Catalytic Pyrolysis of Chilean Oak: Influence of Brønsted Acid Sites of Chilean Natural Zeolite
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biomass and Natural Zeolite Characterization
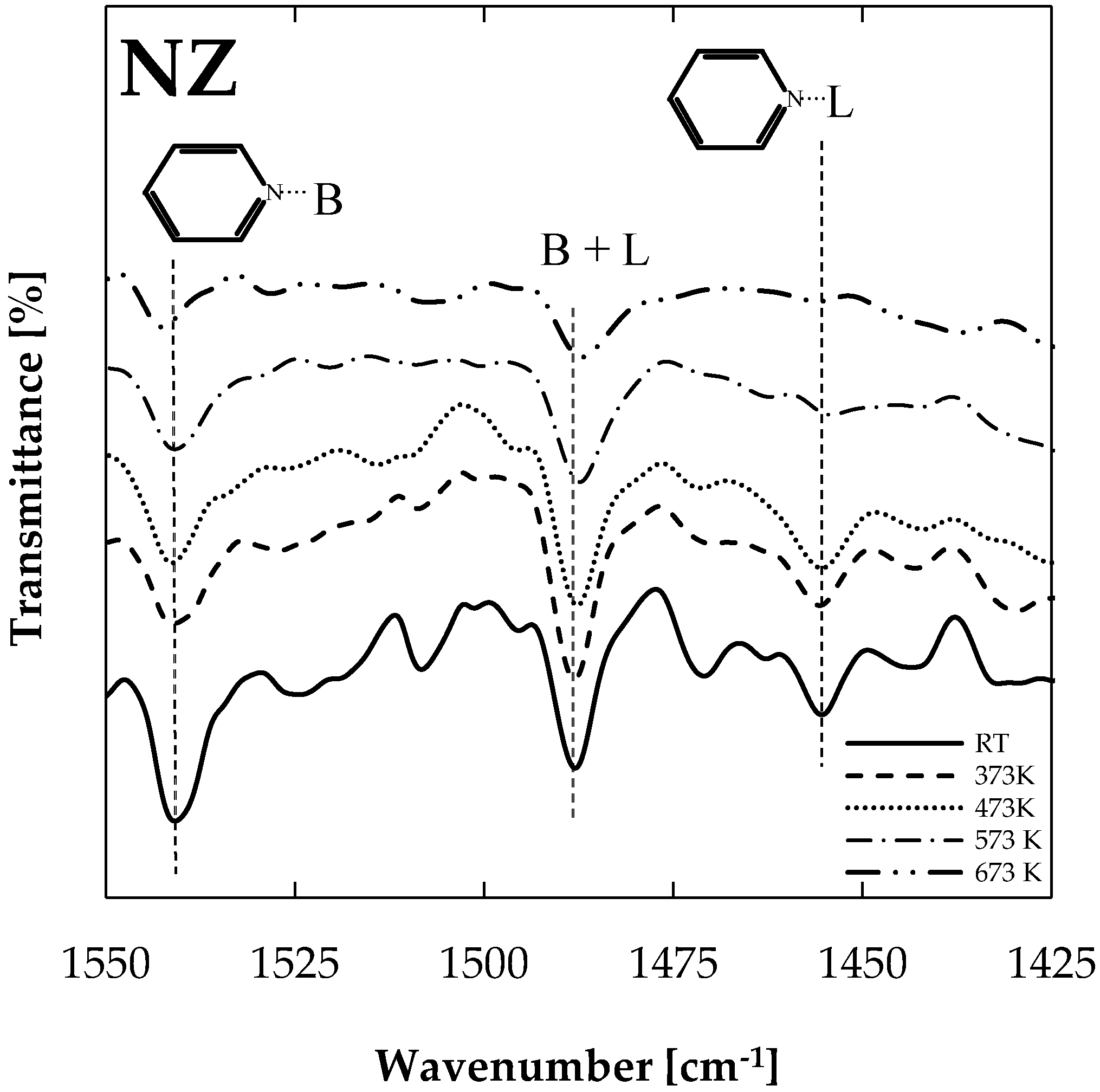
2.2. Pyridine Adsorption Followed by DRIFT
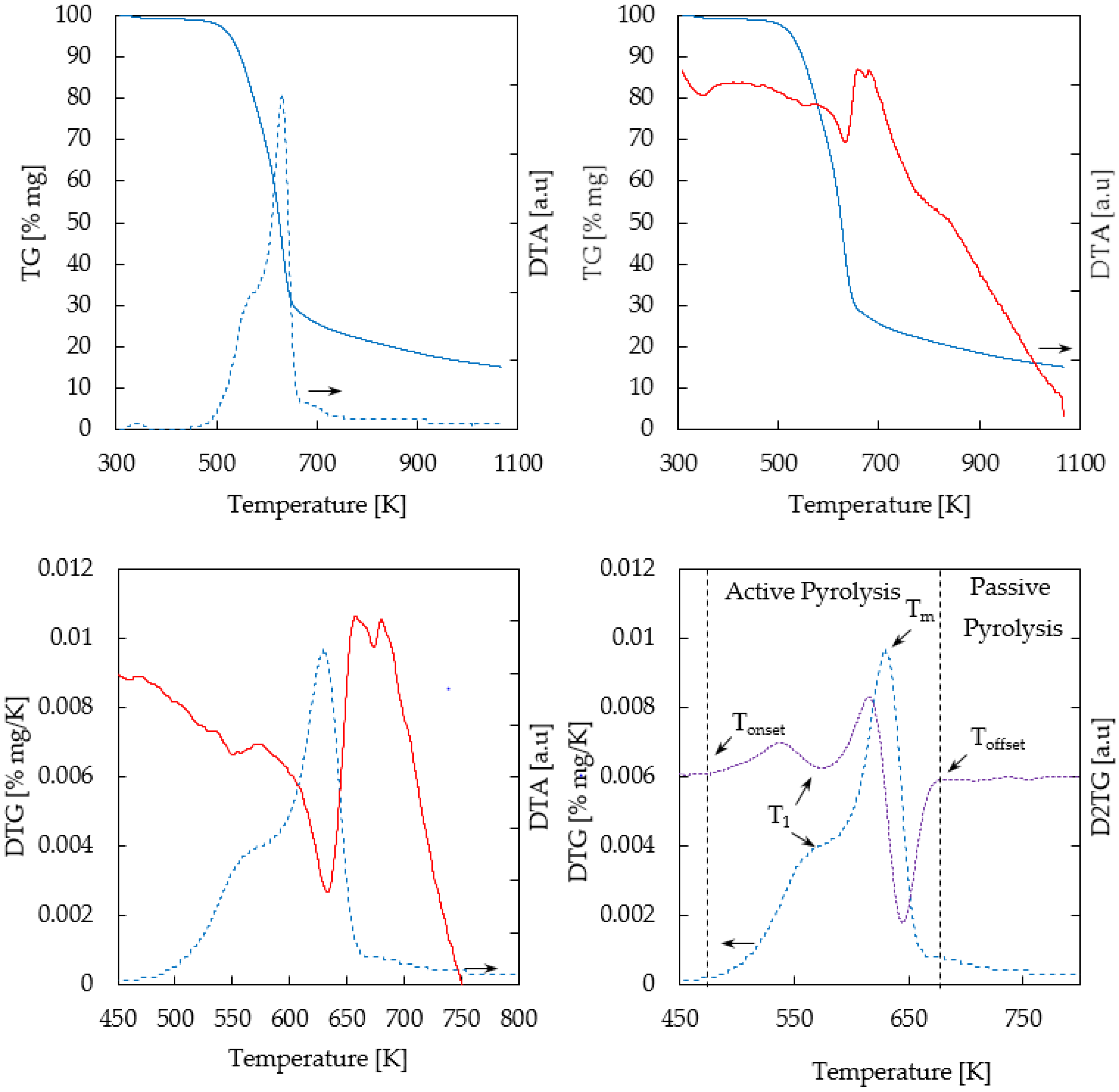
2.3. Thermal Behavior of Biomass
2.4. Product Distribution during Chilean Oak Pyrolysis
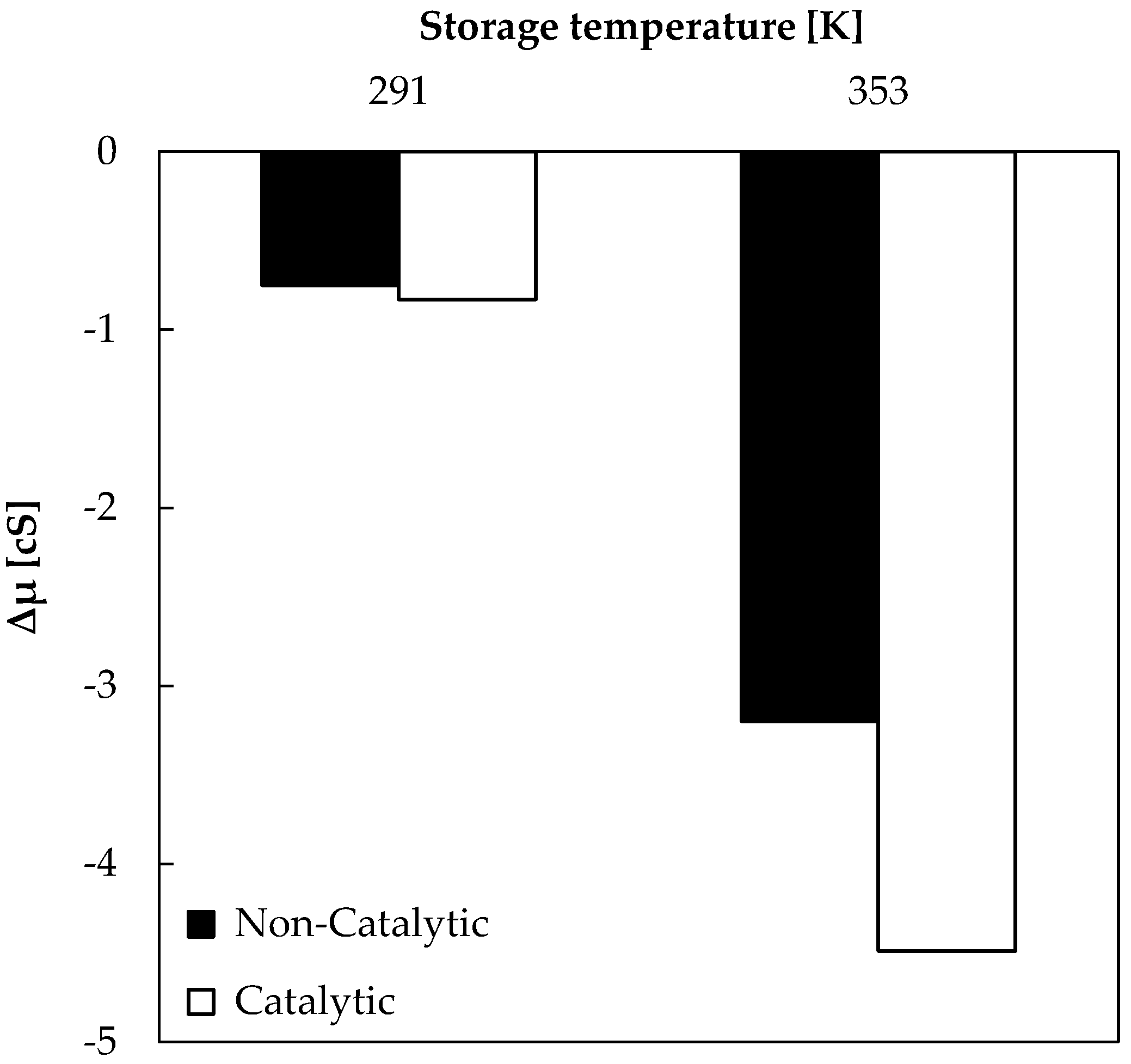
2.5. Viscosity Variation during Bio-Oil Storage at Different Temperatures
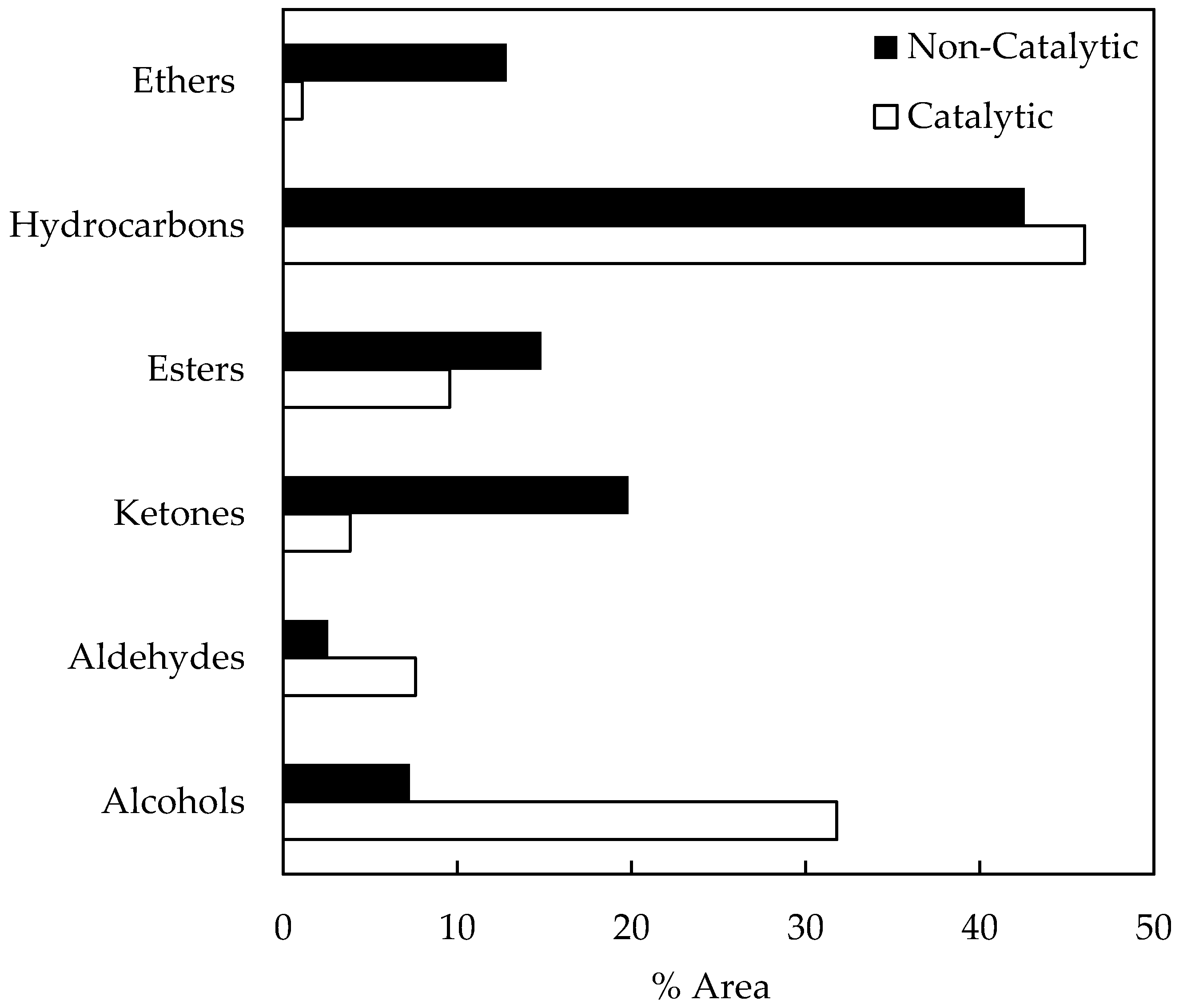
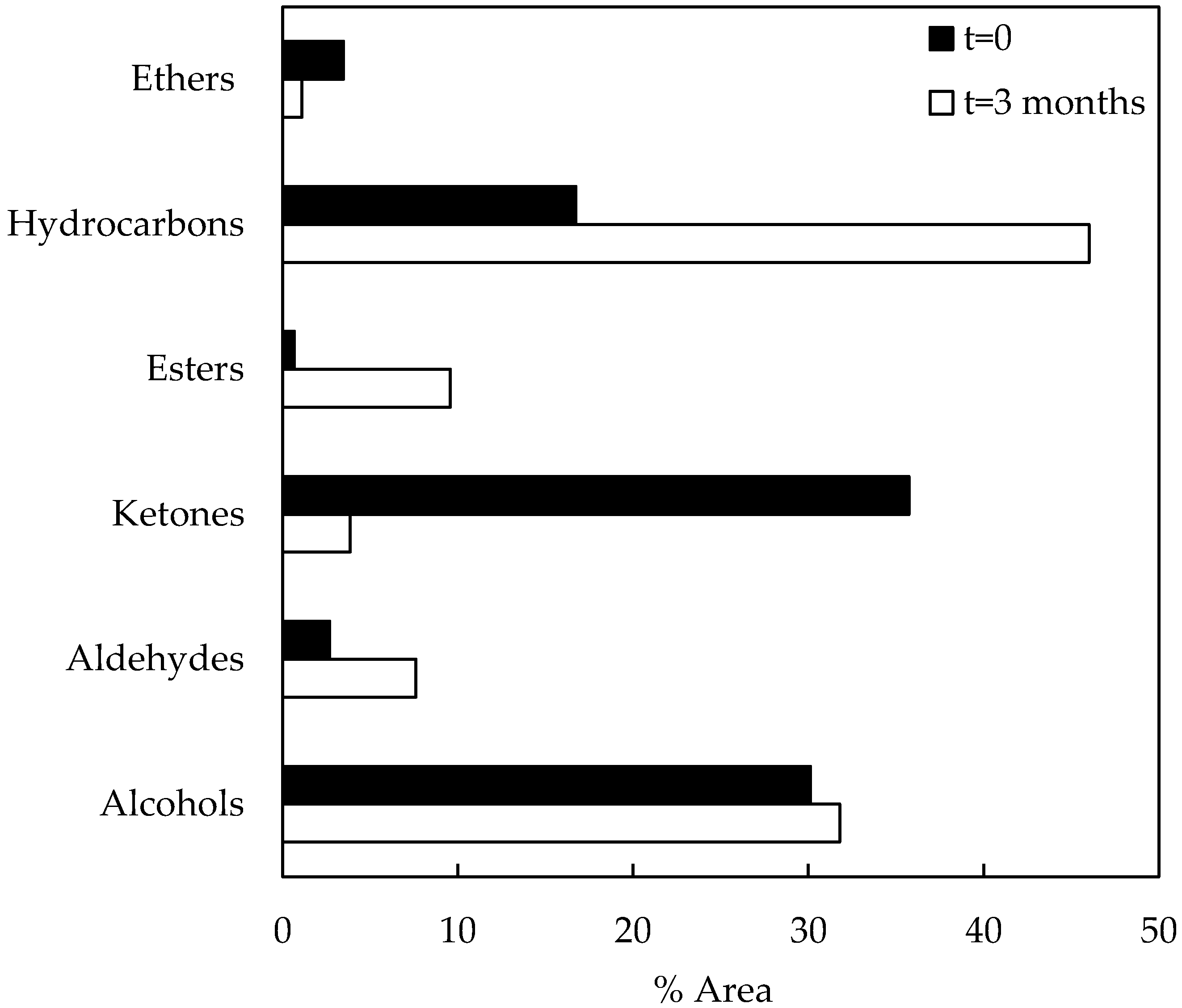
2.6. Chemical Composition of Stored Bio-Oil Samples
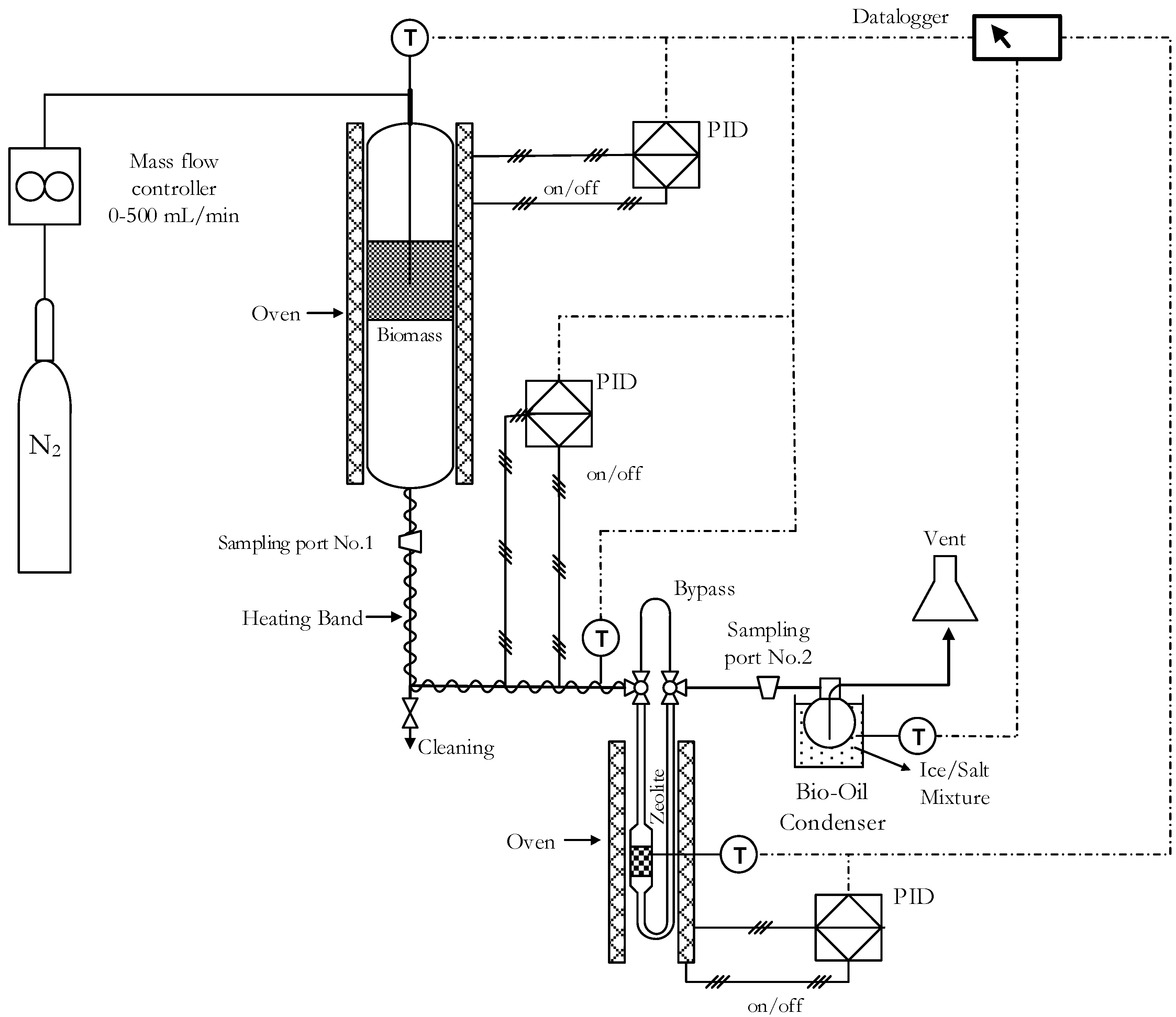
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, Y.; Pan, T.; Zuo, Y.; Guo, Q.-X.; Fu, Y. Production of aromatic hydrocarbons through catalytic pyrolysis of 5-hydroxymethylfurfural from biomass. Bioresour. Technol. 2013, 147, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y. Comparative sugar recovery data from laboratory scale application of leading pretreatment technologies to corn stover. Bioresour. Technol. 2005, 96, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A.; Arin, G. An overview of biomass pyrolysis. Energy Source 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Zanuttini, M.S.; Lago, C.D.; Querini, C.A.; Peralta, M.A. Deoxygenation of m-cresol on pt/γ-Al2O3 catalysts. Catal. Today 2013, 213, 9–17. [Google Scholar] [CrossRef]
- Messina, L.I.G.; Bonelli, P.R.; Cukierman, A.L. In-situ catalytic pyrolysis of peanut shells using modified natural zeolite. Fuel Process. Technol. 2017, 159, 160–167. [Google Scholar] [CrossRef]
- Mohammed, I.Y.; Kazi, F.K.; Yusup, S.; Alaba, P.A.; Sani, Y.M.; Abakr, Y.A. Catalytic intermediate pyrolysis of napier grass in a fixed bed reactor with ZSM-5, HZSM-5 and zinc-exchanged zeolite-A as the catalyst. Energies 2016, 9, 246. [Google Scholar] [CrossRef]
- Imran, A.; Bramer, E.A.; Seshan, K.; Brem, G. Catalytic flash pyrolysis of biomass using different types of zeolite and online vapor fractionation. Energies 2016, 9, 187. [Google Scholar] [CrossRef]
- Ateş, F.; Putun, A.E.; Putun, E. Fixed bed pyrolysis of euphorbia rigida with different catalysts. Energy Convers. Manag. 2005, 46, 421–432. [Google Scholar] [CrossRef]
- Putun, E.; Uzun, B.B.; Putun, A.E. Fixed-bed catalytic pyrolysis of cotton-seed cake: Effects of pyrolysis temperature, natural zeolite content and sweeping gas flow rate. Bioresour. Technol. 2006, 97, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Aho, A.; Kumar, N.; Eranen, K.; Salmi, T.; Hupa, M.; Murzin, D.Y. Catalytic pyrolysis of woody biomass in a fluidized bed reactor: Influence of the zeolite structure. Fuel 2008, 87, 2493–2501. [Google Scholar] [CrossRef]
- Rajić, N.; Logar, N.Z.; Rečnik, A.; El-Roz, M.; Thibault-Starzyk, F.; Sprenger, P.; Hannevold, L.; Andersen, A.; Stöcker, M. Hardwood lignin pyrolysis in the presence of nano-oxide particles embedded onto natural clinoptilolite. Microporous Mesoporous Mater. 2013, 176, 162–167. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Alejandro, S.; Valdés, H.; Zaror, C.A. Natural zeolite reactivity towards ozone: The role of acid surface sites. J. Adv. Oxid. Technol. 2011, 14, 182–189. [Google Scholar] [CrossRef]
- Leliveld, B.R.G.; Kerkhoffs, M.J.H.V.; Broersma, F.A.; van Dillen, J.A.J.; Geus, J.W.; Koningsberger, D.C. Acidic properties of synthetic saponites studied by pyridine ir and tpd[ndash]tg of n-propylamine. J. Chem. Soc. Faraday Trans. 1998, 94, 315–321. [Google Scholar] [CrossRef]
- Konan, K.L.; Peyratout, C.; Smith, A.; Bonnet, J.P.; Magnoux, P.; Ayrault, P. Surface modifications of illite in concentrated lime solutions investigated by pyridine adsorption. J. Colloid Interface Sci. 2012, 382, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Simon-Masseron, A.; Marques, J.P.; Lopes, J.M.; Ribeiro, F.R.A.; Gener, I.; Guisnet, M. Influence of the si/al ratio and crystal size on the acidity and activity of hbea zeolites. Appl. Catal. A Gen. 2007, 316, 75–82. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, Y.-M.; Lee, B.; Kim, S.; Jae, J.; Jung, S.-C.; Kim, T.-W.; Park, Y.-K. Catalytic copyrolysis of torrefied cork oak and high density polyethylene over a mesoporous hy catalyst. Catal. Today 2017, in press. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, J.; Zhang, Q.; Zhu, X. Evaluation methods and research progresses in bio-oil storage stability. Renew. Sustain. Energy Rev. 2014, 40, 69–79. [Google Scholar] [CrossRef]
- Ba, T.; Chaala, A.; Garcia-Perez, M.; Roy, C. Colloidal properties of bio-oils obtained by vacuum pyrolysis of softwood bark. Storage stability. Energy Fuels 2004, 18, 188–201. [Google Scholar] [CrossRef]
- Holladay, J.E.; White, J.F.; Bozell, J.J.; Johnson, D. Top Value Added Chemicals from Biomass—Volume II, Results of Screening for Potential Candidates from Biorefinery Lignin; Pacific Northwest National Lab. (PNNL): Richland, WA, USA; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2007.
- Imam, T.; Capareda, S. Characterization of bio-oil, syn-gas and bio-char from switchgrass pyrolysis at various temperatures. J. Anal. Appl. Pyrolysis 2012, 93, 170–177. [Google Scholar] [CrossRef]
- Cardoso, C.A.L.; Machado, M.E.; Caramão, E.B. Characterization of bio-oils obtained from pyrolysis of bocaiuva residues. Renew. Energy 2016, 91, 21–31. [Google Scholar] [CrossRef]
- Lazzari, E.; Schena, T.; Primaz, C.T.; da Silva Maciel, G.P.; Machado, M.E.; Cardoso, C.A.L.; Jacques, R.A.; Caramão, E.B. Production and chromatographic characterization of bio-oil from the pyrolysis of mango seed waste. Ind. Crops Prod. 2016, 83, 529–536. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Lou, H. In situ catalytic conversion of biomass fast pyrolysis vapors on HZSM-5. J. Energy Chem. 2016, 25, 427–433. [Google Scholar] [CrossRef]
- Mihalcik, D.J.; Mullen, C.A.; Boateng, A.A. Screening acidic zeolites for catalytic fast pyrolysis of biomass and its components. J. Anal. Appl. Pyrolysis 2011, 92, 224–232. [Google Scholar] [CrossRef]
- Zanuttini, M.S.; Peralta, M.A.; Querini, C.A. Deoxygenation of m-cresol: Deactivation and regeneration of pt/γ-Al2O3 catalysts. Ind. Eng. Chem. Res. 2015, 54, 4929–4939. [Google Scholar] [CrossRef]
- Aho, A.; Kumar, N.; Eranen, K.; Salmi, T.; Hupa, M.; Murzin, D.Y. Catalytic pyrolysis of biomass in a fluidized bed reactor: Influence of the acidity of h-beta zeolite. Process Saf. Environ. Prot. 2007, 85, 473–480. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Wang, T.; Xu, Y. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers. Manag. 2007, 48, 87–92. [Google Scholar] [CrossRef]
- Sipilä, K.; Kuoppala, E.; Fagernäs, L.; Oasmaa, A. Characterization of biomass-based flash pyrolysis oils. Biomass Bioenergy 1998, 14, 103–113. [Google Scholar] [CrossRef]
- Diebold, J.P. A Review of the Chemical and Physical Mechanisms of the Storage Stability of Fast Pyrolysis Bio-Oils; National Renewable Energy Lab.: Golden, CO, USA, 1999.
- Boucher, M.; Chaala, A.; Roy, C. Bio-oils obtained by vacuum pyrolysis of softwood bark as a liquid fuel for gas turbines. Part I: Properties of bio-oil and its blends with methanol and a pyrolytic aqueous phase. Biomass Bioenergy 2000, 19, 337–350. [Google Scholar] [CrossRef]
- Yu, F.; Deng, S.; Chen, P.; Liu, Y.; Wan, Y.; Olson, A.; Kittelson, D.; Ruan, R. Physical and chemical properties of bio-oils from microwave pyrolysis of corn stover. Appl. Biochem. Biotechnol. 2007, 137, 957–970. [Google Scholar] [PubMed]
- Adam, J.; Blazsó, M.; Mészáros, E.; Stocker, M.; Nilsen, M.H.; Bouzga, A.; Hustad, J.E.; Gronli, M.; Oye, G. Pyrolysis of biomass in the presence of al-mcm-41 type catalysts. Fuel 2005, 84, 1494–1502. [Google Scholar] [CrossRef]
- Wang, S.; Gu, Y.; Liu, Q.; Yao, Y.; Guo, Z.; Luo, Z.; Cen, K. Separation of bio-oil by molecular distillation. Fuel Process. Technol. 2009, 90, 738–745. [Google Scholar] [CrossRef]
- Erol, M.; Haykiri-Acma, H.; Küçükbayrak, S. Calorific value estimation of biomass from their proximate analyses data. Renew. Energy 2010, 35, 170–173. [Google Scholar] [CrossRef]
- Parikh, J.; Channiwala, S.A.; Ghosal, G.K. A correlation for calculating hhv from proximate analysis of solid fuels. Fuel 2005, 84, 487–494. [Google Scholar] [CrossRef]
- Valdés, H.; Farfán, V.J.; Manoli, J.A.; Zaror, C.A. Catalytic ozone aqueous decomposition promoted by natural zeolite and volcanic sand. J. Hazard. Mater. 2009, 165, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Valdés, H.; Alejandro, S.; Zaror, C.A. Natural zeolite reactivity towards ozone: The role of compensating cations. J. Hazard. Mater. 2012, 227–228, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, M.; Chaala, A.; Pakdel, H.; Kretschmer, D.; Roy, C. Characterization of bio-oils in chemical families. Biomass Bioenergy 2007, 31, 222–242. [Google Scholar] [CrossRef]
- Kanaujia, P.K.; Sharma, Y.K.; Garg, M.O.; Tripathi, D.; Singh, R. Review of analytical strategies in the production and upgrading of bio-oils derived from lignocellulosic biomass. J. Anal. Appl. Pyrolysis 2014, 105, 55–74. [Google Scholar] [CrossRef]
- Dey, A.; Riaz, S. Viscosity measurement of mould fluxes using inclined plane test and development of mathematical model. Ironmak. Steelmak. 2012, 39, 391–397. [Google Scholar] [CrossRef]
| Volatile Matter [wt %] | Fixed Carbon [wt %] | Ash [wt %] | GCV [MJ kg−1] a | GCV [MJ kg−1] b | Cellu Lose [%] | Extrac Tives [%] | Lignin [%] | Hemi Cellulose [%] c | C | H | O | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 85.74 | 12.62 | 1.64 | 20.72 | 24.93 | 35.38 | 1.97 | 27.10 | 35.55 | 47.3 | 6.36 | 46.34 | - |
| S [m2 g−1] | SiO2 a | Al2O3 a | Na2O a | CaO a | K2Oa | MgO a | TiO2 a | Fe2O3 a | MnO a | P2O5 a | CuO a | Si/Al |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 168.17 | 71.61 | 15.18 | 2.0 | 3.43 | 2.03 | 0.74 | 0.61 | 3.99 | 0.06 | 0.12 | 0.03 | 4.72 |
| Product Yield [% w] | |||
|---|---|---|---|
| Bio-Oil | Bio-Char | Permanent Gases | |
| Non-Catalytic | 37.58 | 31.44 | 30.98 |
| Catalytic | 44.18 | 29.78 | 26.03 |
| Oak Pyrolysis | ||
|---|---|---|
| Non-Catalytic | Catalytic | |
| Compounds registered on chromatogram | 90 | 117 |
| Oxygenated compounds | 43 | 54 |
| Non-oxygenated compounds | 15 | 14 |
| Unknown | 32 | 49 |
| * Fam. | Compounds | % Area | Compounds | % Area | ||
|---|---|---|---|---|---|---|
| N–C | Cat. | N–C | Cat. | |||
| Alc | 1,2-Benzenediol | 1.43 | (S)-3-Ethyl-4-methylpentanol | 22.54 | ||
| 1,2-Benzenediol, 3-methoxy- | 1.25 | 3-Furanmethanol | 1.45 | |||
| Cerulignol | 12.75 | 0.57 | p-Ethyl guaiacol | 19.07 | ||
| Guaiacol | 3.02 | 10.92 | 1-Heneicosanol | 34.96 | ||
| 4-Ethylguaiacol | 3.01 | 2-Methoxy-4-vinylphenol | 2.44 | |||
| 4-Vinylguaiacol | 2.05 | 5-tert-Butylpyrogallol | 23.11 | |||
| Homoguaiacol | 3.82 | Furyl alcohol | 1.73 | 4.66 | ||
| Isocreosol | 0.27 | 13.99 | o-Guaiacol | 2.7 | ||
| Methoxyeugenol | 5.31 | 46.09 | p-Methylguaiacol | 3.12 | ||
| o-Benzenediol | 1.14 | Pyrogallol 1-methyl ether | 1.18 | |||
| Syringol | 4.02 | 29.94 | ||||
| Ald | 2-Heptadecenal | 35.96 | Butanedial (Succinaldehyde) | 0.3 | 3.61 | |
| Furfural | 8.76 | 9.4 | Syringaldehyde | 1.53 | 0.74 | |
| Vanillin | 1.38 | 0.35 | Furfural, 5-methyl | 1.61 | 1.75 | |
| K | 1,2-Cyclopentanedione, 3-methyl | 1.15 | 10.42 | 2,4,6-Tris(1,1-dimethylethyl)-4-methylcyclohexa-2,5-dien-1-one | 5.34 | |
| 2-Cyclopenten-1-one, 2-hydroxy | 1.05 | 6.23 | Acetosyringone | 1.65 | ||
| Ethanone, 1-(3-hydroxy-4-methoxyphenyl) | 16.1 | 2-Hydroxy-2-methyl-4-pentanone/Tyranton | 82.39 | 4.34 | ||
| 4-Hydroxy-2-pentanone | 2.72 | 4-Heptanone, 3-methyl- | 0.76 | |||
| 1,2-Cyclopentanedione | 1.34 | |||||
| Es | Acetol acetate | 0.32 | 0.42 | Isopentyl 2-methylpropanoate | 61.68 | |
| Butanoic acid, 3-methylbutyl ester | 21.15 | Isopropyl acetate | 3.89 | |||
| Propanoic acid, 2-methyl-, 2-methylbutyl ester | 59.91 | |||||
| Et | 3,4,5-Trimethoxytoluene | 0.2 | Furan, tetrahydro-2,5-dimethoxy- | 0.1 | ||
| Benzene, 1,4-dimethoxy | 0.8 | Propane, 2-ethoxy- | 69.75 | |||
| Benzene, 1,2,3-trimethoxy-5-methyl- | 7.73 | |||||
| HC | Docosane | 28.25 | 29.22 | Pentane, 2-methyl- | 3.02 | |
| Heneicosane | 4.11 | 3.18 | Tetracosane | 45.17 | 42.68 | |
| Heptacosane | 20.58 | 19.93 | Tricosane | 32.51 | 28.87 | |
| Hexacosane | 26.71 | 30.26 | Undecane | 2.56 | 2.59 | |
| Hexadecane | 4.9 | Triacontane | 2.71 | |||
| Icosane | 13.85 | 10.55 | Butane, 2,2-dimethyl- | 79.01 | ||
| Nonacosane | 9.39 | 10.21 | Cetane | 4.91 | ||
| Octacosane | 13.07 | 14.52 | 2-Hexene, 3,5,5-trimethyl | 15.78 | ||
| Pentacosane | 26.18 | 29.32 | Hexane, 2-methyl- | 2.02 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alejandro Martín, S.; Cerda-Barrera, C.; Montecinos, A. Catalytic Pyrolysis of Chilean Oak: Influence of Brønsted Acid Sites of Chilean Natural Zeolite. Catalysts 2017, 7, 356. https://doi.org/10.3390/catal7120356
Alejandro Martín S, Cerda-Barrera C, Montecinos A. Catalytic Pyrolysis of Chilean Oak: Influence of Brønsted Acid Sites of Chilean Natural Zeolite. Catalysts. 2017; 7(12):356. https://doi.org/10.3390/catal7120356
Chicago/Turabian StyleAlejandro Martín, Serguei, Cristian Cerda-Barrera, and Adan Montecinos. 2017. "Catalytic Pyrolysis of Chilean Oak: Influence of Brønsted Acid Sites of Chilean Natural Zeolite" Catalysts 7, no. 12: 356. https://doi.org/10.3390/catal7120356





