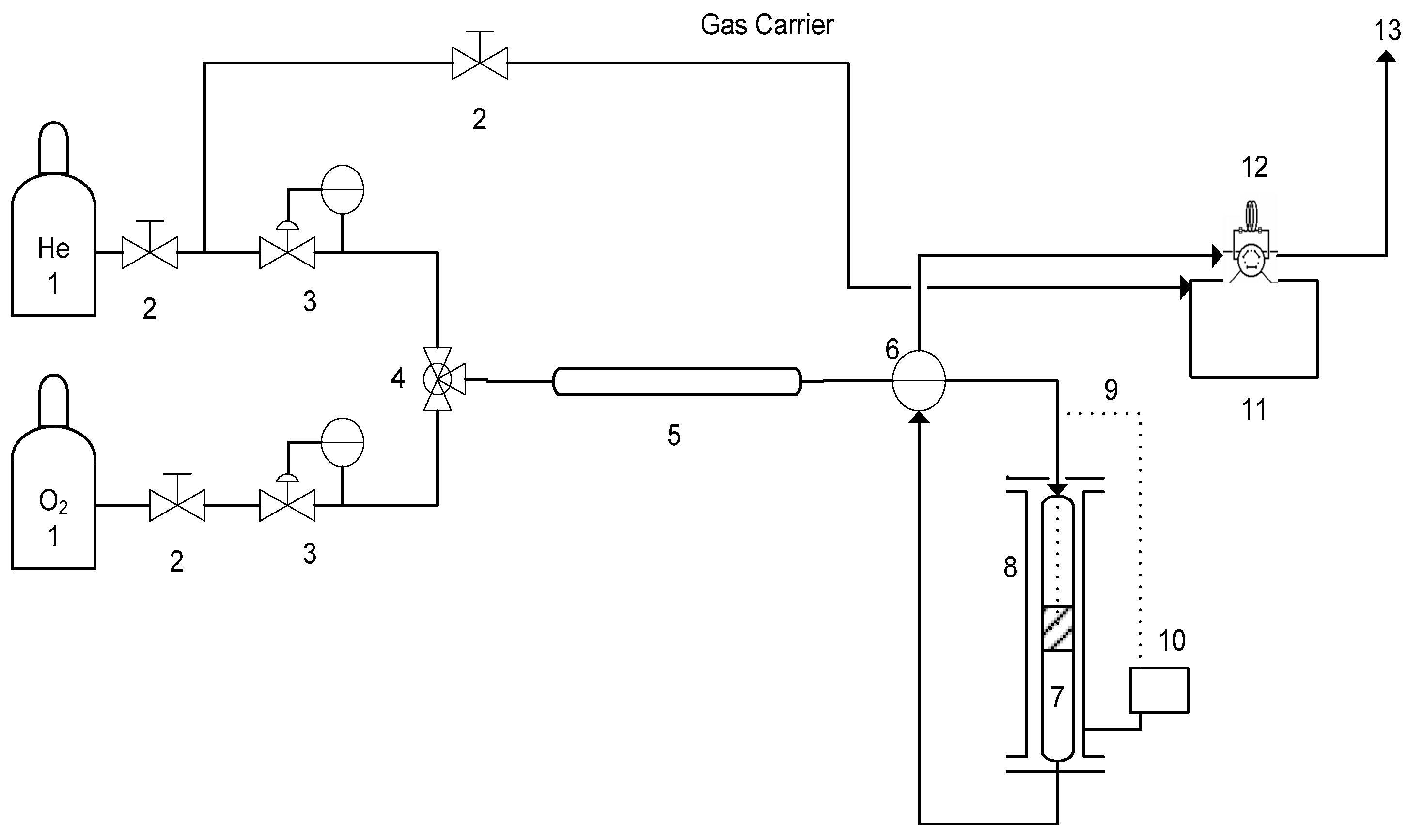
3. Experimental Section
3.1. Material Preparation
3.2. Catalyst Characterization
3.3. Catalytic Activity for Naphthalene Oxidation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Demoulin, O.; Clef, B.L.; Navez, M.; Ruiz, P. Combustion of methane, ethane and propane and of mixtures of methane with ethane or propane on Pd/γ-Al2O3 catalysts. Appl. Catal. A 2008, 344, 1–9. [Google Scholar] [CrossRef]
- Solsona, B.; Garcia, T.; Hutchings, G.J.; Taylor, S.H.; Makkee, M. TAP reactor study of the deep oxidation of propane using cobalt oxide and gold-containing cobalt oxide catalysts. Appl. Catal. A 2009, 365, 222–230. [Google Scholar] [CrossRef]
- Onda, A.; Suzuki, Y.; Takemasa, S.; Kajiyosh, K.; Yanagisawa, K. Catalytic wet oxidations of aromatic compounds over supported copper oxides. J. Mater. Sci. 2008, 43, 4230–4235. [Google Scholar] [CrossRef]
- Okal, J.; Zawadzki, M. Combustion of propane over novel zinc aluminate-supported ruthenium catalysts. Appl. Catal. B Environ. 2011, 105, 182–190. [Google Scholar] [CrossRef]
- Ntainjua, E.N.; Davies, T.E.; García, T.; Solsona, B.; Taylor, S.H. The Influence of Platinum Addition on Nano-Crystalline Ceria. Catalysts for the Total Oxidation of Naphthalene a Model Polycyclic Aromatic Hydrocarbon. Catal. Lett. 2011, 14, 1732–1738. [Google Scholar] [CrossRef]
- Baranowska, K.; Okal, J. Bimetallic Ru-Re/γ-Al2O3 catalysts for the catalytic combustion of propane: Effect of the Re addition. Appl. Catal. A 2015, 499, 158–167. [Google Scholar] [CrossRef]
- García, T.; Agouram, S.; Taylor, S.H.; Morgan, D.; Dejoz, A.; Vázquez, I.; Solsona, B. Total oxidation of propane in vanadia-promoted platinum-alumina catalysts: Influence of the order of impregnation. Catal. Today 2015, 254, 12–20. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Ataloglou, T.; Vakros, J.; Bourikas, K.; Fountzoula, C.; Kordulis, C.; Lycourghiotis, A. Influence of the preparation method on the structure–activity of cobalt oxide catalysts supported on alumina for complete benzene oxidation. Appl. Catal. B Environ. 2005, 57, 299–312. [Google Scholar] [CrossRef]
- García, T.; Solsona, B.; Taylor, S.H. Naphthalene total oxidation over metal oxide catalysts. Appl. Catal. B Environ. 2006, 66, 92–99. [Google Scholar] [CrossRef]
- Aranda, A.; López, J.M.; Murillo, R.; Mastral, A.M.; Dejoz, A.; Vázquez, I.; Solsona, B.; Taylor, S.H.; García, T. Total oxidation of naphthalene with high selectivity using a ceria catalyst prepared by a combustion method employing ethyleneglicol. J. Hazard. Mater. 2009, 171, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Genuino, H.C.; Dharmarathna, S.; Njagi, E.C.; Mei, M.C.; Suib, S.L. Gas-Phase Total Oxidation of Benzene, Toluene, Ethylbenzene, and Xylenes Using Shape-Selective Manganese Oxide and Copper Manganese Oxide Catalysts. J. Phys. Chem. C 2012, 116, 12066–12078. [Google Scholar] [CrossRef]
- Leguizamón Aparicio, M.S.; Lick, I.D. Total oxidation of propane and naphthalene from emission sources with supported cobalt catalysts. React. Kinet. Catal. Lett. 2016, 119, 469–479. [Google Scholar] [CrossRef]
- Leguizamón Aparicio, M.S.; Canafoglia, M.E.; Ocsachoque, M.A.; Lick, I.D.; Botto, I.L. Co-Rh modified natural zeolites as new catalytic materials to oxidize propane and naphthalene from emission sources. Open Chem. 2016, 14, 335–342. [Google Scholar] [CrossRef]
- Ojala, S.; Pitkäaho, S.; Laitinen, T.; Koivikko, N.N.; Brahmi, R.; Gaálová, J.; Matejova, L.; Kucherov, A.; Päivärinta, S.; Hirschmann, C.; et al. Catalysis in VOC Abatement. Top. Catal. 2011, 54, 1224–1256. [Google Scholar] [CrossRef]
- Tomatis, M.; Xu, H.-H.; He, J.; Zhang, X.-D. Recent Development of Catalysts for Removal of Volatile Organic Compounds in Flue Gas by Combustion: A Review. J. Chem. 2016, 2016, 15. [Google Scholar] [CrossRef]
- Clarke, T.J.; Kondrat, S.A.; Taylor, S.H. Total oxidation of naphthalene using copper manganese oxide catalysts. Catal. Today 2015, 258, 610–615. [Google Scholar] [CrossRef]
- Wyrwalski, F.; Lamonier, J.-F.; Siffert, S.; Aboukaïs, A. Additional effects of cobalt precursor and zirconia support modifications for the design of efficient VOC oxidation catalysts. Appl. Catal. B 2007, 70, 393–399. [Google Scholar] [CrossRef]
- Pecchi, G.; Reyes, P.; Gómez, R.; López, T.; Fierro, J.L.G. Methane combustion on Rh/ZrO2 catalysts. Appl. Catal. B Environ. 1998, 17, L7–L13. [Google Scholar] [CrossRef]
- Labaki, M.; Siffert, S.; Lamonier, J.-F.; Zhilinskaya, E.A.; Aboukaïs, A. Total oxidation of propene and toluene in the presence ofzirconia doped by copper and yttrium Role of anionic vacancies. Appl. Catal. B Environ. 2003, 43, 261–271. [Google Scholar] [CrossRef]
- Gómez, L.E.; Tiscornia, I.S.; Boix, A.V.; Miró, E.E. Co/ZrO2 catalysts coated on cordierite monoliths for CO preferential oxidation. Appl. Catal. A 2011, 401, 124–133. [Google Scholar] [CrossRef]
- Gutiérrez-Ortiz, J.I.; Rivas, B.D.; López-Fonseca, R.; González-Velasco, J.R. Catalytic purification of waste gases containing VOC mixtures with Ce/Zr solid solutions. Appl. Catal. B Environ. 2006, 65, 191–200. [Google Scholar] [CrossRef]
- De Rivas, B.; Sampedro, C.; García-Real, M.; López-Fonseca, R.; Gutiérrez-Ortiz, J.I. Promoted activity of sulphated Ce/Zr mixed oxides for chlorinated VOC oxidative abatement. Appl. Catal. B Environ. 2013, 129, 225–235. [Google Scholar] [CrossRef]
- Yamaguchi, T. Application of ZrO2 as a catalyst and a catalyst support. Catal. Today 1994, 20, 199–218. [Google Scholar] [CrossRef]
- Ray, J.C.; Saha, C.R.; Pramanik, P. Stabilized nanoparticles of metastable ZrO2with Cr3+/Cr4+ cations: Preparation from a polymer precursor and the study of the thermal and structural properties. J. Eur. Ceram. Soc. 2002, 22, 851–862. [Google Scholar] [CrossRef]
- Shie, J.-L.; Chang, C.-Y.; Chen, J.-H.; Tsai, W.-T.; Chen, Y.-H.; Chiou, C.-S.; Chang, C.-F. Catalytic oxidation of naphthalene using a Pt/Al2O3 catalyst. Appl. Catal. B Environ. 2005, 58, 289–297. [Google Scholar] [CrossRef]
- Ntainjua, N.E.; Carley, A.F.; Taylor, S.H. The role of support on the performance of platinum-based catalysts for the total oxidation of polycyclic aromatic hydro-carbons. Catal. Today 2008, 137, 362–366. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Shen, S.-C.; Yu, L.E.; Kawi, S.; Hidajat, K.; Simon Ng, K.Y. Oxidative decomposition of naphthalene by supported metal catalysts. Appl. Catal. A Gen. 2003, 250, 341–352. [Google Scholar] [CrossRef]
- Garcia, T.; Solsona, B.; Taylor, S.H. Nano-crystalline Ceria Catalysts for the Abatement of Polycyclic Aromatic Hydrocarbons. Catal. Lett. 2005, 105, 183–189. [Google Scholar] [CrossRef]
- Bampenrat, A.; Meeyoo, V.; Kitiyanan, B.; Rangsunvigit, P.; Rirksomboon, T. Catalytic oxidation of naphthalene over CeO2–ZrO2 mixed oxide catalysts. Catal. Commun. 2008, 9, 2349–2352. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, Y. Spontaneous monolayer dispersion of oxides and salts onto surfaces of supports: Applications to heterogeneous catalysis. Adv. Catal. 1990, 37, 1–43. [Google Scholar]
- Liu, Z.; Amiridis, M.D.; Yi, C. Characterization of CuO Supported on Tetragonal ZrO2 Catalysts for N2O Decomposition to N2. J. Phys. Chem. B 2005, 109, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Alavi, S.M.; Sahebdelfar, S.; Xinmei, L.; Qian, L.; Yan, Z. CO2-CH4 Reforming over Nickel Catalysts Supported on Mesoporous Nanocrystalline Zirconia with High Surface Area. Energy Fuels 2007, 21, 581–589. [Google Scholar] [CrossRef]
- Valigi, M.; Gazzoli, D.; Dragone, R.; Gherardi, M.; Minelli, G. Nichel oxide-zirconium oxide: Ni2+ incorporation and its influence on the phase transition and sintering of zirconia. J. Mater. Chem. 1995, 5, 183–189. [Google Scholar] [CrossRef]
- Koo, K.Y.; Roh, H.S.; Jung, U.H.; Wang, L.Y. CeO2 Promoted Ni/Al2O3Catalyst in Combined Steam and Carbon Dioxide Reforming of Methane for Gas to Liquid (GTL) Process. Catal. Lett. 2009, 130, 217–221. [Google Scholar] [CrossRef]
- De Sousa, H.S.A.; da Silva, A.N.; Castro, A.J.R.; Campos, A.; Filho, J.M.; Oliveira, A.C. Mesoporous catalysts for dry reforming of methane: Correlation between structure and deactivation behaviour of Ni-containing catalysts. Int. J. Hydrogen Energy 2012, 37, 12281–12291. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.C.; Chen, M.; Cao, Y.; He, H.Y.; Fan, K.N. Dry citrate-precursor synthesized nanocrystalline cobalt oxide as highly active catalyst for total oxidation of propane. J. Catal. 2009, 263, 104–113. [Google Scholar] [CrossRef]
- Trigueiro, F.E.; Ferreira, C.M.; Volta, J.C.; González, W.A.; Pries de Oliveria, P.G. Effect of niobium addition to Co/γAl2O3 catalyst on methane combustion. Catal. Today 2006, 118, 425–432. [Google Scholar] [CrossRef]
- Solsona, B.; Davies, T.E.; García, T.; Vázquez, I.; Dejoza, A.; Taylor, S.H. Total oxidation of propane using nanocrystalline cobalt oxide and supported cobalt oxide catalysts. Appl. Catal. B Environ. 2008, 84, 176–184. [Google Scholar] [CrossRef]
- Sato, A.G.; Volanti, D.P.; Meira, D.M.; Damyanova, S.; Longo, E.; Bueno, J.M.C. Effect of the ZrO2 phase on the structure and behavior of supported Cu catalysts for ethanol conversion. J. Catal. 2013, 307, 1–17. [Google Scholar] [CrossRef]
- Kyun Park, B.; Jeong, S.; Kim, D.; Moon, J.; Lim, S.; Kim, J.S. Synthesis and size control of monodisperse copper nanoparticles by polyolmethod. J. Colloid Interface Sci. 2007, 311, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Keramidas, V.G.; White, W.B. Raman Scattering Study of the Crystallization and Phase Transformations of ZrO2. J. Am. Ceram. Soc. 1974, 57, 22–24. [Google Scholar] [CrossRef]
- Yashima, M.; Ohtake, K.; Kakihana, M.; Arashi, H.; Yoshimura, M. Determination of tetragonal-cubic phase boundary of Zr1−XRXO2−X2 (R = Nd, Sm, Y, Er and Yb) by Raman scattering. J. Phys. Chem. Solids 1996, 57, 17–24. [Google Scholar] [CrossRef]
- Hagjiev, V.G.; Iliev, M.N.; Vergilov, I.V. The Raman spectra of Co3O4. J. Phys. C 1988, 21, L199–L201. [Google Scholar] [CrossRef]
- Choi, H.C.; Jung, Y.M.; Noda, I.; Kim, S.B. A Study of the Mechanism of the Electrochemical Reaction of Lithium with CoO by Two-Dimensional Soft X-ray Absorption Spectroscopy (2D XAS), 2D Raman, and 2D Heterospectral XAS−Raman Correlation Analysis. J. Phys. Chem. B 2003, 107, 5806–5811. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Steins, I.; Grabis, J.; Sildos, I.; Pärs, M. Raman scattering in nanosized nickel oxide NiO. J. Phys. 2007, 93, 012039–012043. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Xu, C.; Zheng, C.; Wang, G. Synthesis of NiO nanorods by a novel simple precursor thermal decomposition approach. Chem. Phys. Lett. 2002, 362, 119–122. [Google Scholar] [CrossRef]
- Xu, J.F.; Ji, W.; Shen, Z.X.; Li, W.S.; Tang, S.H.; Ye, X.R.; Jia, D.Z.; Xin, X.Q.J. Raman spectra of CuO nanocrystals. Raman Spectrosc. 1999, 30, 413–415. [Google Scholar] [CrossRef]
- Chuah, G.K.; Jaenicke, S. The preparation of high surface area zirconia - Influence of precipitating agent and digestion. Appl. Catal. A 1997, 163, 261–273. [Google Scholar] [CrossRef]
- Song, X.; Sayari, A. Sulfated Zirconia-Based Strong Solid-Acid Catalysts: Recent Progress. Catal. Rev. 1996, 38, 329–412. [Google Scholar] [CrossRef]
- Normair, C.J.; Goulding, P.A.; McAlpine, I. Role of anions in the surface area stabilisation of zirconia. Catal. Today 1994, 20, 313–321. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Dow, W.P.; Huang, T.J. Effects of Oxygen Vacancy of Yttria-Stabilized Zirconia Support on Carbon Monoxide Oxidation over Copper Catalyst. J. Catal. 1994, 147, 322–332. [Google Scholar] [CrossRef]
- Kulyova, S.P.; Lunina, E.V.; Lunin, V.V.; Kostyuk, B.G.; Muravyova, G.P.; Kharlanov, A.N.; Jilinskaya, E.; Aboukaïs, A. Redox Behavior of Y0.05Ce0.1Zr0.85O2 and Y0.1Ce0.1Zr0.8O2 System Catalysts Doped with Copper (II). Chem. Mater. 2001, 13, 1491–1496. [Google Scholar] [CrossRef]
- Nanda, B.; Pradhan, A.C.; Parida, K.M. Fabrication of mesoporous CuO/ZrO2-MCM-41 nanocomposites for photocatalytic reduction of Cr(VI). Chem. Eng. J. 2017, 316, 1122–1135. [Google Scholar] [CrossRef]
- Freitas, I.C.; Damyanova, S.; Oliveira, D.C.; Marques, C.M.P.; Bueno, J.M.C. Effect of Cu content on the surface and catalytic properties of Cu/ZrO2 catalyst for ethanol dehydrogenation. J. Mol. Catal. A Chem. 2014, 381, 26–37. [Google Scholar] [CrossRef]
- Aguila, G.; Valenzuela, A.; Guerrero, S.; Araya, P. WGS activity of a novel Cu–ZrO2 catalyst prepared by a reflux method. Comparison with a conventional impregnation method. Catal. Commun. 2013, 39, 82–85. [Google Scholar] [CrossRef]
- Baeza, P.; Bassi, R.; Villarroel, M.; Ojeda, J.; Araya, P.; Aguila, G. Adsorption of 4,6-dimethyldibenzothiophene over Cu/ZrO2. J. Chil. Chem. Soc. 2015, 60, 2817–2821. [Google Scholar] [CrossRef]
- Juan-Juan, J.; Román-Martínez, M.C.; Illán-Gómez, M.J. Nickel catalyst activation in the carbon dioxide reforming of methane: Effect of pretreatments. Appl. Catal. A Gen. 2009, 355, 27–32. [Google Scholar] [CrossRef]
- Tidahy, H.L.; Siffert, S.; Wyrwalski, F.; Lamonier, J.; Aboukais, A. Catalytic activity of copper and palladium based catalysts for toluene total oxidation. Catal. Today 2007, 119, 317–320. [Google Scholar] [CrossRef]
- Fierro, G.; Lojacono, M.; Inversi, M.; Porta, P.; Lavecchia, R.; Cioci, F. A Study of Anomalous Temperature-Programmed Reduction Profiles of Cu2O, CuO, and CuO-ZnO Catalysts. J. Catal. 1994, 148, 709–721. [Google Scholar] [CrossRef]
- Aissat, A.; Courcot, D.; Cousin, R.; Siffert, S. VOCs removal in the presence of NOx on Cs–Cu/ZrO2 catalysts. Catal. Today 2011, 117, 120–125. [Google Scholar] [CrossRef]
- Centi, G.; Cerrato, G.; D’Angelo, S.; Finardi, U.; Giamello, E.; Morterra, C.; Perathoner, S. Catalytic behavior and nature of active sites in copper-on-zirconia catalysts for the decomposition of N2O. Catal. Today 1996, 27, 265–270. [Google Scholar] [CrossRef]
- Carno, J.; Berg, M.; Jaras, S. Catalytic abatement of emissions from small-scale combustion of wood A comparison of the catalytic effect in model and real flue gases. Fuel 1996, 75, 959–965. [Google Scholar] [CrossRef]
- Aranda, A.; Agouram, S.; López, J.M.; Mastral, A.M.; Sellick, D.R.; Solsona, B.; Taylor, S.H.; García, T. Oxygen defects: The key parameter controlling the activity and selectivity of mesoporous copper-doped ceria for the total oxidation of naphthalene. Appl. Catal. B Environ. 2012, 127, 77–88. [Google Scholar] [CrossRef]
- Solsona, B.; Sanchis, R.; Dejoz, A.M.; García, T.; Ruiz-Rodríguez, L.; López Nieto, J.M.; Cecilia, J.A.; Rodríguez-Castellón, E. Total Oxidation of Propane Using CeO2 and CuO-CeO2 Catalysts Prepared Using Templates of Different Nature. Catalysts 2017, 7, 96. [Google Scholar] [CrossRef]
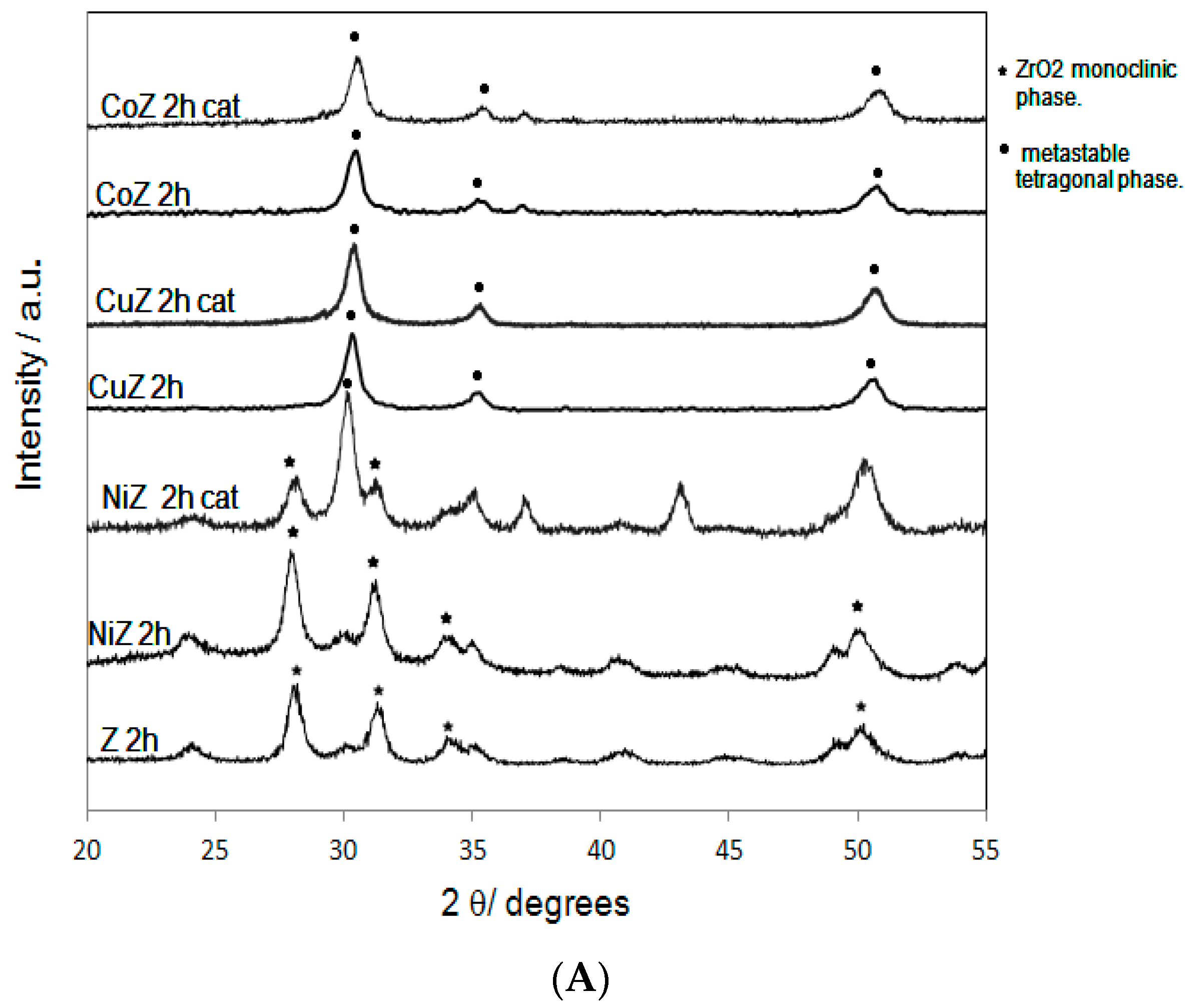
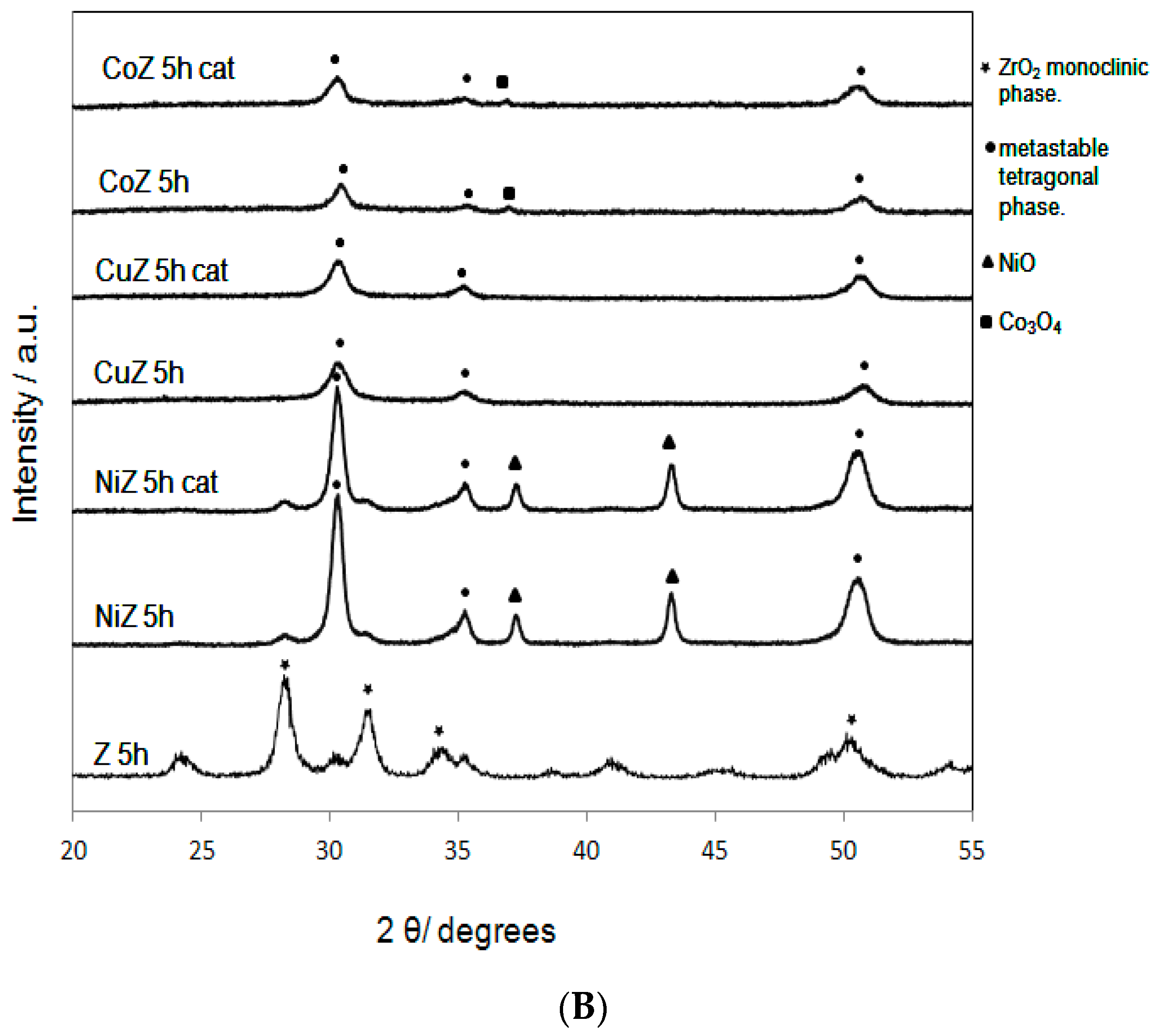


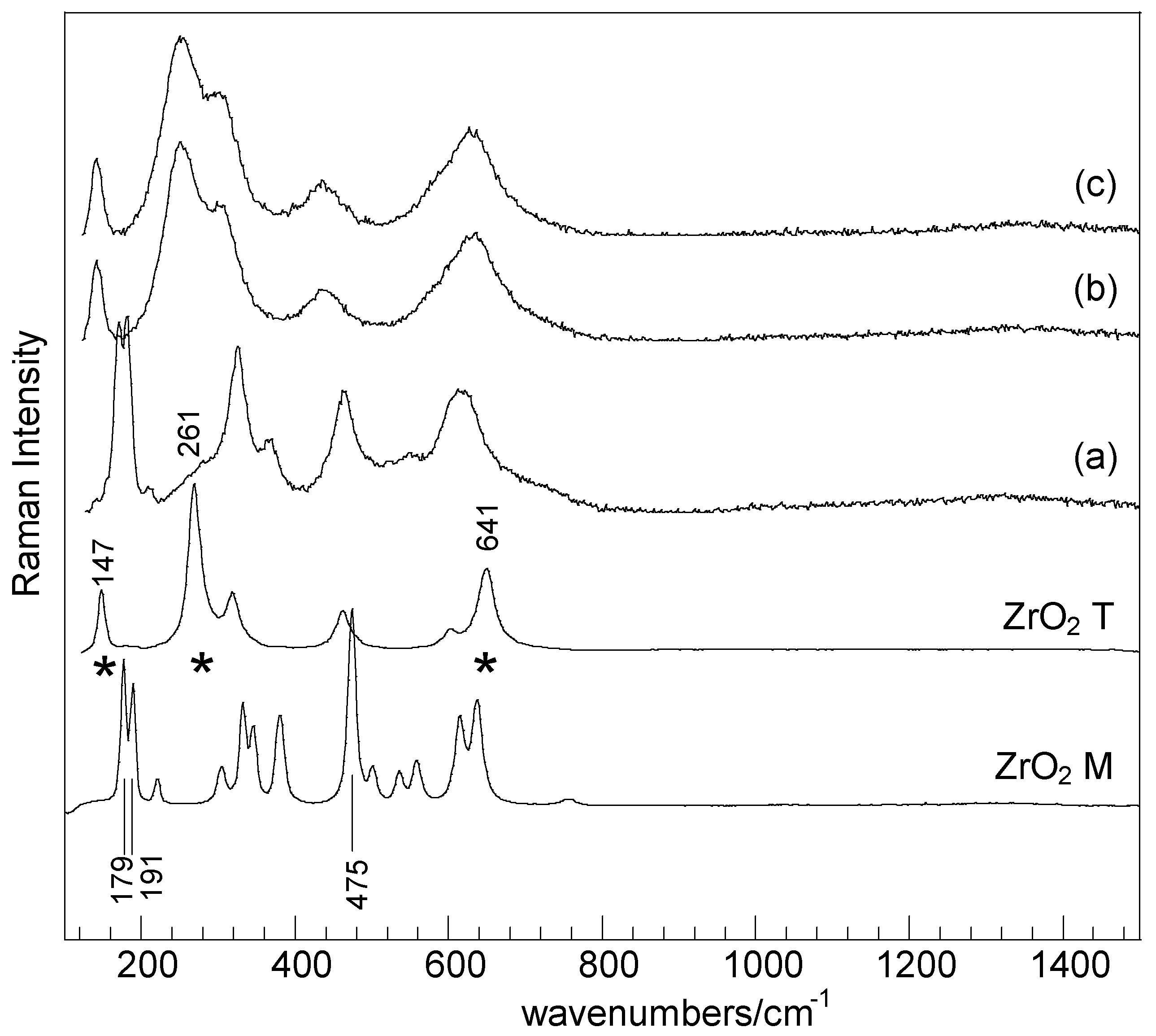
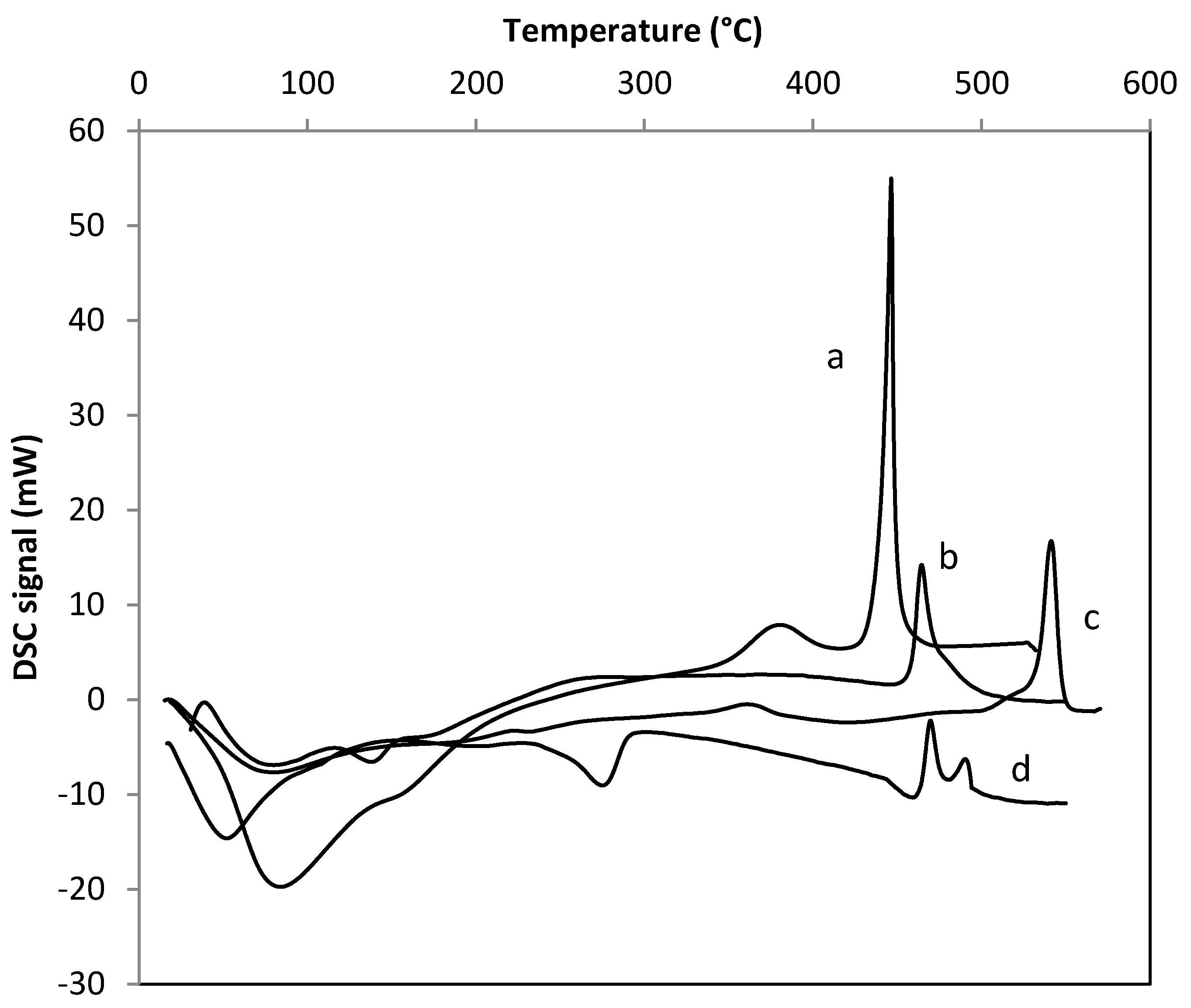
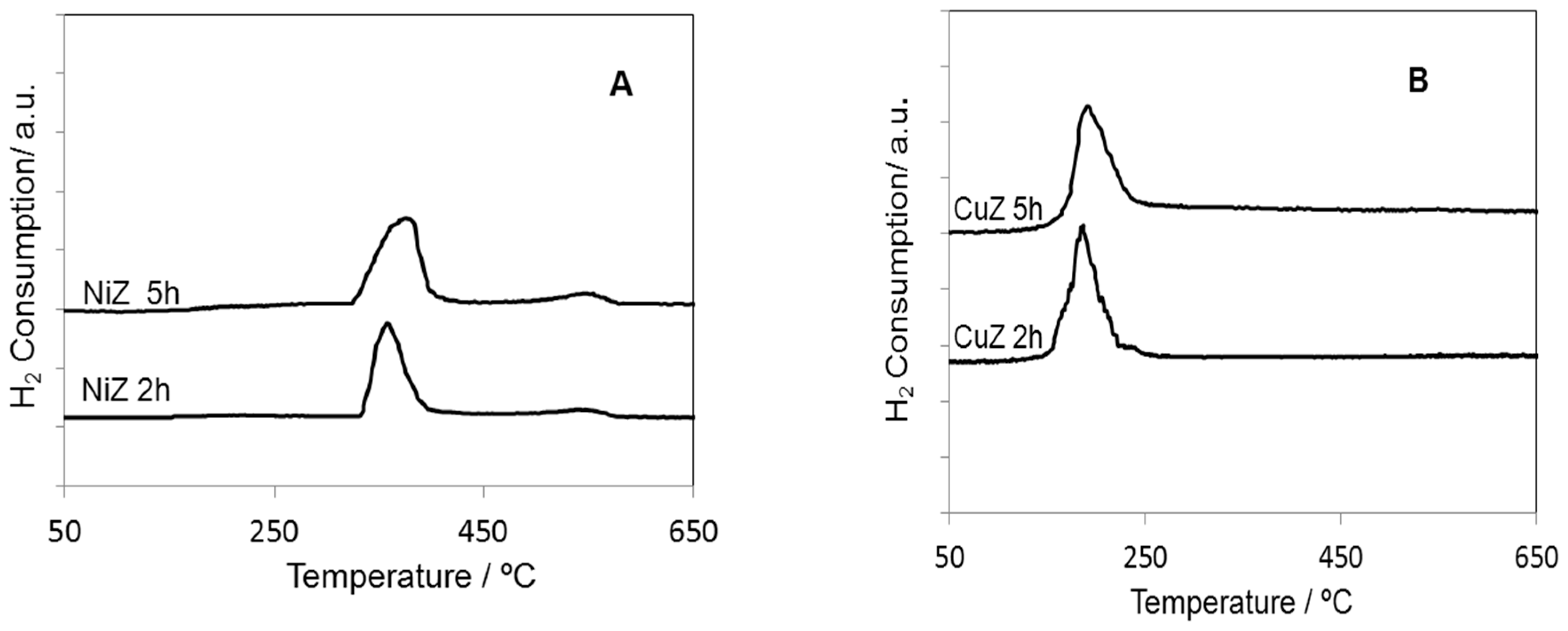
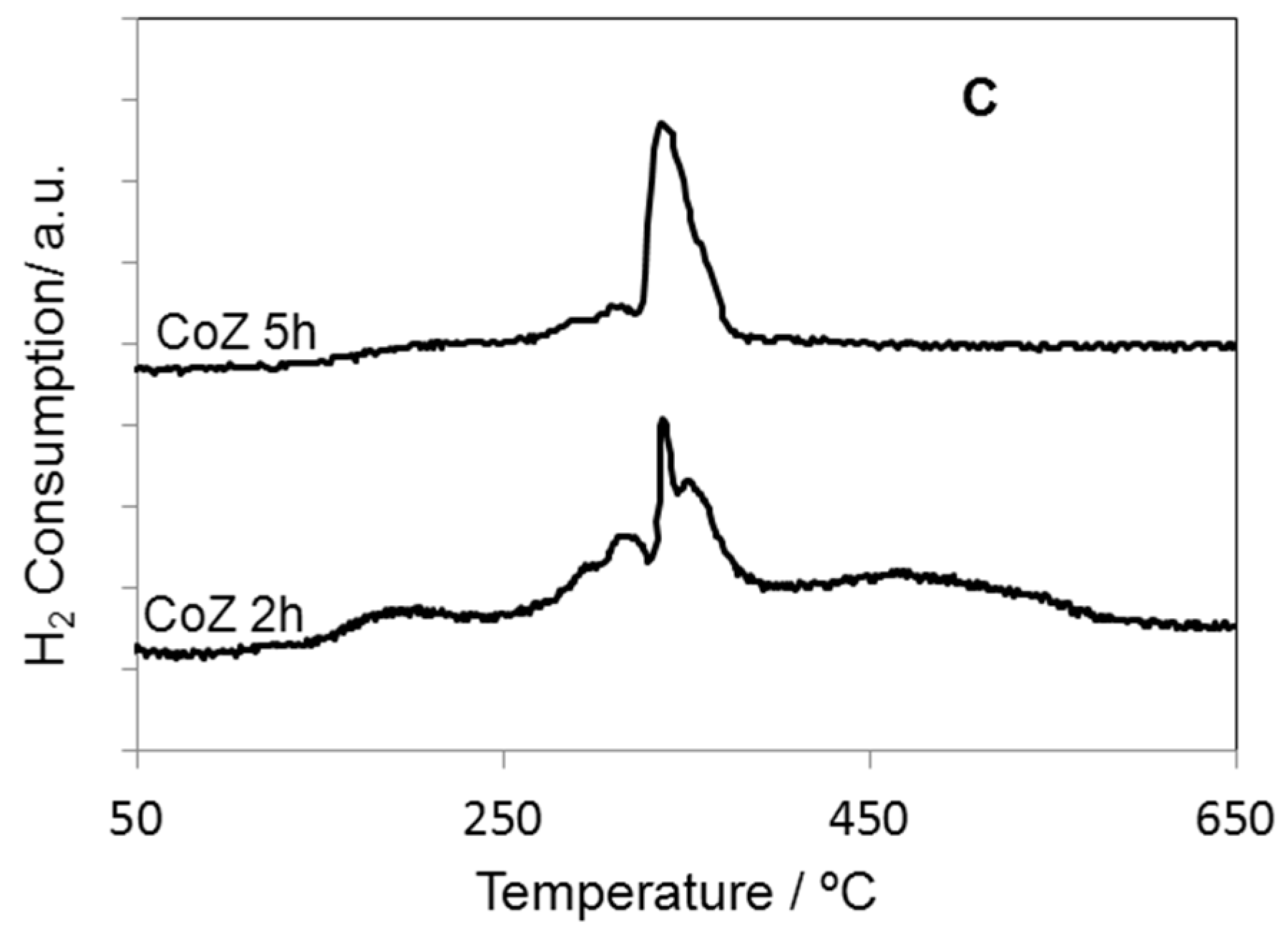
| Samples | SBET (m2·g−1) | Nominal TM/Zr * | EDS TM/Zr | Phases ** | H2 Consumed (mmoles) |
|---|---|---|---|---|---|
| ZrO2·nH2O | 340 | - | - | A | - |
| ZrO2 2 h | 50 | - | - | M | - |
| ZrO2 5 h | 48 | - | - | M | - |
| CoZ 2 h | 37 | 0.110 | 0.362 | T, Co3O4 | 0.029 |
| CuZ 2 h | 52 | 0.102 | 0.331 | T | 0.021 |
| NiZ 2 h | 29 | 0.111 | 0.266 | M | 0.019 |
| CoZ 5 h | 38 | 0.110 | 0.549 | T, Co3O4 | 0.031 |
| CuZ 5 h | 56 | 0.102 | 0.398 | T | 0.023 |
| NiZ 5 h | 26 | 0.111 | 0.334 | T, M (traces), NiO | 0.020 |
| Ion | Coordination | Ionic Radius |
|---|---|---|
| Co2+ | 6 (LS) | 0.65 |
| Co3+ | 6 (LS) | 0.54 |
| Cu2+ | Square plane | 0.57 |
| 6 | 0.73 | |
| Ni2+ | 6 | 0.69 |
| Z4+ | 6 | 0.72 |
| Catalyst | T50 (°C) | T90 (°C) | GHSV (h−1) | Reference |
|---|---|---|---|---|
| Z 5 h | 285 | 350 | 36,000 | This work |
| Z 2 h | 273 | 360 | 36,000 | This work |
| CuZ 2 h | 180 | 193 | 36,000 | This work |
| CuZ 5 h | 160 | 184 | 36,000 | This work |
| CoZ 2 h | 205 | 243 | 36,000 | This work |
| CoZ 5 h | 188 | 213 | 36,000 | This work |
| NiZ 2 h | 218 | 255 | 36,000 | This work |
| NiZ 5 h | 218 | 240 | 36,000 | This work |
| Co3O4 | 245 | 270 | 60,000 | [11] |
| 0.12%Pt-Al2O3 | 204 | 310 | 20,000 | [65] |
| Cu-Mn/Al2O3 | 207 | 20,000 | [65] | |
| Ce0.75Zr0.25O2 | 290 | 320 | - | [31] |
| Mn2O3 | 230 | 250 | 60,000 | [13] |
| CeO2 | 160 | 190 | 25,000 | [30] |
| CeO2-CuO | 75,000 | [66] | ||
| CuMn2O4 | 229 | 238 | [18] | |
| CoOx(15)-SiO2 | 228 | 260 | 18,000 | [14] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leguizamón Aparicio, M.S.; Ocsachoque, M.A.; Gazzoli, D.; Botto, I.L.; Lick, I.D. Total Oxidation of Naphthalene with Zirconia-Supported Cobalt, Copper and Nickel Catalysts. Catalysts 2017, 7, 293. https://doi.org/10.3390/catal7100293
Leguizamón Aparicio MS, Ocsachoque MA, Gazzoli D, Botto IL, Lick ID. Total Oxidation of Naphthalene with Zirconia-Supported Cobalt, Copper and Nickel Catalysts. Catalysts. 2017; 7(10):293. https://doi.org/10.3390/catal7100293
Chicago/Turabian StyleLeguizamón Aparicio, María S., Marco A. Ocsachoque, Delia Gazzoli, Irma L. Botto, and Ileana D. Lick. 2017. "Total Oxidation of Naphthalene with Zirconia-Supported Cobalt, Copper and Nickel Catalysts" Catalysts 7, no. 10: 293. https://doi.org/10.3390/catal7100293





