The Synergy Effect of Ni-M (M = Mo, Fe, Co, Mn or Cr) Bicomponent Catalysts on Partial Methanation Coupling with Water Gas Shift under Low H2/CO Conditions
Abstract
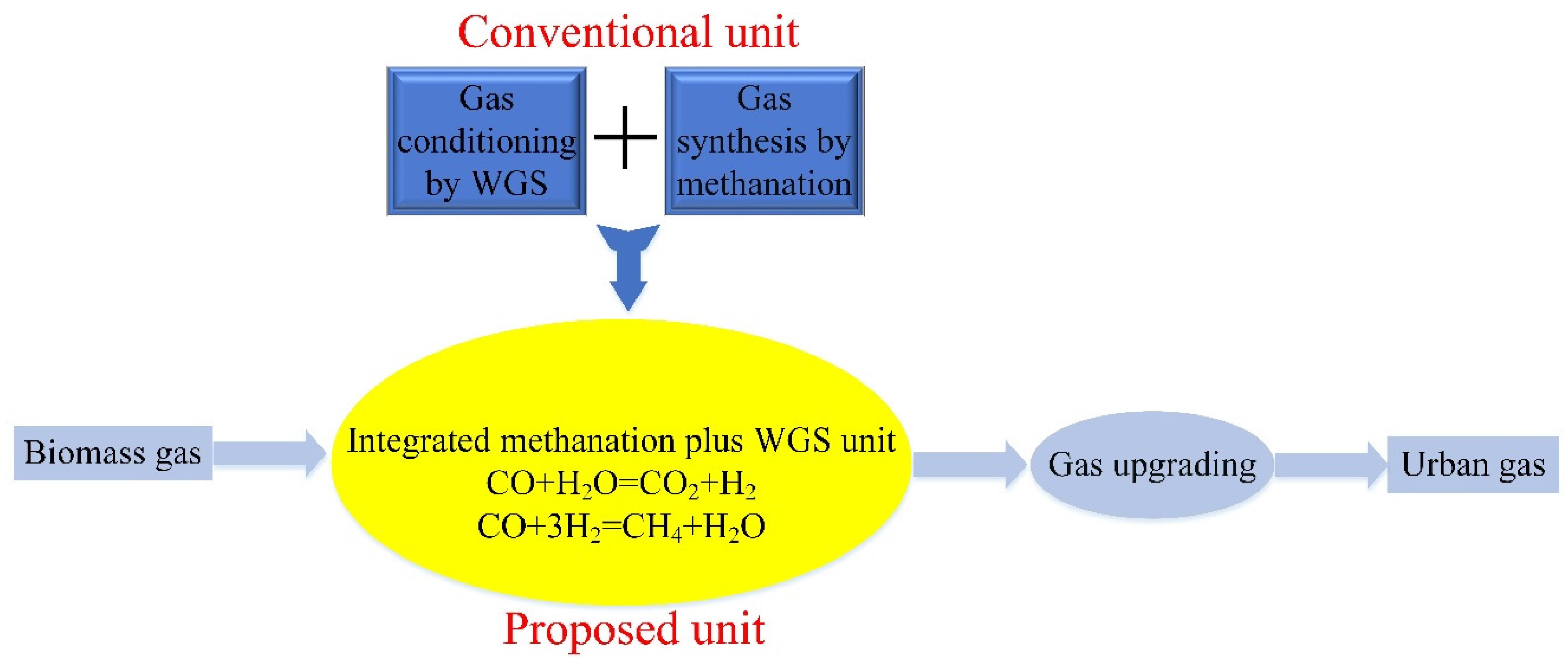
:1. Introduction
2. Results and Discussion
2.1. Characterization Analysis
2.1.1. Physico-Chemical Analysis
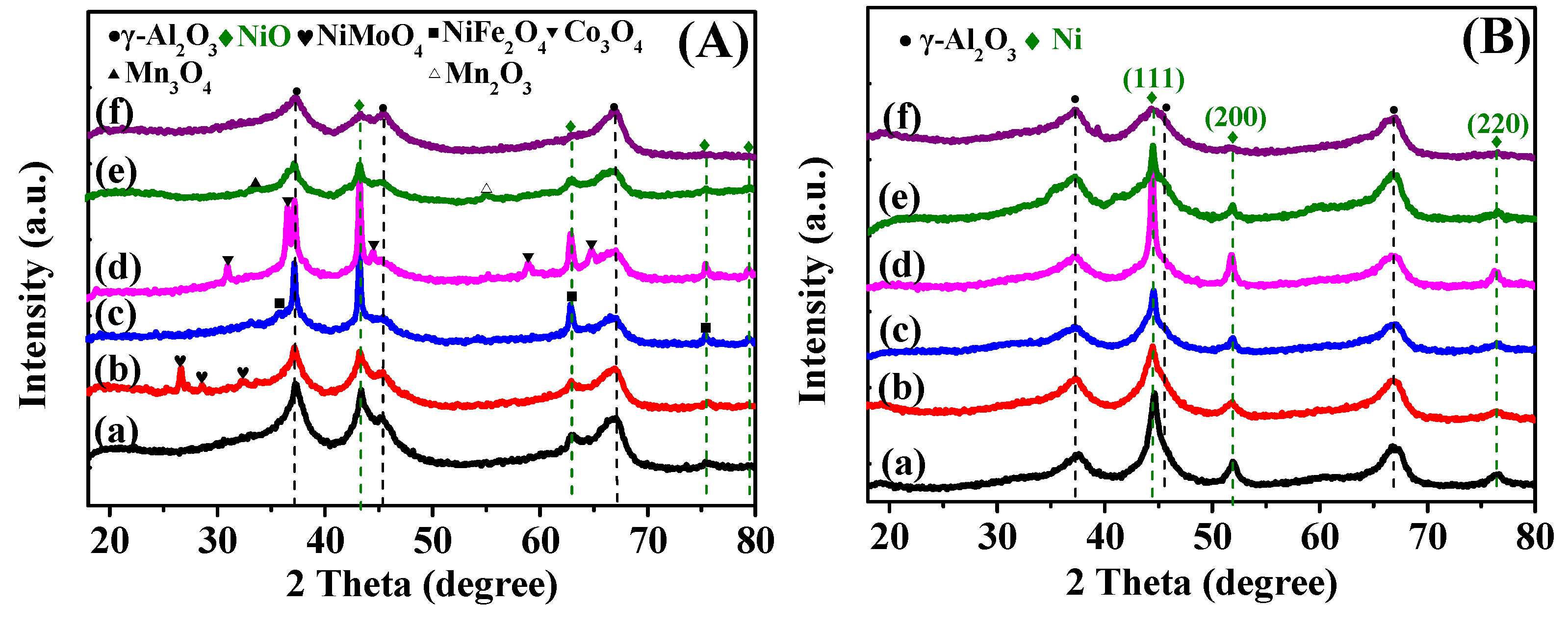
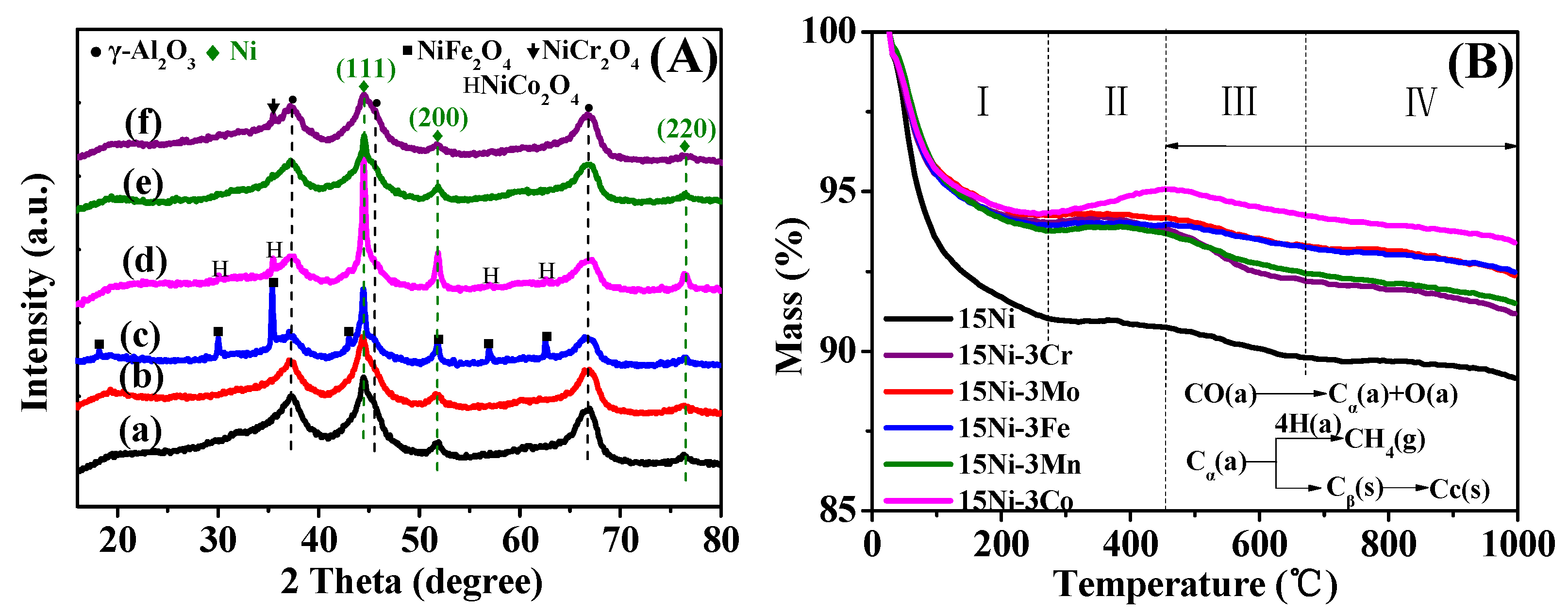
2.1.2. XRD Analysis
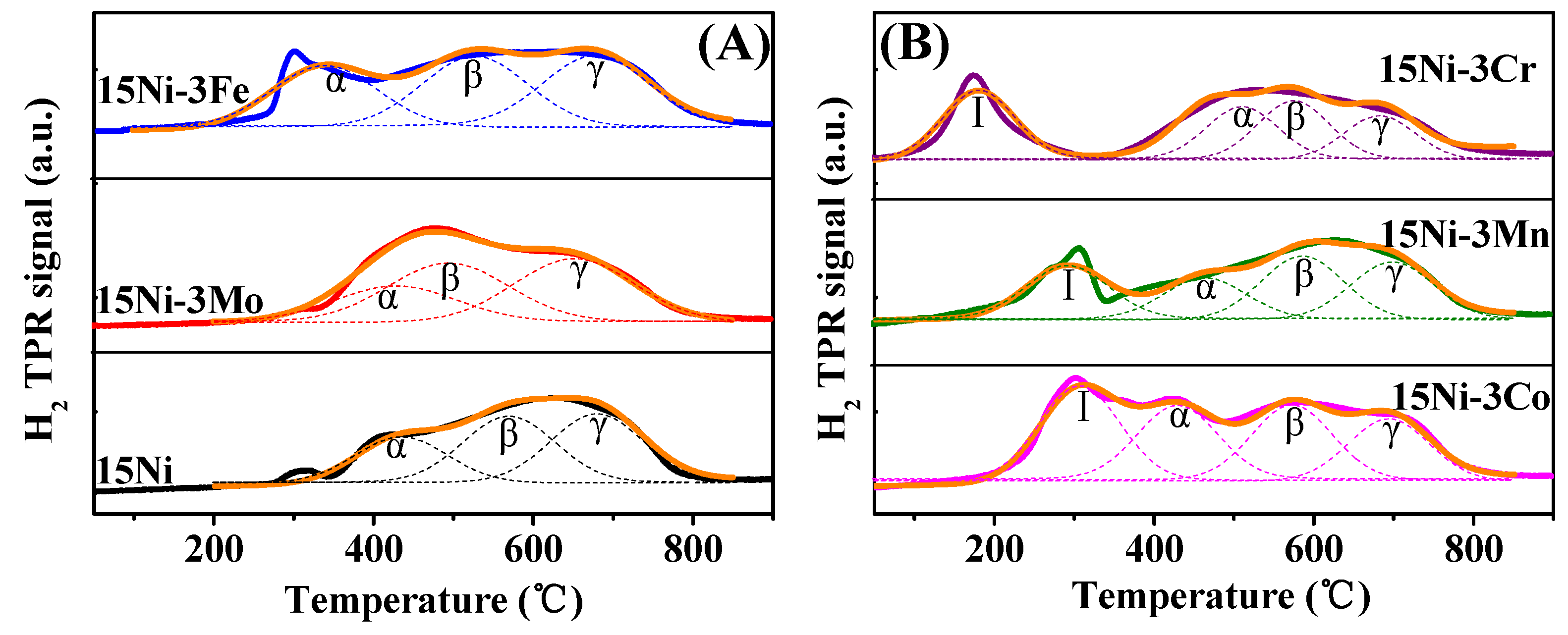
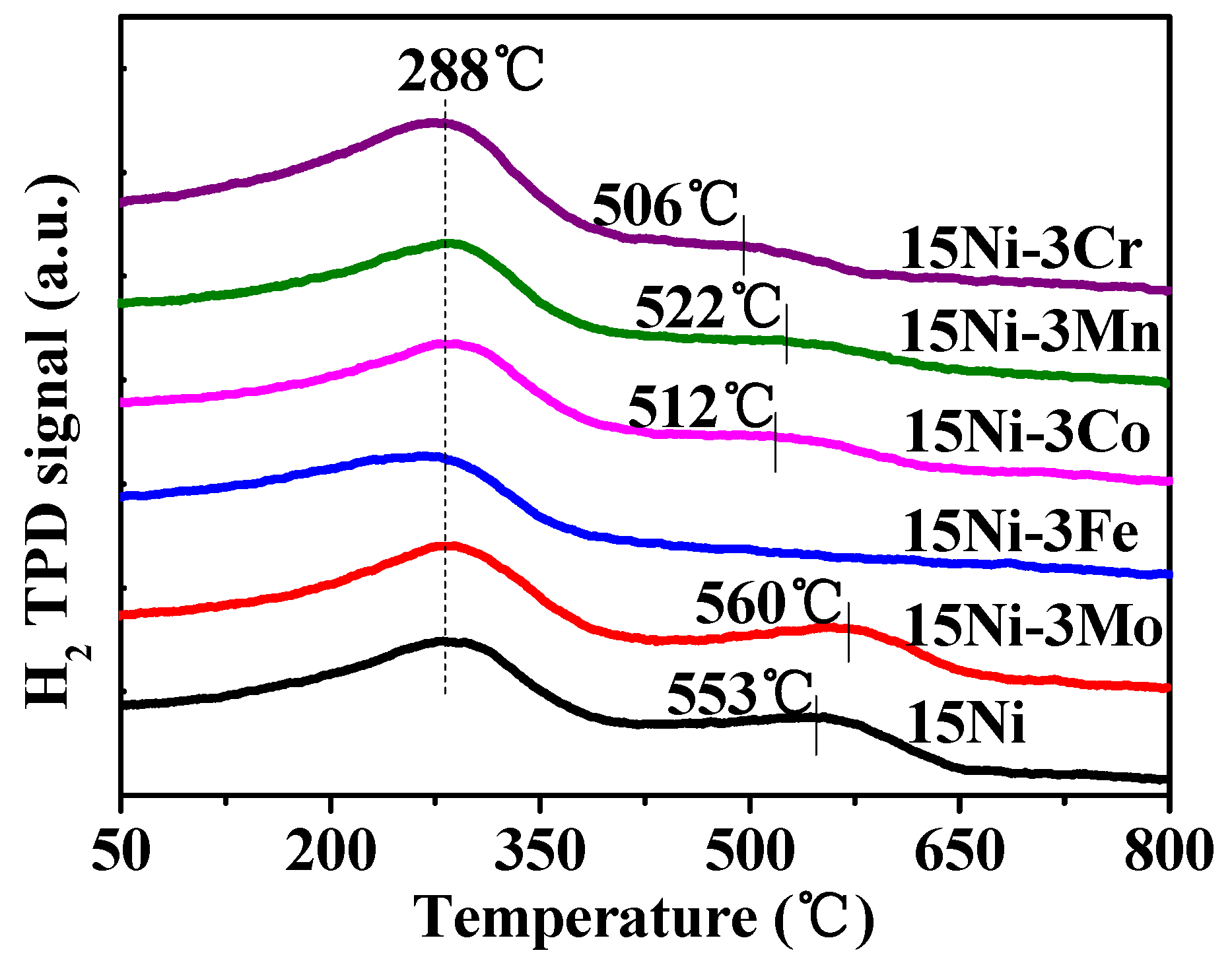
2.1.3. H2-TPR and H2-TPD Analysis
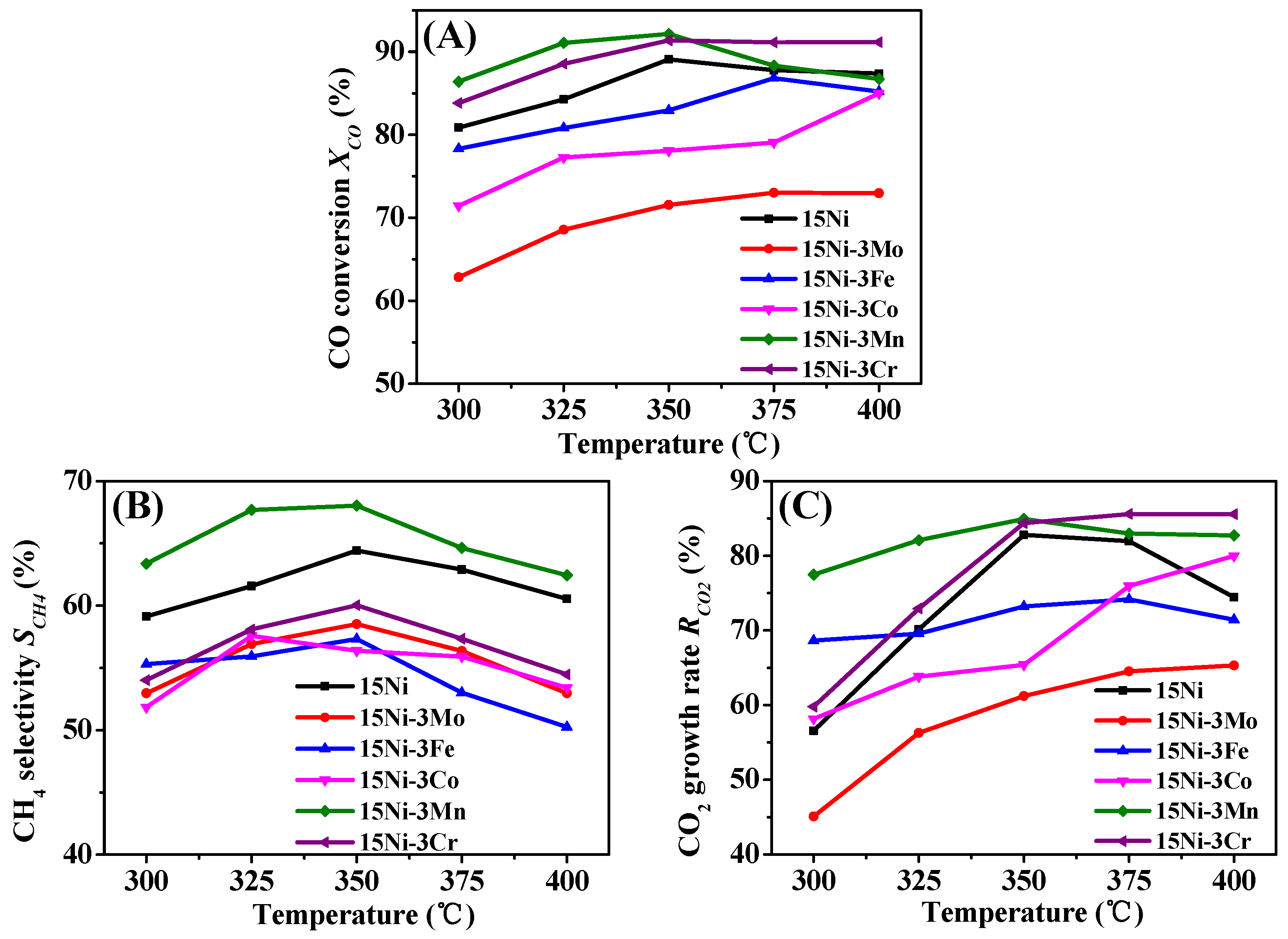
2.2. Catalytic Performances of Catalysts
2.2.1. Performance Tests
2.2.2. XRD and TG Analysis after Performance Tests
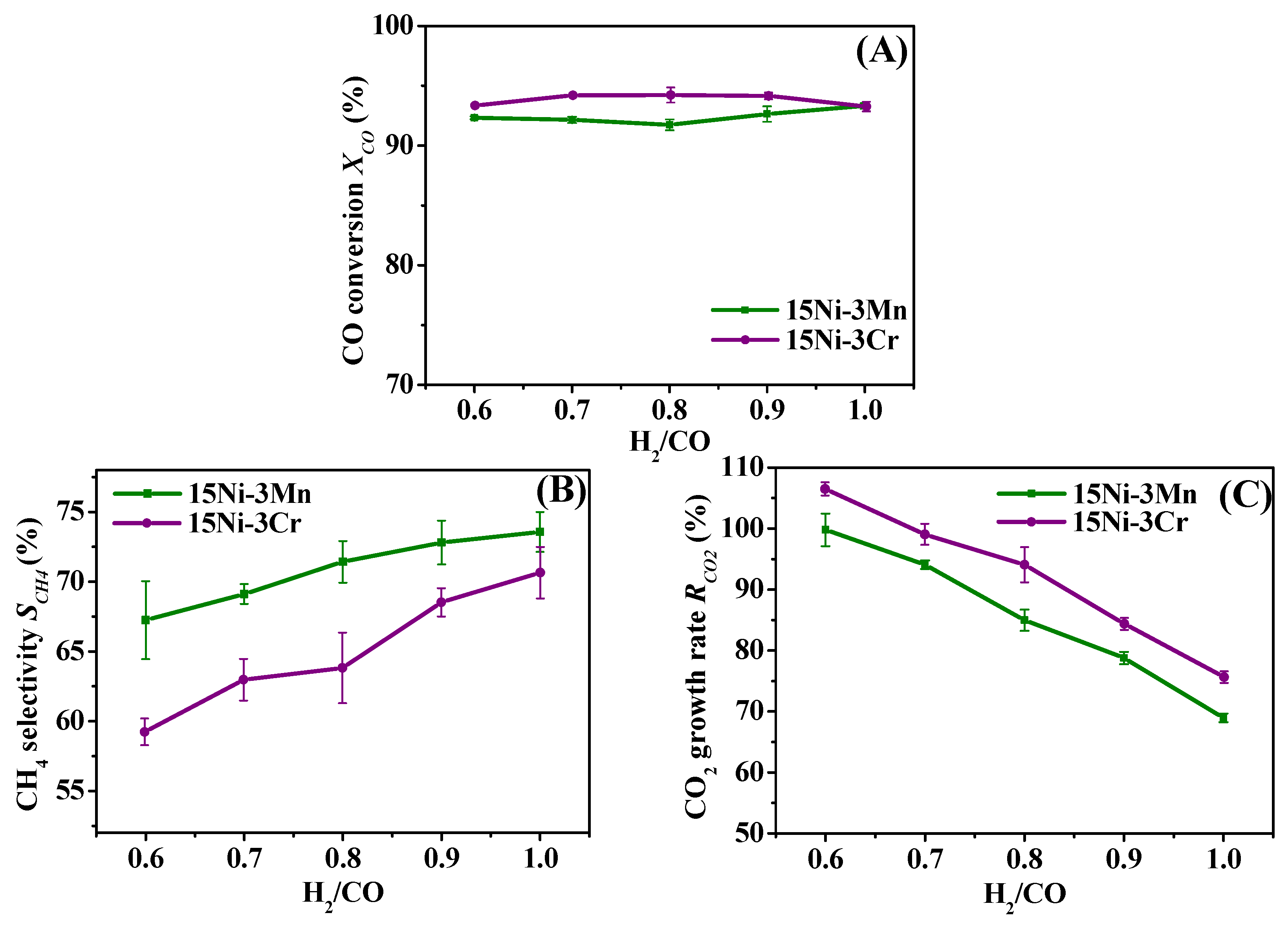
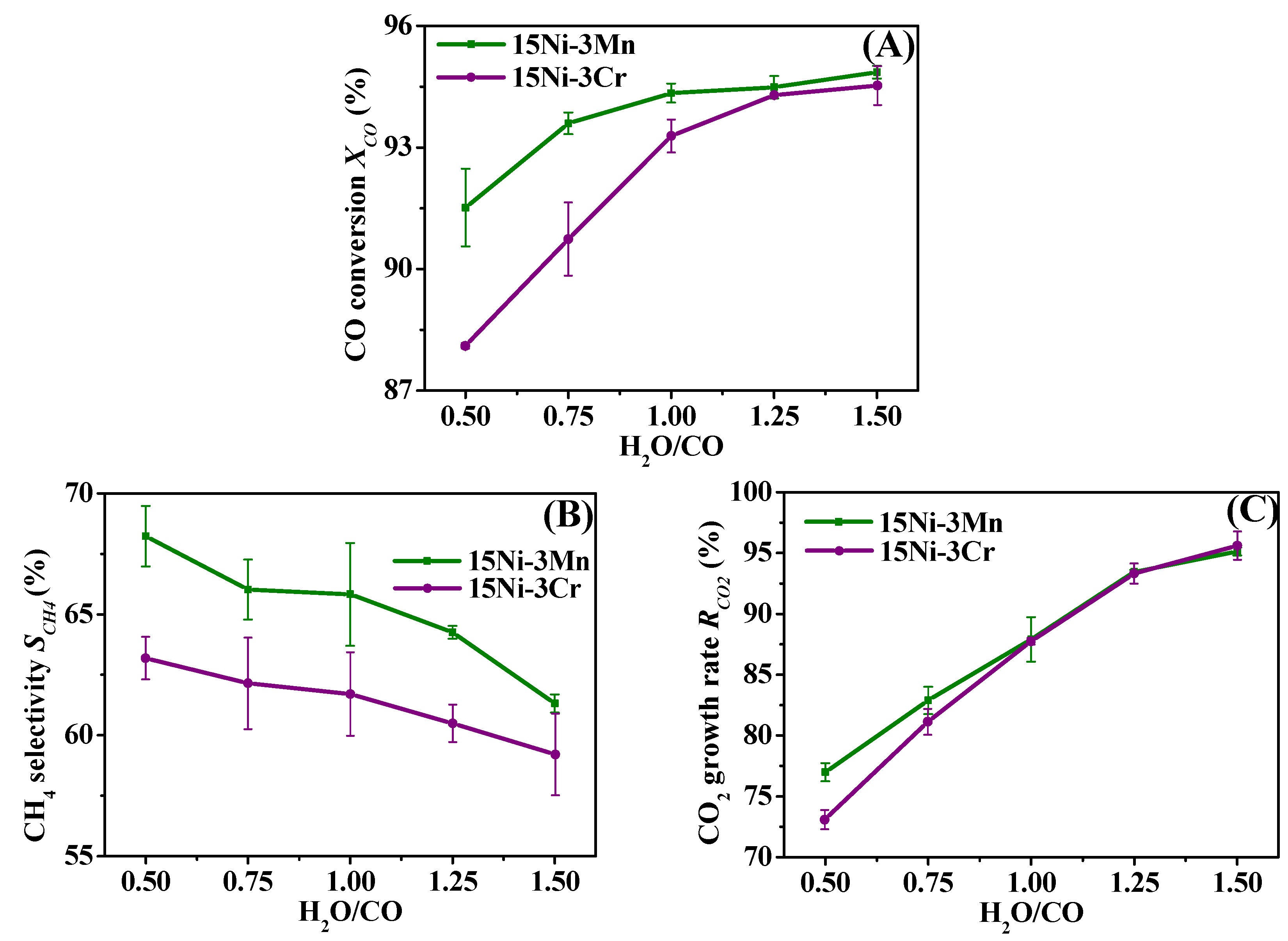
2.3. Comparative Study on 15Ni-3Mn and 15Ni-3Cr
2.3.1. Effect of H2/CO
2.3.2. Effect of H2O/CO
2.3.3. Heating Value Analysis
2.3.4. SEM Analysis after Comparative Study
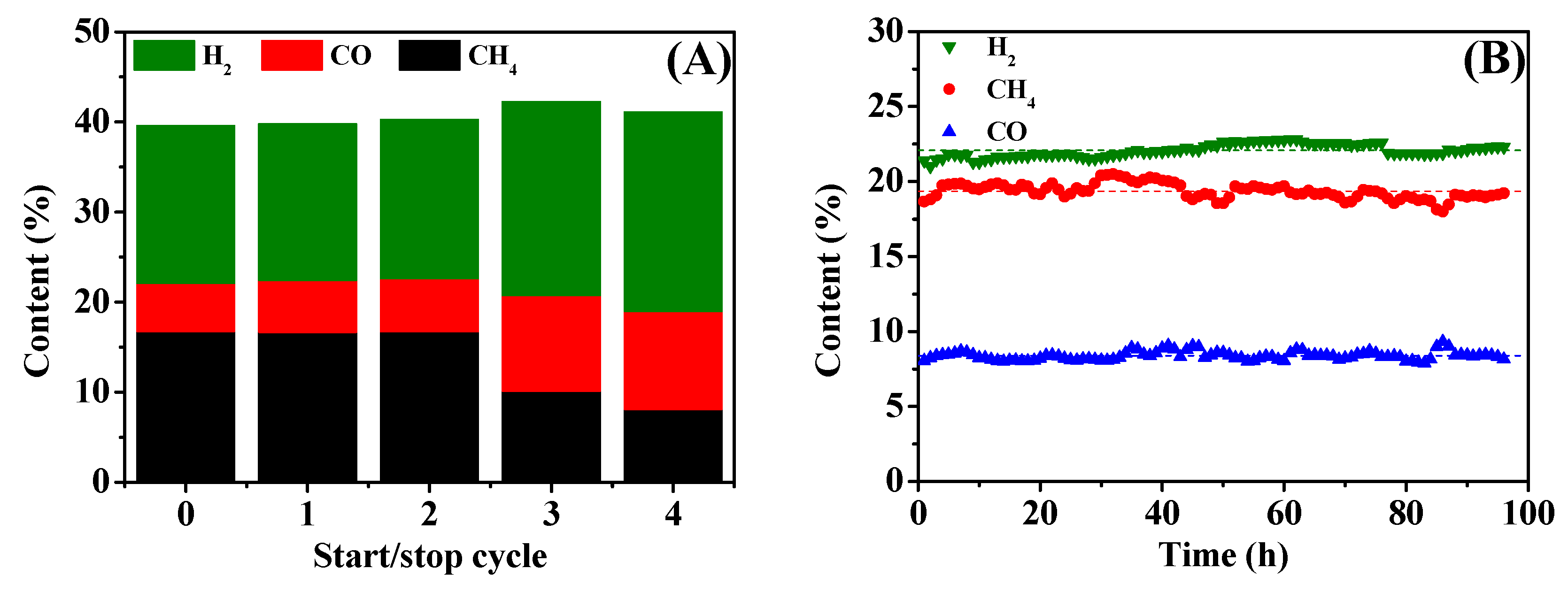
2.4. Stability of 15Ni-3Mn Catalyst
2.5. Synergy Effect between Ni and MnOx Species
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterizations
3.3. Catalyst Testing
4. Conclusions
- (1)
- 15Ni-3Mo, 15Ni-3Fe and 15Ni-3Co catalysts present lower CO conversion, CH4 selectivity and CO2 growth rate than one-component 15Ni catalyst, mainly due to their low Ni dispersion and weak H2 chemisorption ability with the addition of the second component, indicating the deficient synergy effect between Mo/Fe/Co and active Ni particles.
- (2)
- Though both 15Ni-3Mn and 15Ni-3Cr have relative high Ni dispersion and small Ni particle size, as well as comparable H2 chemisorption ability, 15Ni-3Mn is more suitable for our use due to its lower carbon deposition rate, smaller Ni sintering degree and wider adaptability to various H2/CO and H2O/CO conditions in comparison with 15Ni-3Cr.
- (3)
- 15Ni-3Mn is robust to some extent during the start/stop cycle test and has satisfactory stability during a nearly 100-h lifetime test, showing its qualification for industrial and engineering use.
- (4)
- The lower heating value of the low H2/CO ratio biomass gas catalyzed by 15Ni-3Mn through partial methanation coupling with WGS could be over 10 MJ∙Nm−3, which is eligible as urban gas for downstream users.
- (5)
- MnOx species as an additive or second component cooperate well with Ni, for increasing methanation activity, as well as enhancing WGS reaction, indicating its favorable synergy effect with active Ni particle.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M.A. Production of synthetic natural gas (SNG) from coal and dry biomass-A technology review from 1950 to 2009. Fuel 2010, 89, 1763–1783. [Google Scholar] [CrossRef]
- Berrueco, C.; Recari, J.; Abelló, S.; Farriol, X.; Montané, D. Experimental Investigation of Solid Recovered Fuel (SRF) Gasification: Effect of Temperature and Equivalence Ratio on Process Performance and Release of Minor Contaminants. Energy Fuels 2015, 29, 7419–7427. [Google Scholar] [CrossRef]
- Yu, H.; Li, Z.; Yang, X.; Jiang, L.; Zhang, Z.; Chen, D. Experimental research on oxygen-enriched gasification of straw in an entrained-flow gasifier. J. Renew. Sustain. Energy 2013, 5, 053127. [Google Scholar] [CrossRef]
- Kwapinska, M.; Xue, G.; Horvat, A.; Rabou, L.P.L.M.; Dooley, S.; Kwapinski, W.; Leahy, J.J. Fluidized Bed Gasification of Torrefied and Raw Grassy Biomass (Miscanthus ×gigantenus). The Effect of Operating Conditions on Process Performance. Energy Fuels 2015, 29, 7290–7300. [Google Scholar] [CrossRef]
- Biagini, E.; Barontini, F.; Tognotti, L. Gasification of agricultural residues in a demonstrative plant: Vine pruning and rice husks. Bioresour. Technol. 2015, 194, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, T.; Qin, J.; Wu, J.; Li, J.; Zi, Z.; Liu, G.; Wu, J.; Sun, L. Gasification of biomass with oxygen-enriched air in a pilot scale two-stage gasifier. Fuel 2015, 150, 386–393. [Google Scholar] [CrossRef]
- Bukur, D.B.; Todic, B.; Elbashir, N. Role of water-gas-shift reaction in Fischer-Tropsch synthesis on iron catalysts: A review. Catal. Today 2016, 275, 66–75. [Google Scholar] [CrossRef]
- Haro, P.; Johnsson, F.; Thunman, H. Improved syngas processing for enhanced Bio-SNG production: A techno-economic assessment. Energy 2016, 101, 380–389. [Google Scholar] [CrossRef]
- Gao, J.; Qing, L.; Gu, F.; Liu, B.; Zhong, Z.; Su, F. Recent advances in methanation catalysts for the production of synthetic natural gas. RSC Adv. 2015, 5, 22759–22776. [Google Scholar] [CrossRef]
- Sener, C.; Wesley, T.S.; Alba-Rubio, A.C.; Kumbhalkar, M.D.; Hakim, S.H.; Ribeiro, F.H.; Miller, J.T.; Dumesic, J.A. PtMo Bimetallic Catalysts Synthesized by Controlled Surface Reactions for Water Gas Shift. ACS Catal. 2016, 6, 1334–1344. [Google Scholar] [CrossRef]
- Zhu, M.; Wachs, I.E. Iron-Based Catalysts for the High-Temperature Water-Gas Shift (HT-WGS) Reaction: A Review. ACS Catal. 2016, 6, 722–732. [Google Scholar] [CrossRef]
- Ahn, C.; Jeong, D.; Cho, J.M.; Na, H.; Jang, W.; Roh, H.; Choi, J.; Um, S.H.; Bae, J.W. Water gas shift reaction on the Mn-modified ordered mesoporous Co3O4. Microporous Mesoporous Mater. 2016, 221, 204–211. [Google Scholar] [CrossRef]
- He, R.; Wang, D.; Zhi, K.; Wang, B.; Zhong, H.; Jiang, H.; Li, N.; Liu, Q. Cu-Mn catalysts modified by rare earth lantnaum for low temperature water-gas shift reaction. J. Rare Earths 2016, 34, 994–1003. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, X.; Liu, S.; Wan, J.; Li, M.; Weng, D.; Tong, C. Modification of PdO/CeO2–ZrO2 catalyst by MnOx for water–gas shift reaction: Redox property and valence state of Pd. J. Mater. Sci. 2016, 51, 5377–5387. [Google Scholar] [CrossRef]
- Chianese, S.; Loipersböck, J.; Malits, M.; Rauch, R.; Hofbauer, H.; Molino, A.; Musmarra, D. Hydrogen from the high temperature water gas shift reaction with an industrial Fe/Cr catalyst using biomass gasification tar rich synthesis gas. Fuel Process. Technol. 2015, 132, 39–48. [Google Scholar] [CrossRef]
- Ma, S.; Tan, Y.; Han, Y. Water-gas shift coupling with methanation over MOx modified nanorod-NiO/γ-Al2O3 catalysts. J. Ind. Eng. Chem. 2011, 17, 723–726. [Google Scholar] [CrossRef]
- Mei, Z.; Li, Y.; Fan, M.; Argyle, M.D.; Tang, J. The effects of bimetallic Co–Ru nanoparticles on Co/RuO2/Al2O3 catalysts for the water gas shift and methanation. Int. J. Hydrog. Energy 2014, 39, 14808–14816. [Google Scholar] [CrossRef]
- Ding, M.; Tu, J.; Zhang, Q.; Wang, M.; Tsubaki, N.; Wang, T.; Ma, L. Enhancement of methanation of bio-syngas over CeO2-modified Ni/Al2O3 catalysts. Biomass Bioenergy 2016, 85, 12–17. [Google Scholar] [CrossRef]
- Hu, D.; Gao, J.; Ping, Y.; Jia, L.; Gunawan, P.; Zhong, Z.; Xu, G.; Gu, F.; Su, F. Enhanced Investigation of CO Methanation over Ni/Al2O3 Catalysts for Synthetic Natural Gas Production. Ind. Eng. Chem. Res. 2012, 51, 4875–4886. [Google Scholar] [CrossRef]
- Kumar, J.; Deo, G.; Kunzru, D. Preferential oxidation of carbon monoxide on Pt/γ-Al2O3 catalyst: Effect of adding ceria and nickel. Int. J. Hydrog. Energy 2016, 41, 18494–18501. [Google Scholar] [CrossRef]
- Gao, Z.; Cui, L.; Ma, H. Selective methanation of CO over Ni/Al2O3 catalyst: Effects of preparation method and Ru addition. Int. J. Hydrog. Energy 2016, 41, 5484–5493. [Google Scholar] [CrossRef]
- Arbeláez, O.; Reina, T.R.; Ivanova, S.; Bustamante, F.; Villa, A.L.; Centeno, M.A.; Odriozola, J.A. Mono and bimetallic Cu-Ni structured catalysts for the water gas shift reaction. Appl. Catal. A 2015, 497, 1–9. [Google Scholar] [CrossRef]
- Bayat, N.; Rezaei, M.; Meshkani, F. Methane decomposition over Ni–Fe/Al2O3 catalysts for production of COx-free hydrogen and carbon nanofiber. Int. J. Hydrog. Energy 2016, 41, 1574–1584. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.; Gu, F.; Wang, X.; Lu, X.; Li, H.; Xu, G.; Su, F. CO methanation on ordered mesoporous Ni–Cr–Al catalysts: Effects of the catalyst structure and Cr promoter on the catalytic properties. J. Catal. 2016, 337, 221–232. [Google Scholar] [CrossRef]
- Zhao, K.; Li, Z.; Bian, L. CO2 methanation and co-methanation of CO and CO2 over Mn-promoted Ni/Al2O3 catalysts. Front. Chem. Sci. Eng. 2016, 10, 273–280. [Google Scholar] [CrossRef]
- Lu, H.; Yang, X.; Gao, G.; Wang, J.; Han, C.; Liang, X.; Li, C.; Li, Y.; Zhang, W.; Chen, X. Metal (Fe, Co, Ce or La) doped nickel catalyst supported on ZrO2 modified mesoporous clays for CO and CO2 methanation. Fuel 2016, 183, 335–344. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Shi, R.; Yang, M. Effect of metal–support interaction on the catalytic performance of Ni/Al2O3 for selective hydrogenation of isoprene. J. Mol. Catal. A Chem. 2011, 344, 122–127. [Google Scholar] [CrossRef]
- Zhao, A.; Ying, W.; Zhang, H.; Hongfang, M.; Fang, D. Ni/Al2O3 catalysts for syngas methanation: Effect of Mn promoter. J. Nat. Gas Chem. 2012, 21, 170–177. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Jin, X.; Ge, Q.; Li, W. Characterizations and activities of the nano-sized Ni/Al2O3 and Ni/La–Al2O3 catalysts for NH3 decomposition. Appl. Catal. A 2005, 290, 87–96. [Google Scholar] [CrossRef]
- Lu, X.; Gu, F.; Liu, Q.; Gao, J.; Jia, L.; Xu, G.; Zhong, Z.; Su, F. Ni–MnOx Catalysts Supported on Al2O3-Modified Si Waste with Outstanding CO Methanation Catalytic Performance. Ind. Eng. Chem. Res. 2015, 54, 12516–12524. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Chaturvedi, S.; Hanson, J.C.; Brito, J.L. Reaction of H2 and H2S with CoMoO4 and NiMoO4: TPR, XANES, Time-Resolved XRD, and Molecular-Orbital Studies. J. Phys. Chem. B 1999, 103, 770–781. [Google Scholar] [CrossRef]
- Jeong, D.; Jang, W.; Shim, J.; Roh, H. High temperature water–gas shift without pre-reduction over spinel ferrite catalysts synthesized by glycine assisted sol–gel combustion method. Int. J. Hydrog. Energy 2016, 41, 3870–3876. [Google Scholar] [CrossRef]
- Nikparsa, P.; Mirzaei, A.A.; Rauch, R. Impact of Na Promoter on Structural Properties and Catalytic Performance of CoNi/Al2O3 Nanocatalysts for the CO Hydrogenation Process: Fischer–Tropsch Technology. Catal. Lett. 2016, 146, 61–71. [Google Scholar] [CrossRef]
- Velu, S.; Gangwal, S. Synthesis of alumina supported nickel nanoparticle catalysts and evaluation of nickel metal dispersions by temperature programmed desorption. Solid State Ion. 2006, 177, 803–811. [Google Scholar] [CrossRef]
- Gatica, J. M.; Chen, X.; Zerrad, S.; Vidal, H.; Ali, A.B. Simultaneous water gas shift and methanation reactions on Ru/Ce0.8Tb0.2O2−x based catalysts. Catal. Today 2012, 180, 42–50. [Google Scholar] [CrossRef]
- Senanayake, S.D.; Evans, J.; Agnoli, S.; Barrio, L.; Chen, T.; Hrbek, J.; Rodriguez, J.A. Water-Gas Shift and CO Methanation Reactions over Ni–CeO2(111) Catalysts. Top. Catal. 2011, 54, 34–41. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, J.Y.; Lee, D.; Moon, D.J.; Lee, K. The effect of Zn addition into NiFe2O4 catalyst for high-temperature shift reaction of natural gas reformate assuming no external steam addition. Int. J. Hydrog. Energy 2012, 37, 11218–11226. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Yousef, A.M.I.; Eldrainy, Y.A.; El-Maghlany, W.M.; Attia, A. Upgrading biogas by a low-temperature CO2 removal technique. Alex. Eng. J. 2016, 55, 1143–1150. [Google Scholar] [CrossRef]
- Campo, M.C.; Ribeiro, A.M.; Ferreira, A.F.P.; Santos, J.C.; Lutz, C.; Loureiro, J.M.; Rodrigues, A.E. Carbon dioxide removal for methane upgrade by a VSA process using an improved 13X zeolite. Fuel Process. Technol. 2016, 143, 185–194. [Google Scholar] [CrossRef]
- Lestinsky, P.; Vecer, M.; Navratil, P.; Stehlik, P. The removal of CO2 from biogas using a laboratory PSA unit: Design using breakthrough curves. Clean Technol. Environ. Policy 2015, 17, 1281–1289. [Google Scholar] [CrossRef]
- Guo, F.; Xu, J.; Chu, W. CO2 reforming of methane over Mn promoted Ni/Al2O3 catalyst treated by N2 glow discharge plasma. Catal. Today 2015, 256, 124–129. [Google Scholar] [CrossRef]
- Yao, L.; Zhu, J.; Peng, X.; Tong, D.; Hu, C. Comparative study on the promotion effect of Mn and Zr on the stability of Ni/SiO2 catalyst for CO2 reforming of methane. Int. J. Hydrog. Energy 2013, 38, 7268–7279. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, J.; Zhang, M.; Li, H.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. Highly active and stable Ni/γ-Al2O3 catalysts selectively deposited with CeO2 for CO methanation. RSC Adv. 2014, 4, 16094–16103. [Google Scholar] [CrossRef]


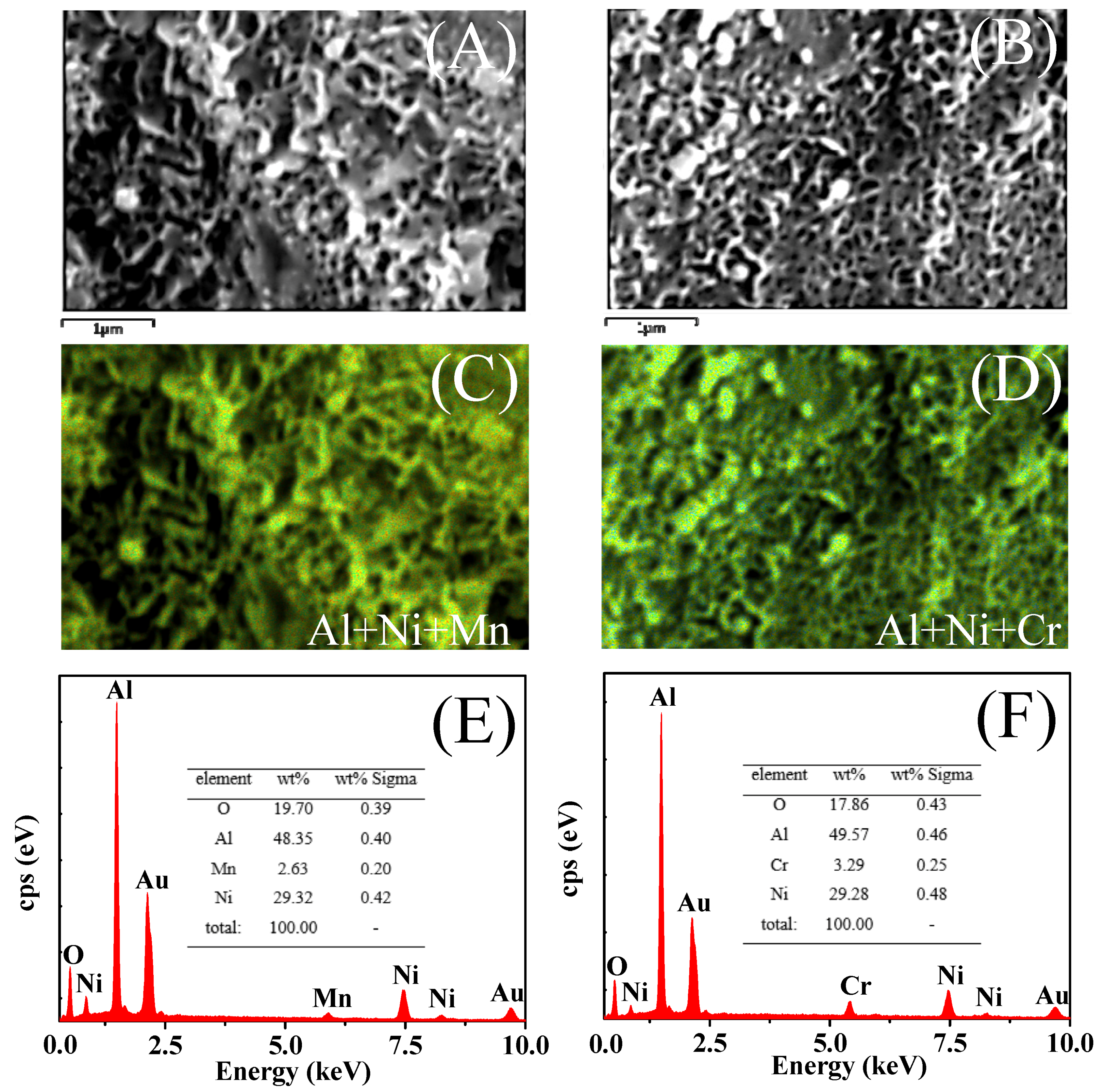

| Samples | Ni wt % a | M wt % a | SBET (m2∙g−1) b | Vp (cm3∙g−1) c | dp,peak (nm) d | Average Pore Size (nm) e | Ni Size (nm) f | H2 Uptake (μmol∙g−1) | D (%) g | TOFCO (s−1) h | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reduced | Spent | ||||||||||
| γ-Al2O3 | - | - | 241.46 | 0.34 | 3.82 | 6.62 | - | - | - | - | - |
| 15Ni | 14.6 | - | 179.15 | 0.31 | 3.72 | 7.02 | 17.0 | 18.3 | 385.0 | 36.1 | 0.0025 |
| 15Ni-3Mo | 13.7 | 2.7 | 167.07 | 0.27 | 3.79 | 6.35 | 16.5 | 17.1 | 294.5 | 31.2 | 0.0024 |
| 15Ni-3Fe | 14.4 | 3.2 | 162.18 | 0.29 | 3.68 | 7.26 | 22.6 | 23.3 | 235.1 | 21.2 | 0.0020 |
| 15Ni-3Co | 13.8 | 2.5 | 160.13 | 0.28 | 3.68 | 7.06 | 22.8 | 23.8 | 342.3 | 27.1 | 0.0023 |
| 15Ni-3Mn | 14.7 | 3.1 | 159.01 | 0.28 | 3.68 | 7.03 | 13.2 | 13.4 | 416.8 | 41.0 | 0.0040 |
| 15Ni-3Cr | 15.5 | 3.1 | 179.96 | 0.27 | 3.79 | 7.10 | 12.1 | 13.7 | 395.5 | 42.8 | 0.0031 |
| Samples | Carbon Deposition Rate a (mmol∙min−1) | Carbon Deposition b (wt %) |
|---|---|---|
| 15Ni | 4.882 | 1.82 |
| 15Ni-3Mo | 6.425 | 1.95 |
| 15Ni-3Fe | 3.870 | 1.54 |
| 15Ni-3Co | 4.212 | 1.69 |
| 15Ni-3Mn | 5.305 | 2.40 |
| 15Ni-3Cr | 7.450 | 3.06 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Song, M.; Jin, B.; Zhou, Z.; Yang, X. The Synergy Effect of Ni-M (M = Mo, Fe, Co, Mn or Cr) Bicomponent Catalysts on Partial Methanation Coupling with Water Gas Shift under Low H2/CO Conditions. Catalysts 2017, 7, 51. https://doi.org/10.3390/catal7020051
Dong X, Song M, Jin B, Zhou Z, Yang X. The Synergy Effect of Ni-M (M = Mo, Fe, Co, Mn or Cr) Bicomponent Catalysts on Partial Methanation Coupling with Water Gas Shift under Low H2/CO Conditions. Catalysts. 2017; 7(2):51. https://doi.org/10.3390/catal7020051
Chicago/Turabian StyleDong, Xinxin, Min Song, Baosheng Jin, Zheng Zhou, and Xu Yang. 2017. "The Synergy Effect of Ni-M (M = Mo, Fe, Co, Mn or Cr) Bicomponent Catalysts on Partial Methanation Coupling with Water Gas Shift under Low H2/CO Conditions" Catalysts 7, no. 2: 51. https://doi.org/10.3390/catal7020051
APA StyleDong, X., Song, M., Jin, B., Zhou, Z., & Yang, X. (2017). The Synergy Effect of Ni-M (M = Mo, Fe, Co, Mn or Cr) Bicomponent Catalysts on Partial Methanation Coupling with Water Gas Shift under Low H2/CO Conditions. Catalysts, 7(2), 51. https://doi.org/10.3390/catal7020051






