Morphology-Dependent Properties of Cu/CeO2 Catalysts for the Water-Gas Shift Reaction
Abstract
:1. Introduction
2. Results and Discussion
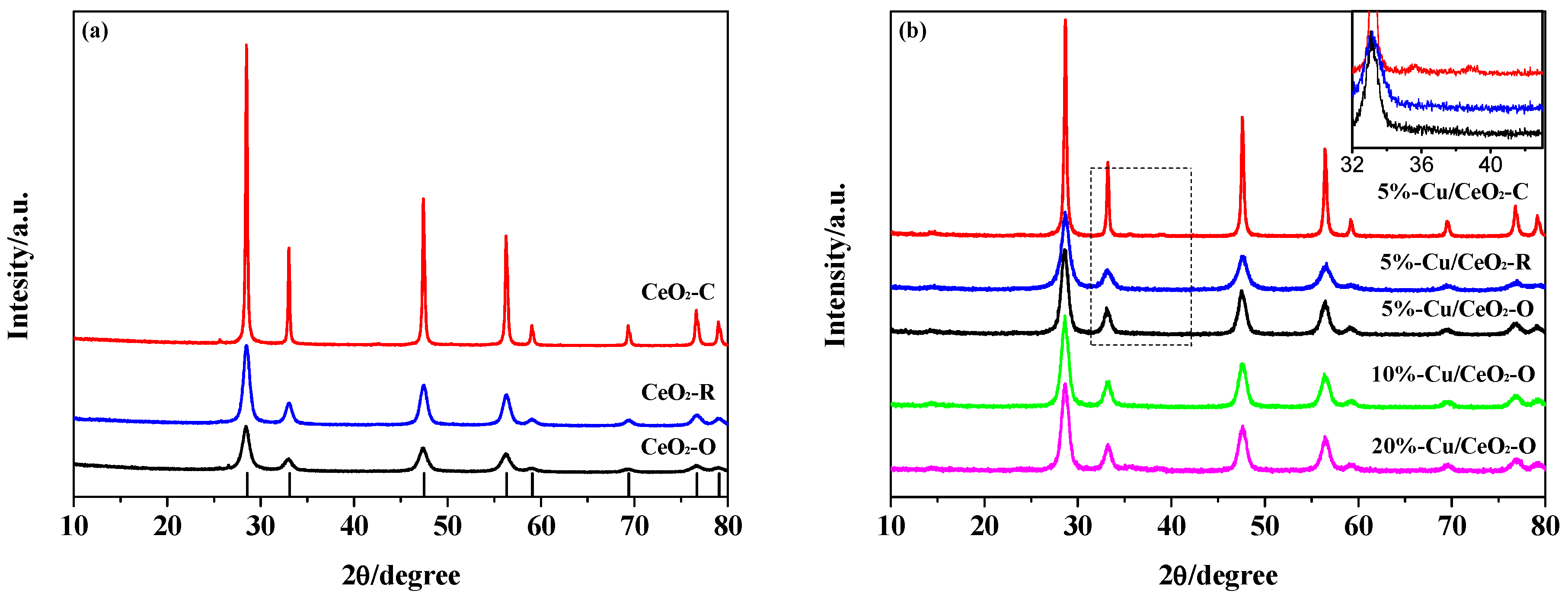
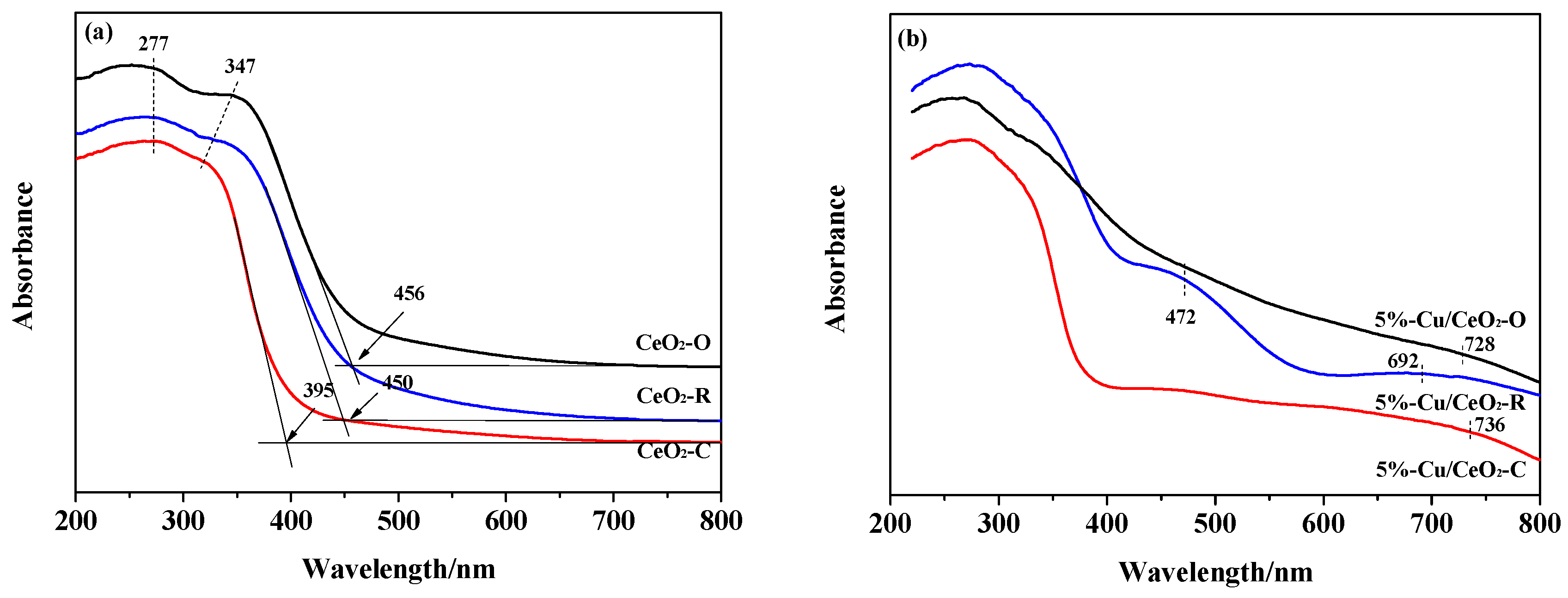
2.1. Structural Characterization of the Catalysts
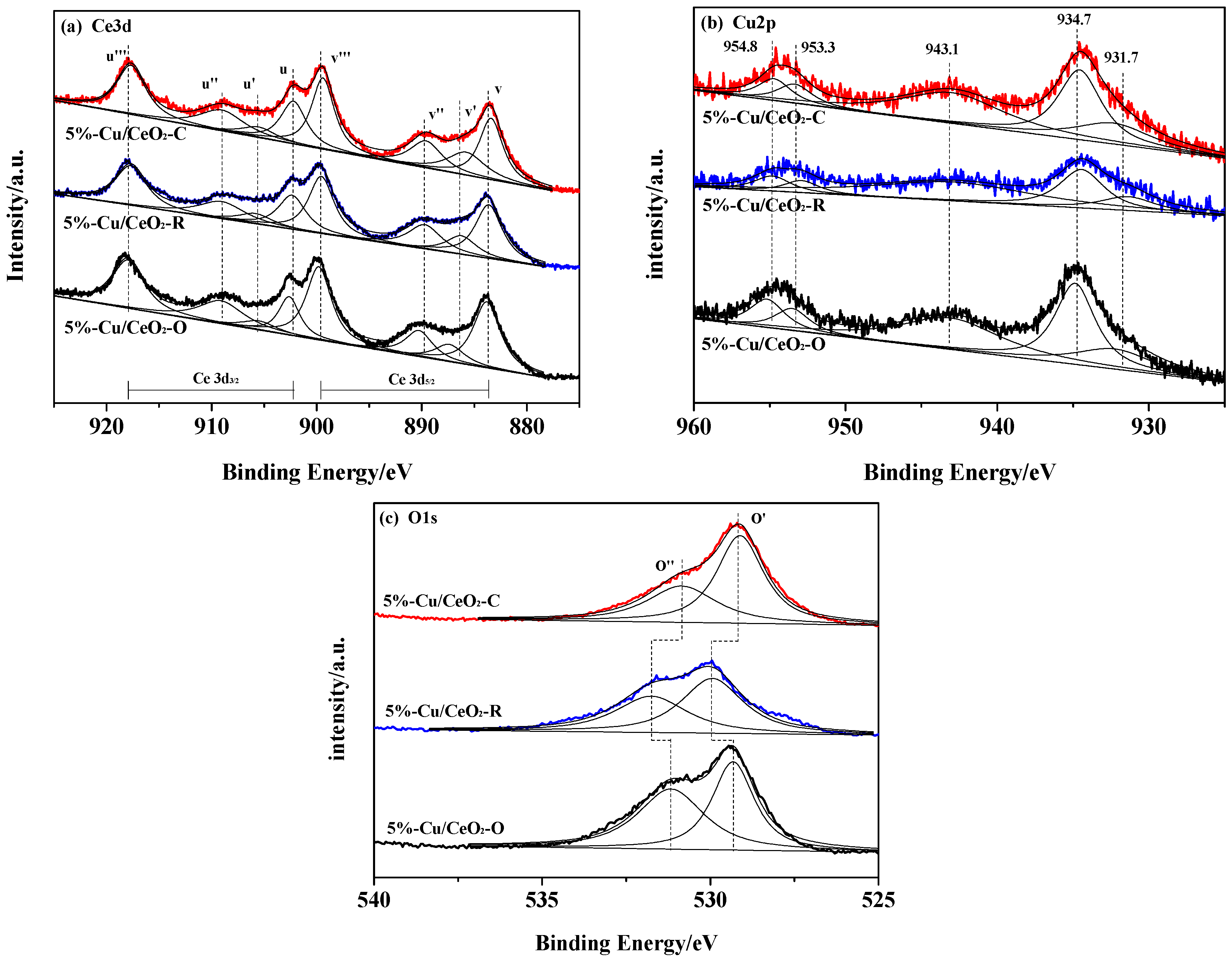
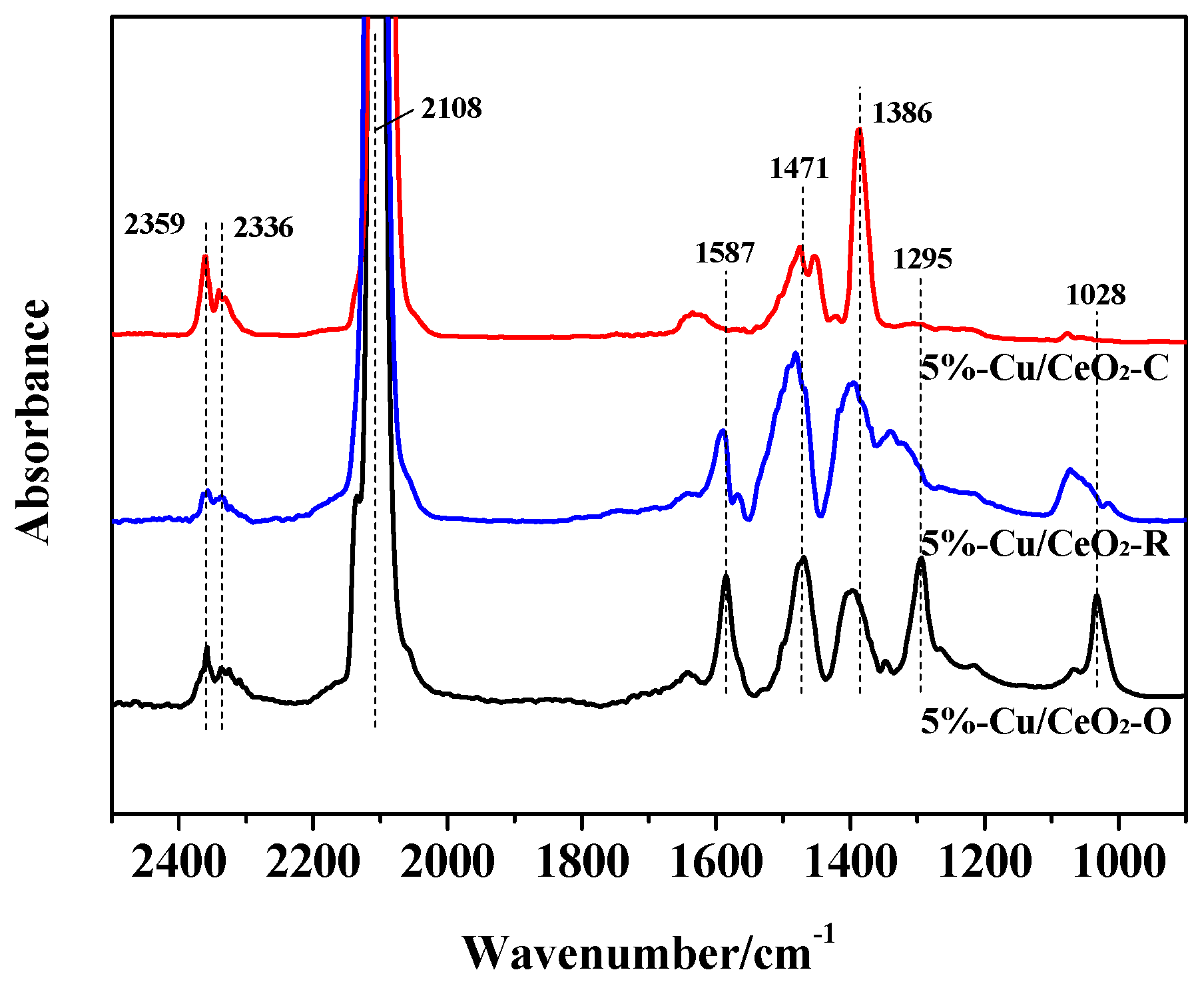
2.2. Chemical States of the Catalysts
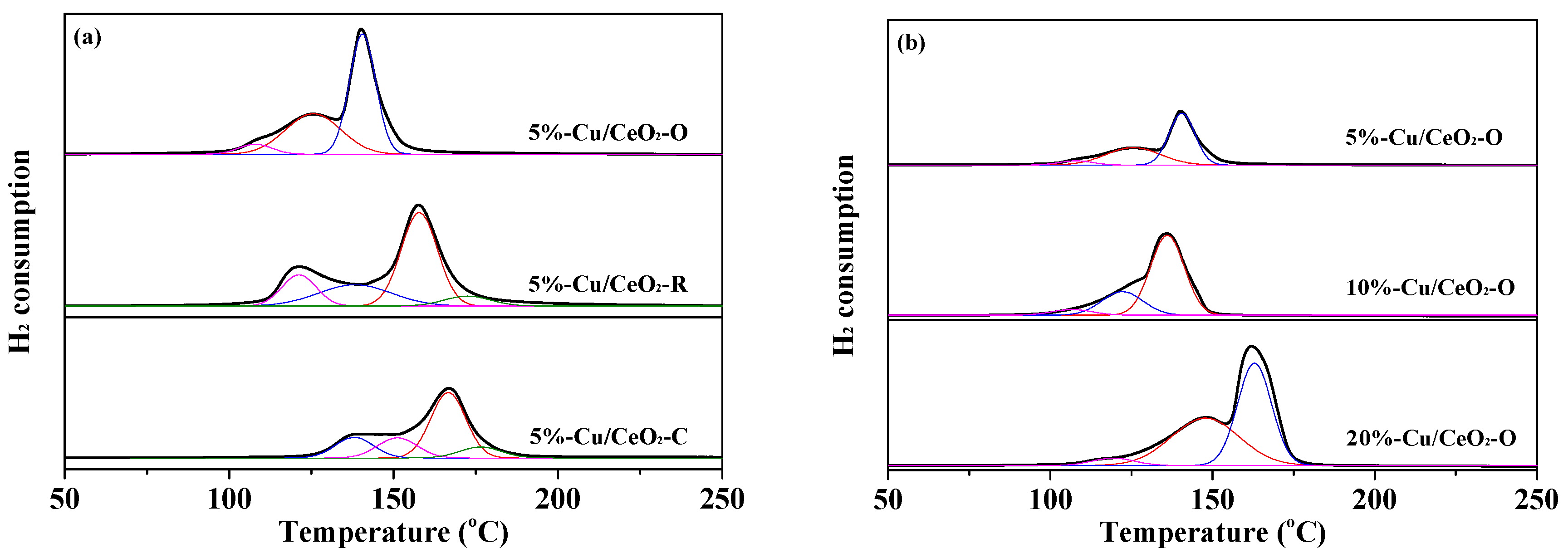
2.3. Reductive Properties of the Catalysts
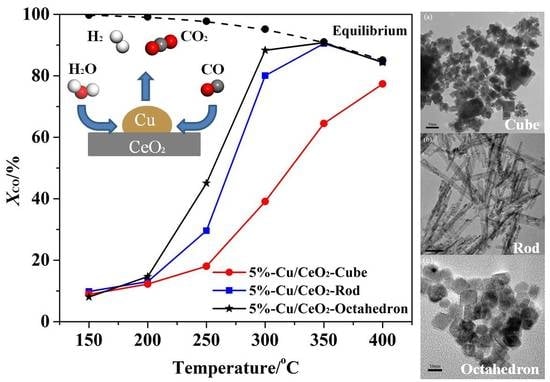
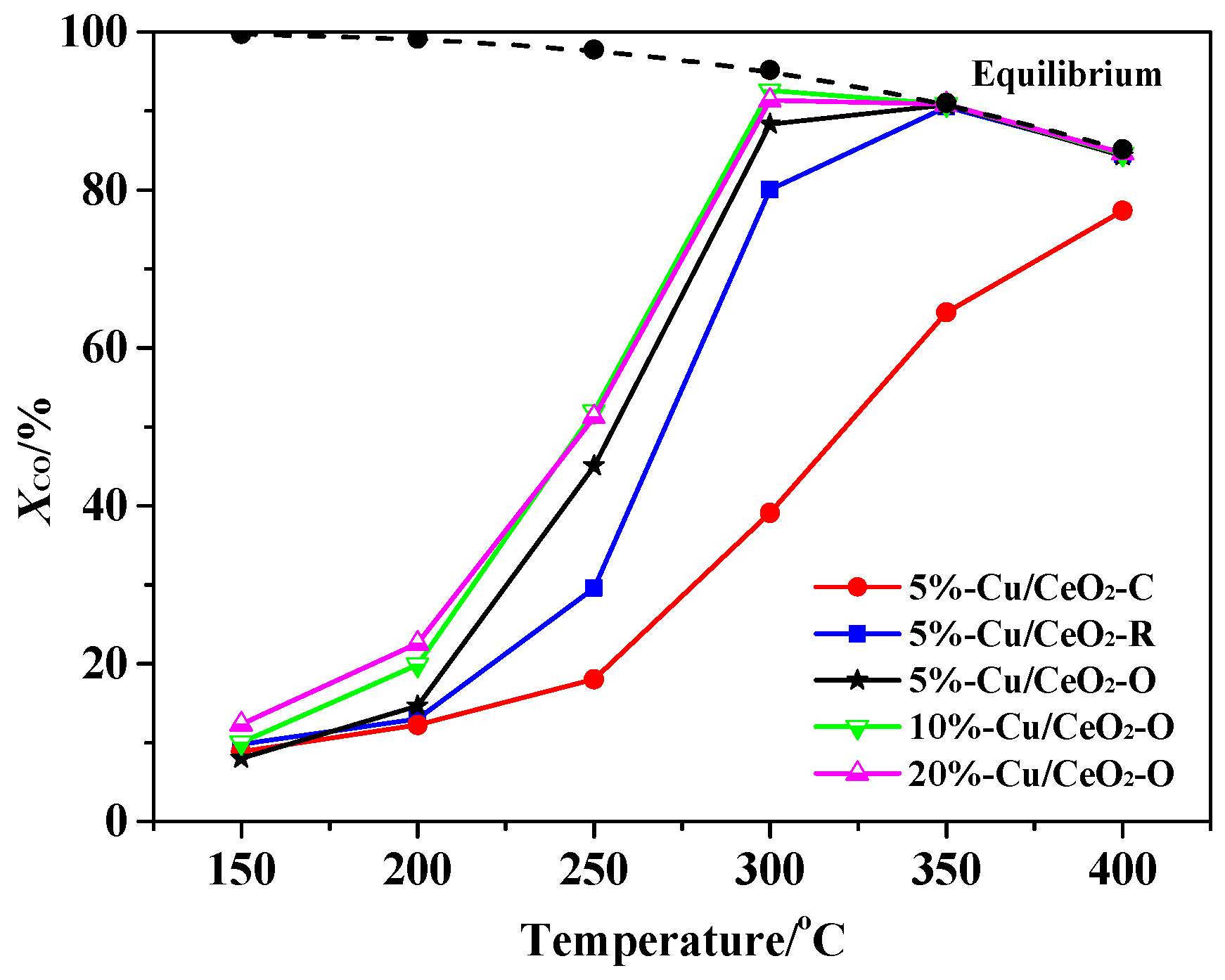
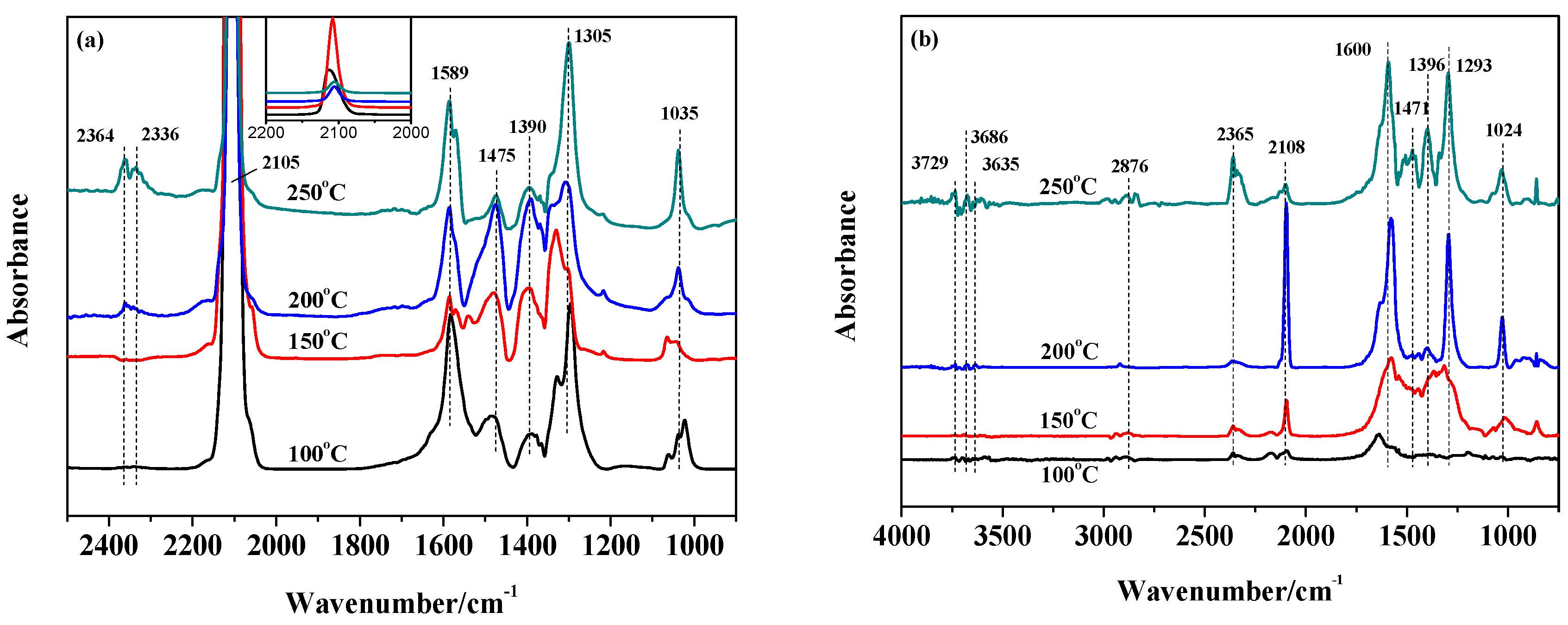
2.4. The Catalytic Activities of the Catalysts
3. Materials and Methods
3.1. Synthesis of CeO2 Supports
3.2. Synthesis of Cu/CeO2 Catalysts
3.3. Characterization
3.4. Catalytic Activity Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Andrews, J.; Shabani, B. Re-envisioning the role of hydrogen in a sustainable energy economy. Int. J. Hydrogen Energy 2012, 37, 1184–1203. [Google Scholar] [CrossRef]
- Navarro, R.; Pena, M.; Fierro, J. Hydrogen production reactions from carbon feedstocks: Fossil fuels and biomass. Chem. Rev. 2007, 107, 3952–3991. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Ma, Z. Water-gas shift on gold catalysts: Catalyst systems and fundamental studies. Phys. Chem. Chem. Phys. 2013, 15, 15260–15270. [Google Scholar] [CrossRef] [PubMed]
- Soykal, I.I.; Bayram, B.; Sohn, H.; Gawade, P.; Miller, J.T.; Ozkan, U.S. Ethanol steam reforming over Co/CeO2 catalysts: Investigation of the effect of ceria morphology. Appl. Catal. A 2012, 449, 47–58. [Google Scholar] [CrossRef]
- Zou, W.X.; Ge, C.Y.; Lu, M.Y.; Wu, S.G.; Wang, Y.Z.; Sun, J.F.; Pu, Y.; Tang, C.J.; Gao, F.; Dong, L. Engineering the NiO/CeO2 interface to enhance the catalytic performance for CO oxidation. Rsc Adv. 2015, 5, 98335–98343. [Google Scholar] [CrossRef]
- Si, R.; Flytzani-Stephanopoulos, M. Shape and crystal-plane effects of nanoscale ceria on the activity of Au-CeO2 catalysts for the water-gas shift reaction. Angew. Chem. 2008, 120, 2926–2929. [Google Scholar] [CrossRef]
- Zhang, D.F.; Zhang, C.S.; Chen, Y.M.; Wang, Q.F.; Bian, L.Y.; Miao, J. Support shape effect on the catalytic performance of Pt/CeO2 nanostructures for methanol electrooxidation. Electrochim. Acta 2014, 139, 42–47. [Google Scholar] [CrossRef]
- Gorte, R.J. Ceria in catalysis: From automotive applications to the water–gas shift reaction. AIChE J. 2010, 56, 1126–1135. [Google Scholar] [CrossRef]
- Huang, W.X.; Gao, Y.X. Morphology-dependent surface chemistry and catalysis of CeO2 nanocrystals. Catal. Sci. Technol. 2014, 4, 3772–3784. [Google Scholar] [CrossRef]
- Liu, L.J.; Yao, Z.J.; Deng, Y.; Gao, F.; Liu, B.; Dong, L. Morphology and crystal-plane effects of nanoscale ceria on the activity of CuO/CeO2 for NO reduction by CO. ChemCatChem 2011, 3, 978–989. [Google Scholar] [CrossRef]
- Huang, X.S.; Sun, H.; Wang, L.C.; Liu, Y.M.; Fan, K.N.; Cao, Y. Morphology effects of nanoscale ceria on the activity of Au/CeO2 catalysts for low-temperature CO oxidation. Appl. Catal. B 2009, 90, 224–232. [Google Scholar] [CrossRef]
- Zhang, M.L.; Li, J.; Li, H.J.; Li, Y.; Shen, W.J. Morphology-dependent redox and catalytic properties of CeO2 nanostructures: Nanowires, nanorods and nanoparticles. Catal. Today 2009, 148, 179–183. [Google Scholar]
- Aneggi, E.; Wiater, D.; de Leitenburg, C.; Llorca, J.; Trovarelli, A. Shape-dependent activity of ceria in soot combustion. ACS Catal. 2013, 4, 172–181. [Google Scholar] [CrossRef]
- Désaunay, T.; Bonura, G.; Chiodo, V.; Freni, S.; Couzinié, J.-P.; Bourgon, J.; Ringuedé, A.; Labat, F.; Adamo, C.; Cassir, M. Surface-dependent oxidation of H2 on CeO2 surfaces. J. Catal. 2013, 297, 193–201. [Google Scholar] [CrossRef]
- Monte, M.; Gamarra, D.; Cámara, A.L.; Rasmussen, S.B.; Gyorffy, N.; Schay, Z.; Martínez-Arias, A.; Conesa, J. Preferential oxidation of CO in excess H2 over CuO/CeO2 catalysts: Performance as a function of the copper coverage and exposed face present in the CeO2 support. Catal. Today 2014, 229, 104–113. [Google Scholar] [CrossRef]
- Gawade, P.; Mirkelamoglu, B.; Ozkan, U.S. The role of support morphology and impregnation medium on the water gas shift activity of ceria-supported copper catalysts. J. Phys. Chem. C 2010, 114, 18173–18181. [Google Scholar] [CrossRef]
- Si, R.; Raitano, J.; Yi, N.; Zhang, L.H.; Chan, S.W.; Flytzani-Stephanopoulos, M. Structure sensitivity of the low-temperature water-gas shift reaction on Cu–CeO2 catalysts. Catal. Today 2012, 180, 68–80. [Google Scholar] [CrossRef]
- Han, J.; Kim, H.J.; Yoon, S.; Lee, H. Shape effect of ceria in Cu/ceria catalysts for preferential CO oxidation. J. Mol. Catal. A 2011, 335, 82–88. [Google Scholar] [CrossRef]
- Mai, H.X.; Sun, L.D.; Zhang, Y.W.; Si, R.; Feng, W.; Zhang, H.P.; Liu, H.C.; Yan, C.H. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. [Google Scholar] [CrossRef] [PubMed]
- Zabilskiy, M.; Djinovic, P.; Tchernychova, E.; Tkachenko, O.P.; Kustov, L.M.; Pintar, A. Nanoshaped CuO/CeO2 materials: Effect of the exposed ceria surfaces on catalytic activity in N2O decomposition reaction. ACS Catal. 2015, 5, 5357–5365. [Google Scholar] [CrossRef]
- Yao, S.Y.; Xu, W.Q.; Johnston-Peck, A.C.; Zhao, F.Z.; Liu, Z.Y.; Luo, S.; Senanayake, S.D.; Martínez-Arias, A.; Liu, W.J.; Rodriguez, J.A. Morphological effects of the nanostructured ceria support on the activity and stability of CuO/CeO2 catalysts for the water-gas shift reaction. Phys. Chem. Chem. Phys. 2014, 16, 17183–17195. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.N.; Bharali, P.; Thrimurthulu, G.; Reddy, B.M. Supported copper-ceria catalysts for low temperature CO oxidation. Catal. Commun. 2010, 11, 863–866. [Google Scholar] [CrossRef]
- He, Y.H.; Liang, X.; Chen, B.H. Surface selective growth of ceria nanocrystals by CO absorption. Chem. Commun. 2013, 49, 9000–9002. [Google Scholar] [CrossRef] [PubMed]
- Pfau, A.; Schierbaum, K. The electronic structure of stoichiometric and reduced CeO2 surfaces: An XPS, UPS and HREELS study. Surf. Sci. 1994, 321, 71–80. [Google Scholar] [CrossRef]
- Gu, T.T.; Liu, Y.; Weng, X.L.; Wang, H.Q.; Wu, Z.B. The enhanced performance of ceria with surface sulfation for selective catalytic reduction of NO by NH3. Catal. Commun. 2010, 12, 310–313. [Google Scholar] [CrossRef]
- Polster, C.S.; Nair, H.; Baertsch, C.D. Study of active sites and mechanism responsible for highly selective CO oxidation in H2 rich atmospheres on a mixed Cu and Ce oxide catalyst. J. Catal. 2009, 266, 308–319. [Google Scholar] [CrossRef]
- Li, L.; Song, L.; Chen, C.Q.; Zhang, Y.J.; Zhan, Y.Y.; Lin, X.Y.; Zheng, Q.; Wang, H.D.; Ma, H.X.; Ding, L.H. Modified precipitation processes and optimized copper content of CuO-CeO2 catalysts for water–gas shift reaction. Int. J. Hydrogen Energy 2014, 39, 19570–19582. [Google Scholar] [CrossRef]
- Sun, J.F.; Zhang, L.; Ge, C.Y.; Tang, C.J.; Dong, L. Comparative study on the catalytic CO oxidation properties of CuO/CeO2 catalysts prepared by solid state and wet impregnation. Chin. J. Catal. 2014, 35, 1347–1358. [Google Scholar] [CrossRef]
- Singhania, N.; Anumol, E.; Ravishankar, N.; Madras, G. Influence of CeO2 morphology on the catalytic activity of CeO2-Pt hybrids for CO oxidation. Dalton Trans. 2013, 42, 15343–15354. [Google Scholar] [CrossRef] [PubMed]
- Cámara, A.L.; Chansai, S.; Hardacre, C.; Martínez-Arias, A. The water–gas shift reaction over CeO2/CuO: Operando SSITKA-DRIFTS-mass spectrometry study of low temperature mechanism. Int. J. Hydrogen Energy 2014, 39, 4095–4101. [Google Scholar] [CrossRef]

| Sample | Crystallite Size (nm) | BET Surface Area 1 (m2/g) | Atomic Ratio 2 (%) | ||
|---|---|---|---|---|---|
| Ce3+/(Ce3+ + Ce4+) | Cu+/(Cu+ + Cu2+) | O″/(O″ + O′) | |||
| CeO2-C | 33.5 | 30.34 | 14.19 | 18.14 | 38.07 |
| CeO2-R | 10.1 | 81.90 | 12.00 | 24.55 | 43.59 |
| CeO2-O | 8.4 | 100.01 | 9.59 | 22.88 | 51.84 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Peng, F.; Li, J.; Liang, X.; Chen, B. Morphology-Dependent Properties of Cu/CeO2 Catalysts for the Water-Gas Shift Reaction. Catalysts 2017, 7, 48. https://doi.org/10.3390/catal7020048
Ren Z, Peng F, Li J, Liang X, Chen B. Morphology-Dependent Properties of Cu/CeO2 Catalysts for the Water-Gas Shift Reaction. Catalysts. 2017; 7(2):48. https://doi.org/10.3390/catal7020048
Chicago/Turabian StyleRen, Zhibo, Fei Peng, Jianwei Li, Xin Liang, and Biaohua Chen. 2017. "Morphology-Dependent Properties of Cu/CeO2 Catalysts for the Water-Gas Shift Reaction" Catalysts 7, no. 2: 48. https://doi.org/10.3390/catal7020048