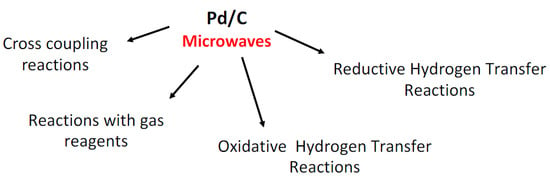
Pd/C Catalysis under Microwave Dielectric Heating
Abstract
:1. Introduction
2. Pd/C in Cross-Coupling Reactions under MW Heating
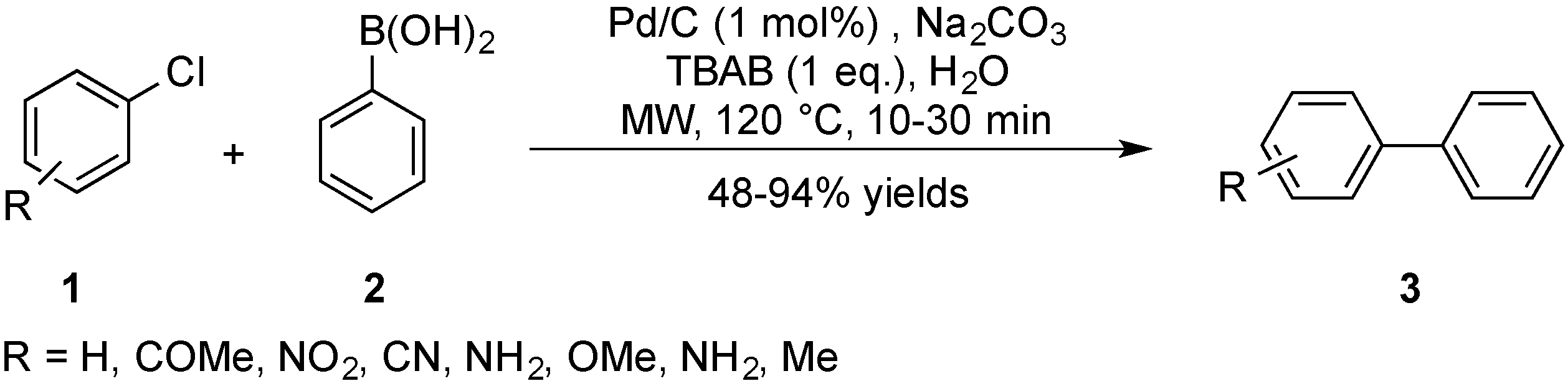
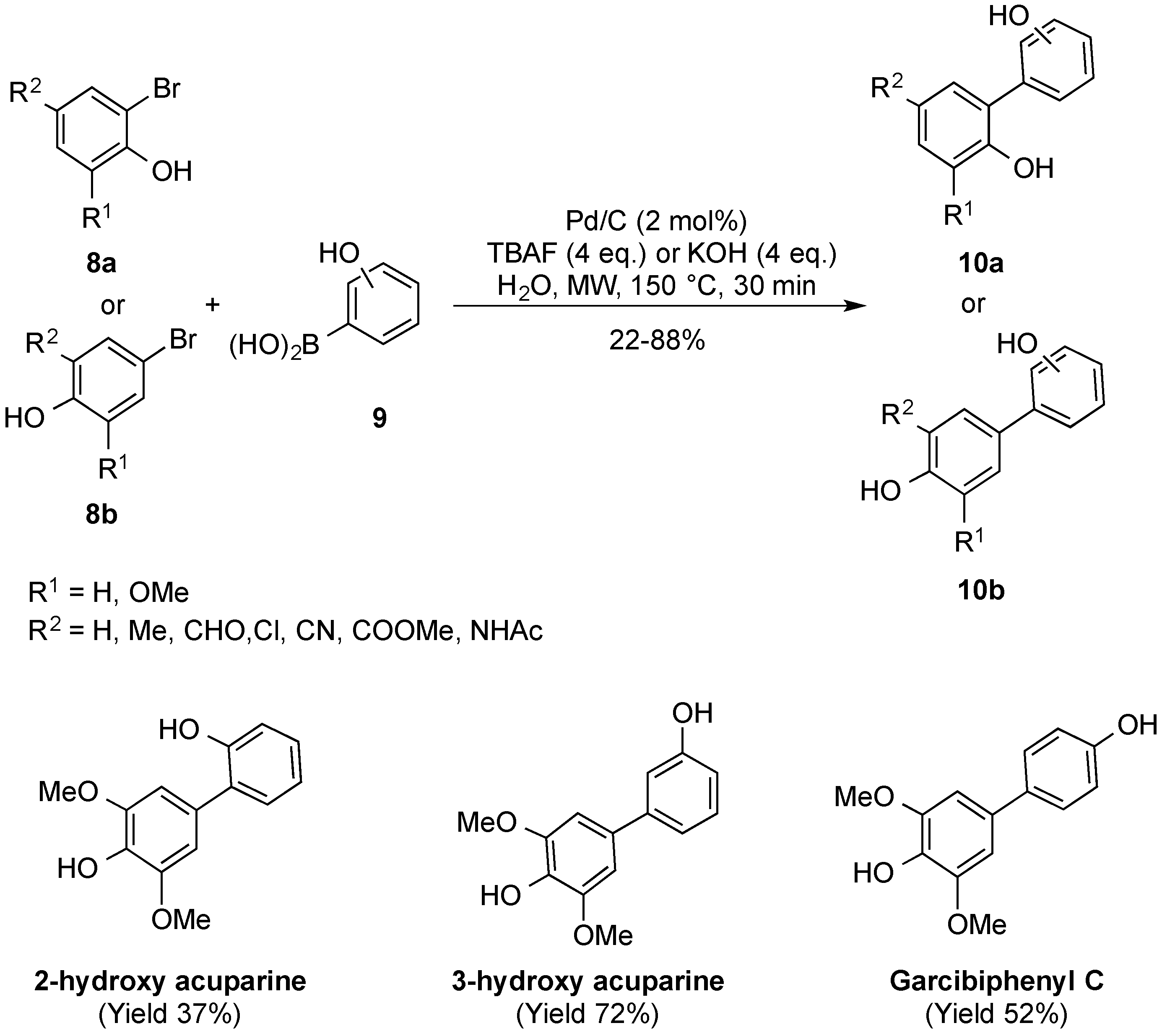
2.1. Suzuki–Miyaura Coupling
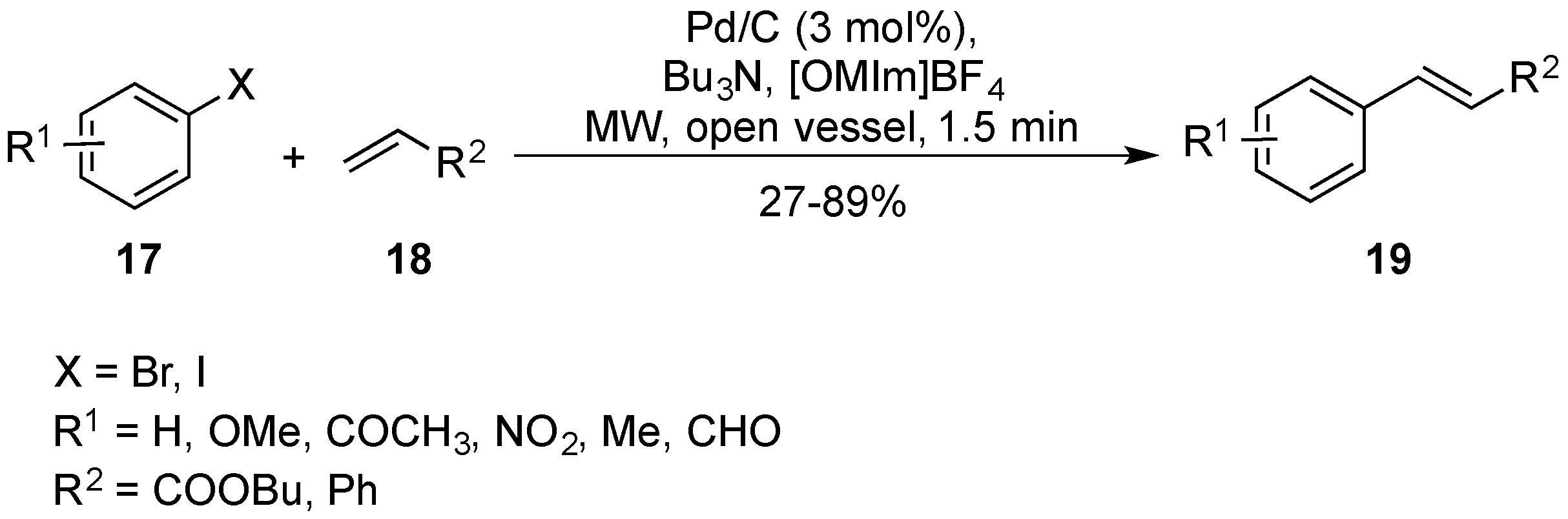
2.2. Pd/C for Mizoroki–Heck under MW Heating
2.3. Pd/C for Sonogashira Coupling under MW Heating
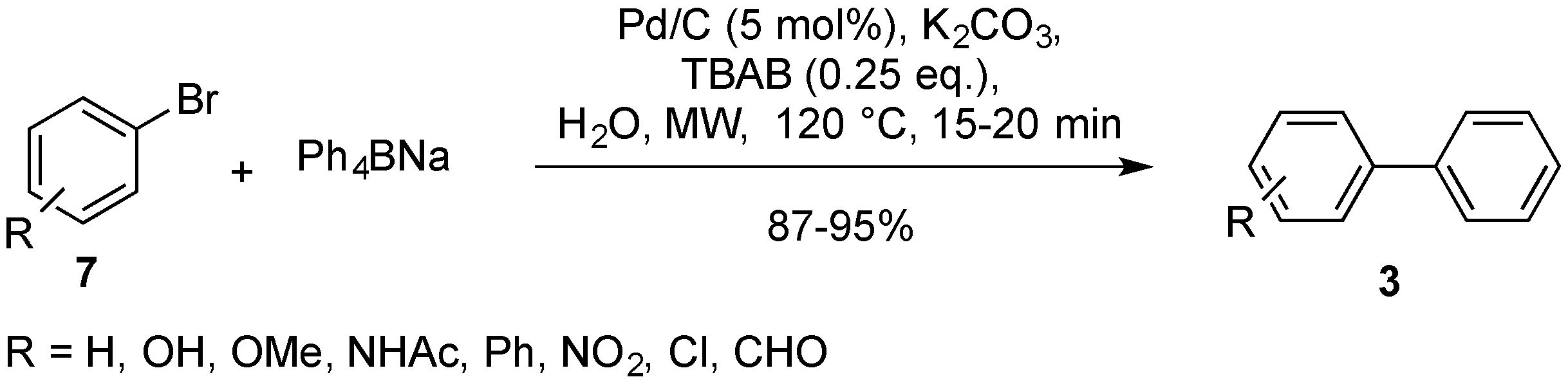
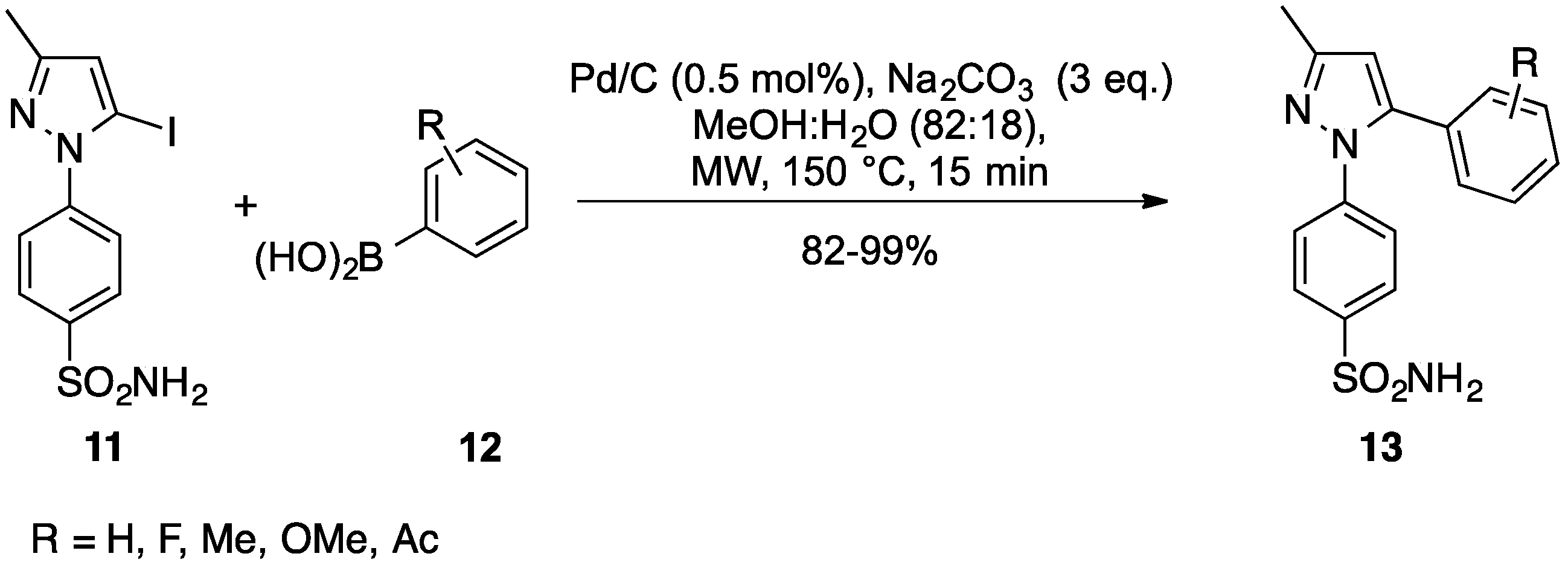
2.4. Other Pd/C-Catalyzed Cross-Couplings
3. Pd/C for MW-Assisted Reactions with Gas Reagents
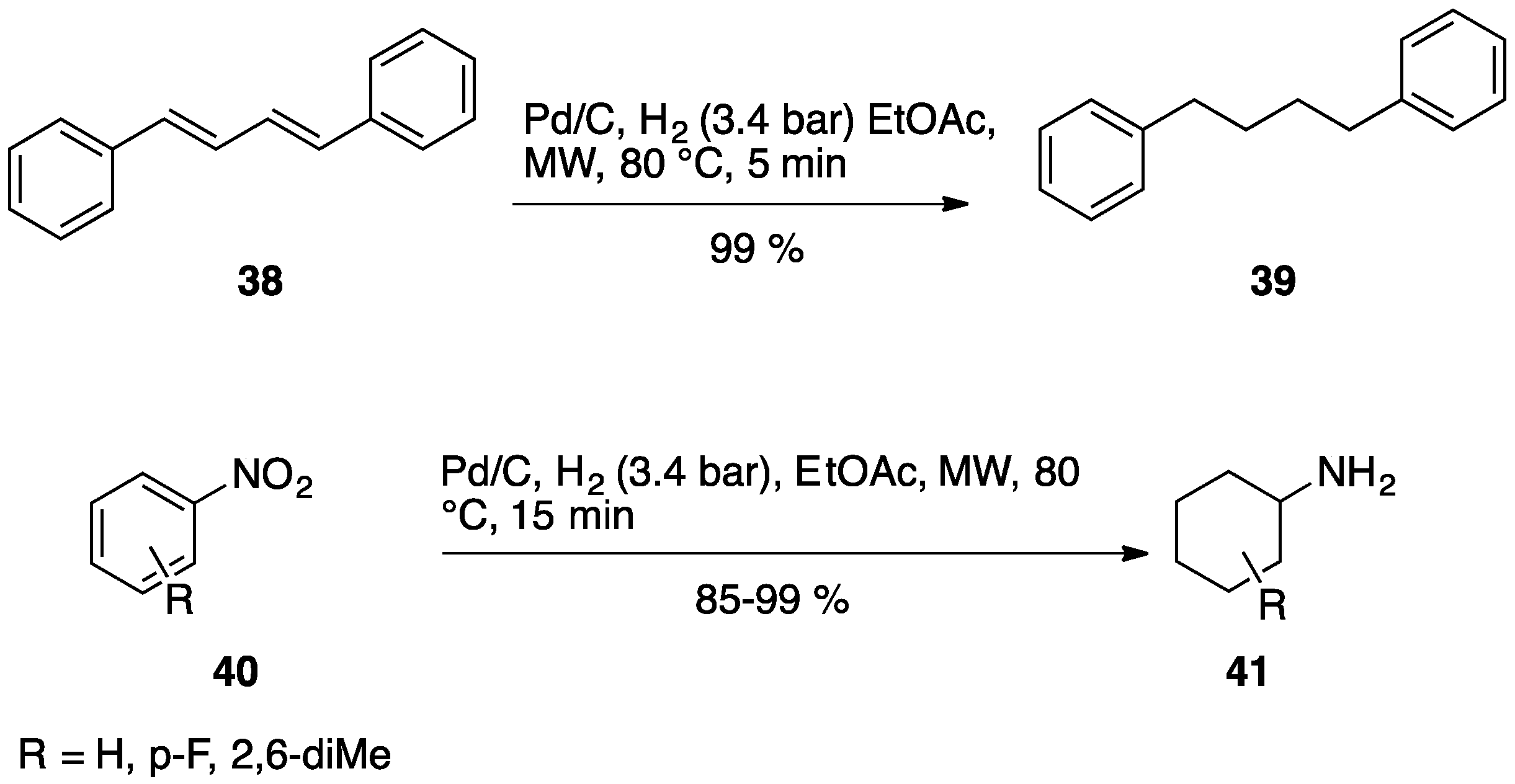
3.1. Hydrogen
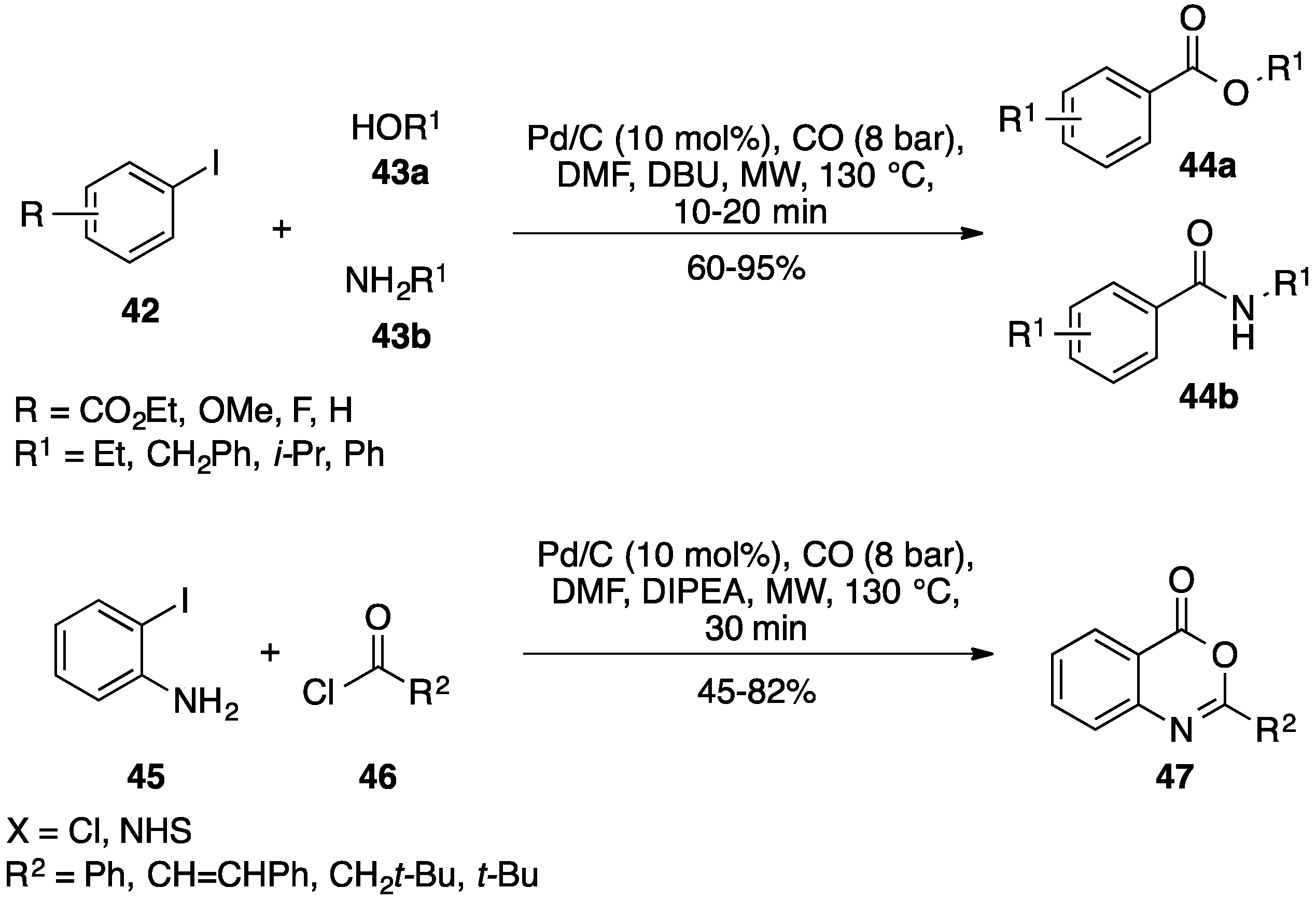
3.2. Carbon Monoxide
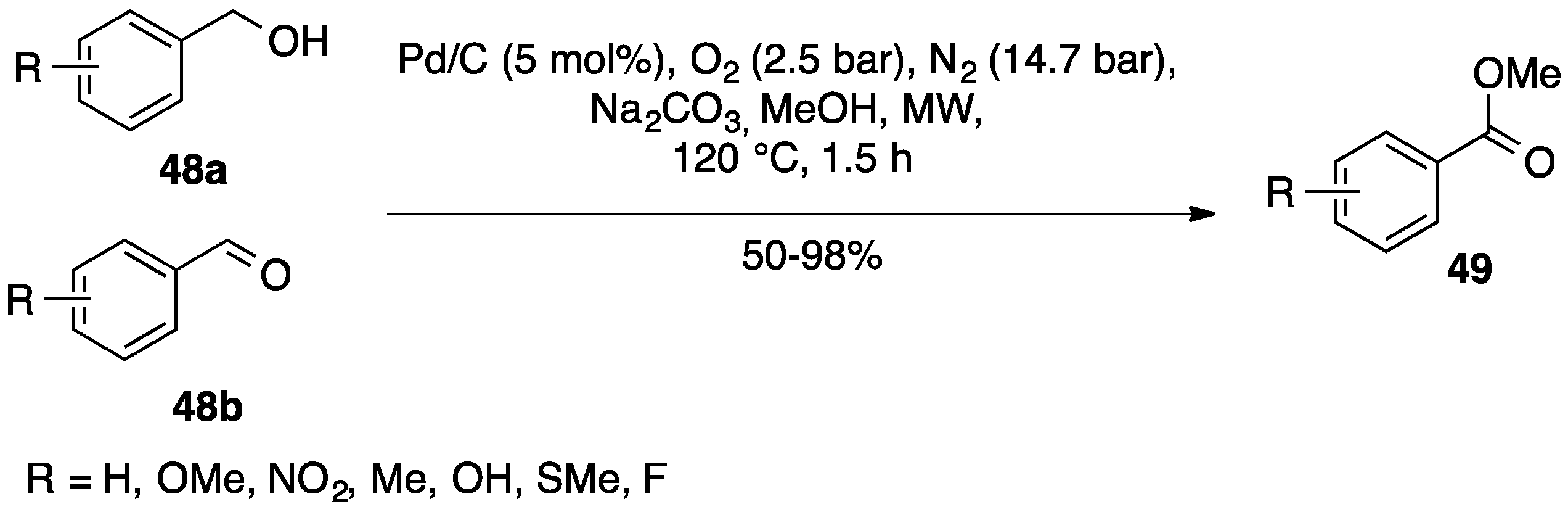
3.3. Oxygen
4. Pd/C for Reductive Hydrogen Transfer Reactions
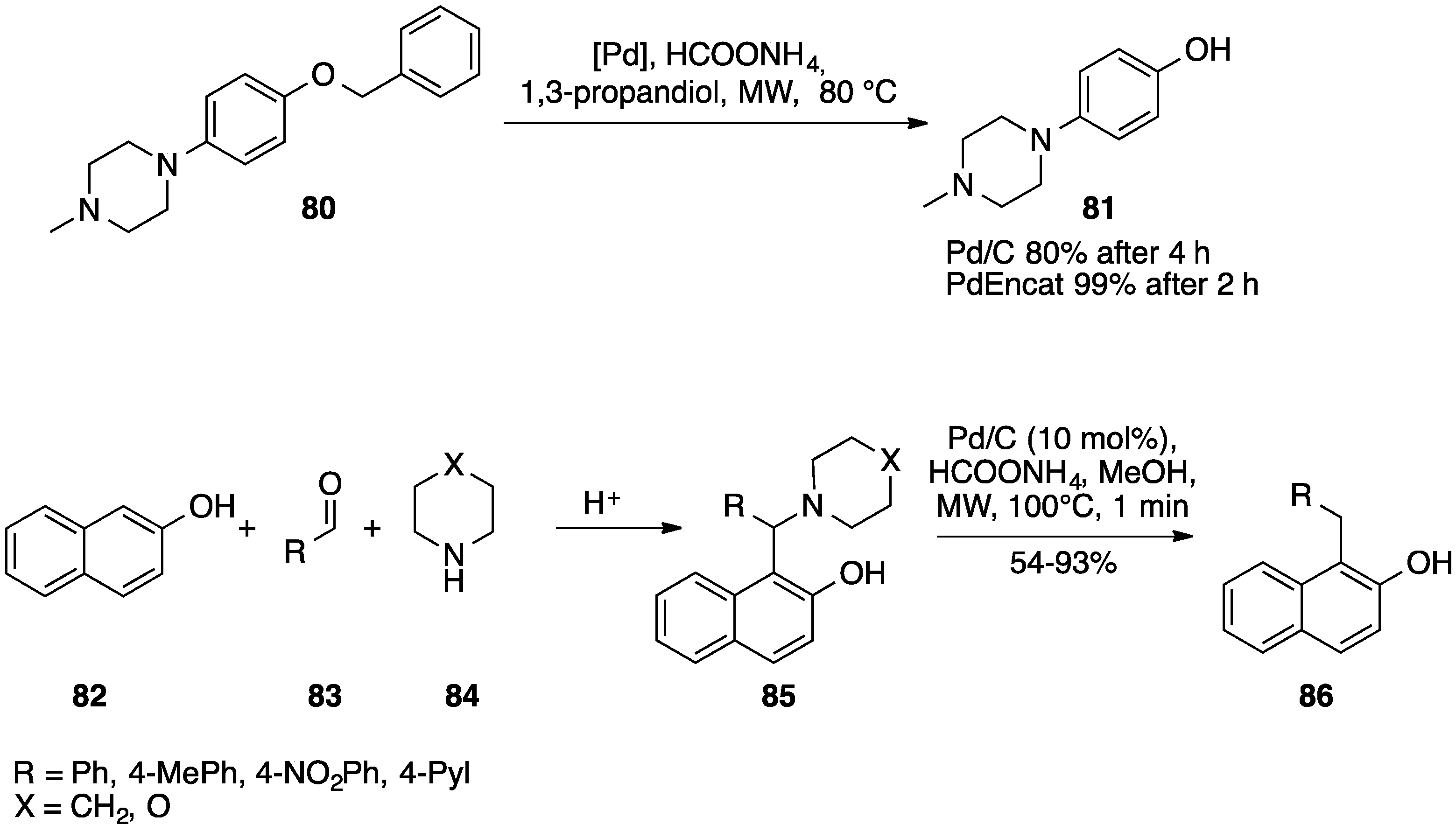
4.1. Formates as Hydrogen Source
- (i)
- some examples of a rapid cinnamate hydrogenation [56]
- (ii)
- the preparation of a key intermediate (57) for the first synthesis of the unusual nitrogen-containing N-(3-carboxylpropyl)-5-amino-2-hydroxy-3-tridecyl-1,4-benzoquinone isolated from Embelia ribes [57];
- (iii)
- the synthesis of novel selective σ1 opioid ligands (59) [58];
- (iv)
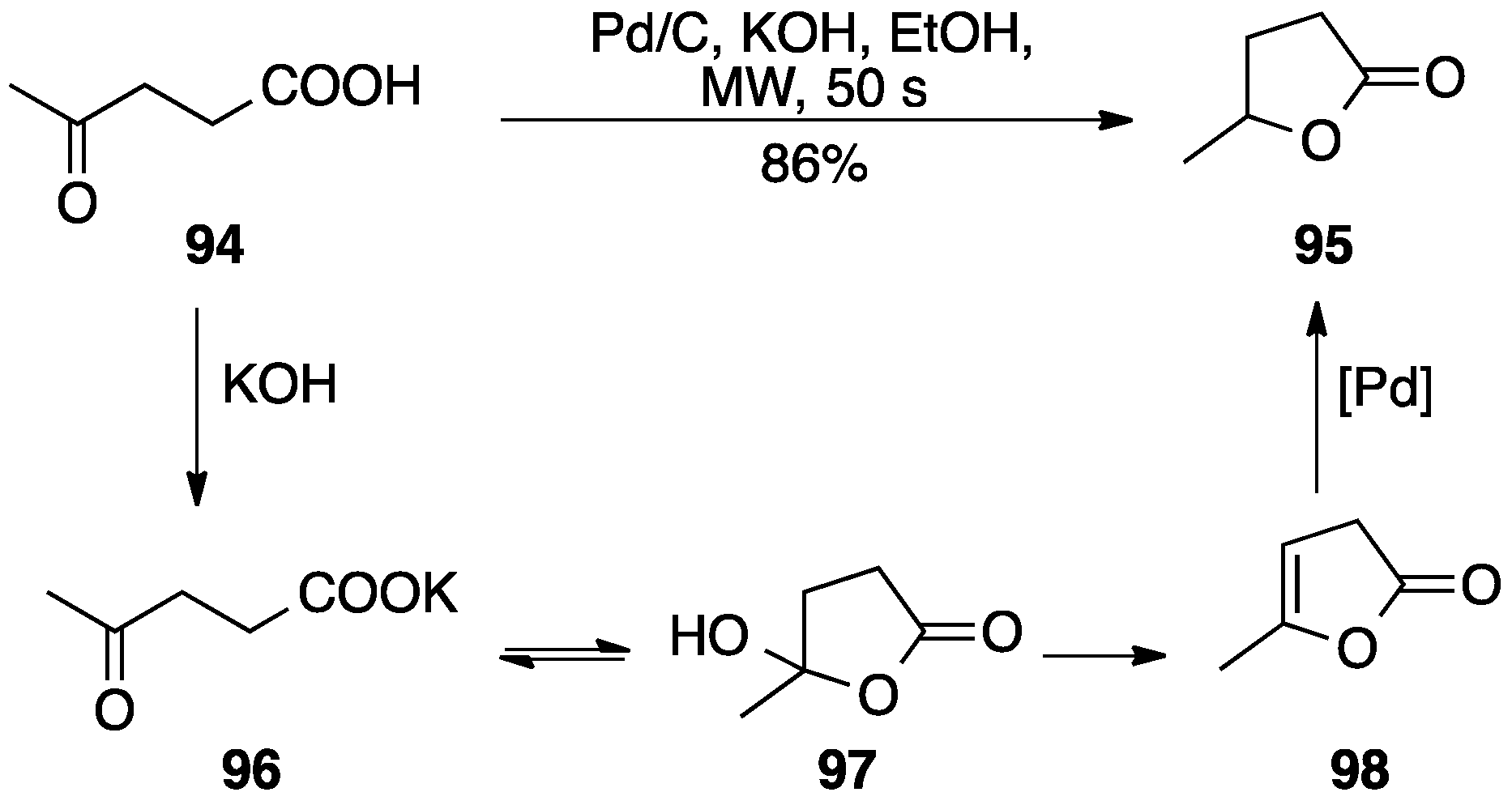
4.2. Other Hydrogen Sources
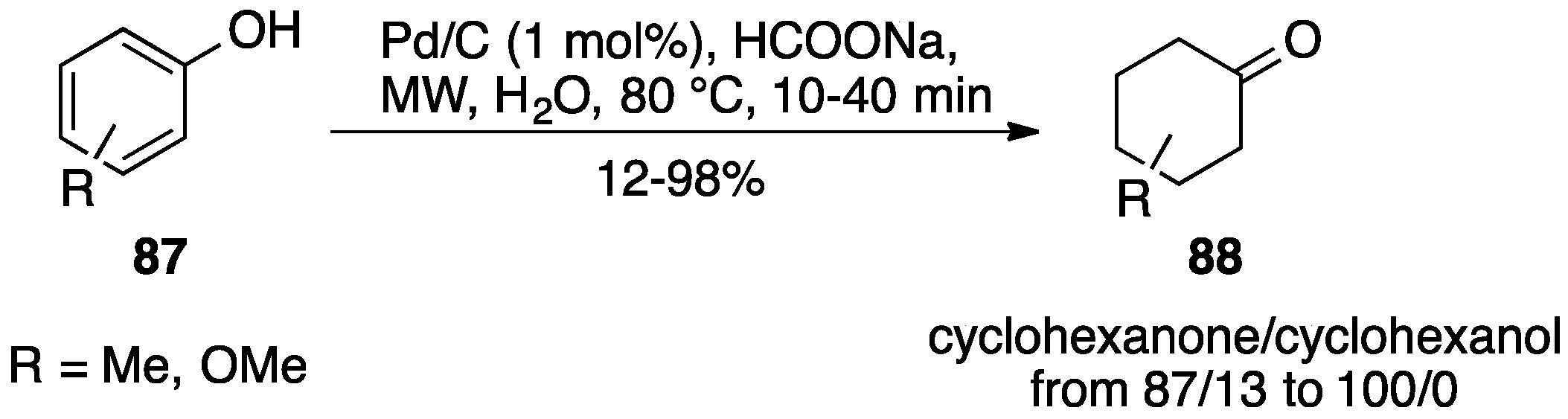
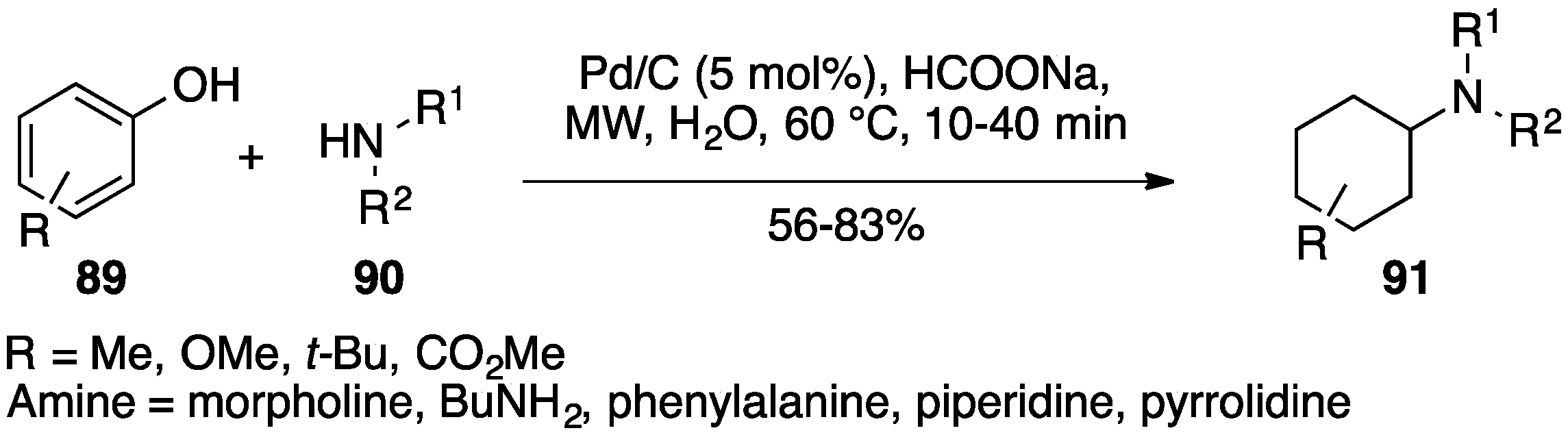
5. Pd/C in Oxidative Hydrogen Transfer Reactions
6. Other Pd/C-Catalyzed Transformations
7. Conclusions
8. Addendum
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thomas, J.M.; John, M.; Thomas, W.J. Principles and Practice of Heterogeneous Catalysis; VCH: Weinheim, Germany, 1997. [Google Scholar]
- Tsuji, J. Palladium Reagents and Catalysts : New Perspectives for the 21st Century; Wiley: Weinheim, Germany, 2004. [Google Scholar]
- Irfan, M.; Fuchs, M.; Glasnov, T.N.; Kappe, C.O. Microwave-assisted cross-coupling and hydrogenation chemistry by using heterogeneous transition-metal catalysts: An evaluation of the role of selective catalyst heating. Chem. A Eur. J. 2009, 15, 11608–11618. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, M.; Möllmann, K.-P.; Karstädt, D. Microwave oven experiments with metals and light sources. Phys. Educ. 2004, 39, 500–508. [Google Scholar] [CrossRef]
- Chen, W.; Gutmann, B.; Kappe, C.O. Characterization of microwave-induced electric discharge phenomena in metal–solvent mixtures. ChemistryOpen 2012, 1, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, A.G.; Mingos, D.M.P. Arcing and other microwave characteristics of metal powders in liquid systems. J. Chem. Soc. Dalt. Trans. 2000, 29, 1521–1526. [Google Scholar] [CrossRef]
- Horikoshi, S.; Osawa, A.; Abe, M.; Serpone, N. On the generation of hot-spots by microwave electric and magnetic fields and their impact on a microwave-assisted heterogeneous reaction in the presence of metallic Pd nanoparticles on an activated carbon support. J. Phys. Chem. C 2011, 115, 23030–23035. [Google Scholar] [CrossRef]
- Horikoshi, S.; Kamata, M.; Mitani, T.; Serpone, N. Control of microwave-generated hot spots. 6. Generation of hot spots in dispersed catalyst particulates and factors that affect catalyzed organic syntheses in heterogeneous media. Ind. Eng. Chem. Res. 2014, 53, 14941–14947. [Google Scholar] [CrossRef]
- Felpin, F.-X. Ten years of adventures with Pd/C catalysts: From reductive processes to coupling reactions. Synlett 2014, 25, 1055–1067. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. Role of microwaves in heterogeneous catalytic systems. Catal. Sci. Technol. 2014, 4, 1197. [Google Scholar] [CrossRef]
- Reay, A.J.; Fairlamb, I.J.S. Catalytic C–H bond functionalisation chemistry: The case for quasi-heterogeneous catalysis. Chem. Commun. 2015, 51, 16289–16307. [Google Scholar] [CrossRef] [PubMed]
- Felpin, F.X.; Ayad, T.; Mitra, S. Pd/C: An old catalyst for new applications—Its use for the Suzuki–Miyaura reaction. Eur. J. Org. Chem. 2006, 2679–2690. [Google Scholar] [CrossRef]
- Marck, G.; Villiger, A.; Buchecker, R. Aryl couplings with heterogeneous palladium catalysts. Tetrahedron Lett. 1994, 35, 3277–3280. [Google Scholar] [CrossRef]
- Conlon, D.A.; Pipik, B.; Ferdinand, S.; LeBlond, C.R.; Sowa, J.R.; Izzo, B.; Collins, P.; Ho, G.-J.; Williams, J.M.; Shi, Y.-J.; Sun, Y. Suzuki–Miyaura Cross-Coupling With Quasi-Heterogeneous Palladium. Adv. Synth. Catal. 2003, 345, 931–935. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Van Der Sluys, M.; Jones, C.W. On the Nature of the Active Species in Palladium Catalyzed Mizoroki–Heck and Suzuki–Miyaura Couplings—Homogeneous or Heterogeneous Catalysis, A Critical Review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Arvela, R.K.; Leadbeater, N.E. Suzuki coupling of aryl chlorides with phenylboronic acid in water, using microwave heating with simultaneous cooling. Org. Lett. 2005, 7, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Hayes, B.L. Recent Advances in Microwave - Assisted Synthesis. Aldrichimica Acta 2004, 37, 66–76. [Google Scholar]
- Bowman, M.D.; Schmink, J.R.; McGowan, C.M.; Kormos, C.M.; Leadbeater, N.E. Scale-up of microwave-promoted reactions to the multigram level using a sealed-vessel microwave apparatus. Org. Process Res. Dev. 2008, 12, 1078–1088. [Google Scholar] [CrossRef]
- Freundlich, J.S.; Landis, H.E. An expeditious aqueous Suzuki–Miyaura method for the arylation of bromophenols. Tetrahedron Lett. 2006, 47, 4275–4279. [Google Scholar] [CrossRef]
- Bai, L. Atom-efficient coupling reaction of aryl bromides with sodium tetraphenylborate catalyzed by reusable Pd/C in water under focused microwave irradiation. Chin. Chem. Lett. 2009, 20, 158–160. [Google Scholar] [CrossRef]
- Schmidt, B.; Riemer, M.; Karras, M. 2,2???-Biphenols Via Protecting Group-Free Thermal or Microwave-Accelerated Suzuki–Miyaura Coupling in Water. J. Org. Chem. 2013, 78, 8680–8688. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Riemer, M. Suzuki–Miyaura coupling of halophenols and phenol boronic acids: Systematic investigation of positional isomer effects and conclusions for the synthesis of phytoalexins from pyrinae. J. Org. Chem. 2014, 79, 4104–4118. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Keilman, J.; Pabba, C.; Chen, Z.J.; Gregg, B.T. Microwave-assisted cross-coupling of 3-chloro-2-pyrazolines and 3-chloro-1-phenyl-1,4,5,6-tetrahydropyridazine with aryl boronic acids. Tetrahedron Lett. 2005, 46, 2631–2634. [Google Scholar] [CrossRef]
- Wannberg, J.; Dallinger, D.; Kappe, C.O.; Larhed, M. Microwave-enhanced and metal-catalyzed functionalizations of the 4-aryl-dihydropyrimidone template. J. Comb. Chem. 2005, 7, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Quagliotto, P.; Barbero, N.; Barolo, C.; Buscaino, R.; Carfora, P.; Prosperini, S.; Viscardi, G. Water based surfactant-assisted synthesis of thienylpyridines and thienylbipyridine intermediates. Dye. Pigment. 2017, 137, 468–479. [Google Scholar] [CrossRef]
- Horikoshi, S.; Osawa, A.; Suttisawat, Y.; Abe, M.; Serpone, N. A novel dewar-like reactor for maintaining constant heat and enhancing product yields during microwave-assisted organic syntheses. Org. Process Res. Dev. 2010, 14, 1453–1456. [Google Scholar] [CrossRef]
- Horikoshi, S.; Osawa, A.; Sakamoto, S.; Serpone, N. Control of microwave-generated hot spots. Part V. Mechanisms of hot-spot generation and aggregation of catalyst in a microwave-assisted reaction in toluene catalyzed by Pd-loaded AC particulates. Appl. Catal. A Gen. 2013, 460–461, 52–60. [Google Scholar] [CrossRef]
- Organ, M.G.; Mayer, S.; Lepifre, F.; N’Zemba, B.; Khatri, J. Combining the use of solid-supported transition metal catalysis with microwave irradiation in solution-phase parallel library synthesis. Mol. Divers. 2003, 7, 211–227. [Google Scholar] [CrossRef] [PubMed]
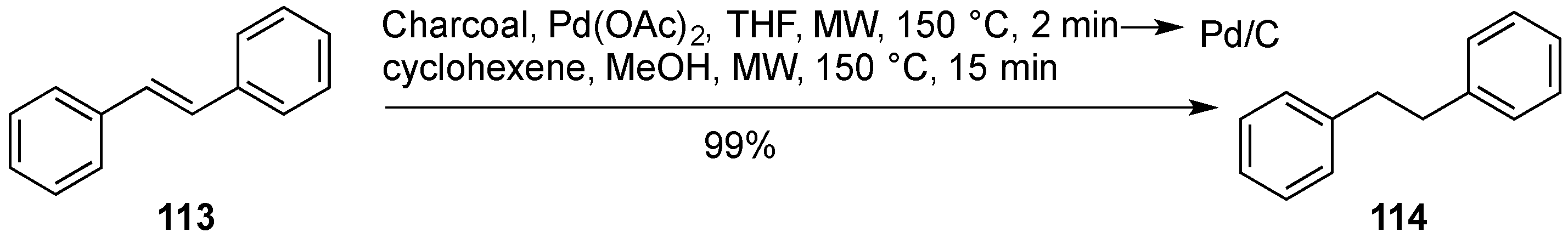
- Cravotto, G.; Beggiato, M.; Penoni, A.; Palmisano, G.; Tollari, S.; Lévêque, J.M.; Bonrath, W. High-intensity ultrasound and microwave, alone or combined, promote Pd/C-catalyzed aryl-aryl couplings. Tetrahedron Lett. 2005, 46, 2267–2271. [Google Scholar] [CrossRef]
- García-Suárez, E.J.; Lara, P.; García, A.B.; Ojeda, M.; Luque, R.; Philippot, K. Efficient and recyclable carbon-supported Pd nanocatalysts for the Suzuki–Miyaura reaction in aqueous-based media: Microwave vs conventional heating. Appl. Catal. A Gen. 2013, 468, 59–67. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, H.; Liu, T.; Cao, R. Monodisperse noble metal nanoparticles stabilized in SBA-15: Synthesis, characterization and application in microwave-assisted Suzuki–Miyaura coupling reaction. J. Catal. 2010, 270, 268–274. [Google Scholar] [CrossRef]
- Wali, A.; Pillai, S.M.; Satish, S. Heterogeneous Pd catalysts and microwave irradiation in heck arylation. React. Kinet. Catal. Lett. 1997, 60, 189–194. [Google Scholar] [CrossRef]
- Xie, X.; Lu, J.; Chen, B.; Han, J.; She, X.; Pan, X. Pd/C-catalyzed Heck reaction in ionic liquid accelerated by microwave heating. Tetrahedron Lett. 2004, 45, 809–811. [Google Scholar] [CrossRef]
- Palmisano, G.; Bonrath, W.; Boffa, L.; Garella, D.; Barge, A.; Cravotto, G. Heck reactions with very low ligandless catalyst loads accelerated by microwaves or simultaneous microwaves/ultrasound irradiation. Adv. Synth. Catal. 2007, 349, 2338–2344. [Google Scholar] [CrossRef]
- Jeffery, T. Palladium-catalysed vinylation of organic halides under solid–liquid phase transfer conditions. J. Chem. Soc., Chem. Commun. 1984, 1287–1289. [Google Scholar] [CrossRef]
- Glasnov, T.N.; Findenig, S.; Kappe, C.O. Heterogeneous versus homogeneous palladium catalysts for ligandless Mizoroki–Heck reactions: A comparison of batch/microwave and continuous-flow processing. Chem. A Eur. J. 2009, 15, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.A.; Kremsner, J.M.; Kappe, C.O. Nonthermal microwave effects revisited: On the importance of internal temperature monitoring and agitation in microwave chemistry. J. Org. Chem. 2008, 73, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Janowska, I.; Chizari, K.; Olivier, J.H.; Ziessel, R.; Ledoux, M.J.; Pham-Huu, C. A new recyclable Pd catalyst supported on vertically aligned carbon nanotubes for microwaves-assisted Heck reactions. Comptes Rendus Chim. 2011, 14, 663–670. [Google Scholar] [CrossRef]
- Begouin, A.; Queiroz, M.J.P. Tandem palladium/charcoal-copper(I) iodide (Pd/C-CuI) catalyzed Sonogashira coupling and intramolecular cyclization from 2-bromonicotinic acid (=2-bromopyridine-3-carboxylic acid) and ethynylarenes to 4-azaphthalides (=furo[3,4-b]pyridin-5(7H)-ones) and 5. Helv. Chim. Acta 2011, 94, 1792–1801. [Google Scholar] [CrossRef]
- Stadler, A.; Kappe, C.O. Rapid formation of triarylphosphines by microwave-assisted transition metal-catalyzed C-P cross-coupling reactions. Org. Lett. 2002, 4, 3541–3543. [Google Scholar] [CrossRef] [PubMed]
- Rummelt, S.M.; Ranocchiari, M.; Van Bokhoven, J.A. Synthesis of water-soluble phosphine oxides by Pd/C-catalyzed P-C coupling in water. Org. Lett. 2012, 14, 2188–2190. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Weng, J.; Zheng, Z.; Zhu, X.; Cai, Y.; Cai, J.; Wan, Y. Pd/C-catalyzed cyanation of aryl halides in aqueous PEG. Eur. J. Org. Chem. 2008, 3524–3528. [Google Scholar] [CrossRef]
- Petricci, E.; Taddei, M. Microwave assisted reactions with gas reagents. Chim. Oggi 2007, 25, 40–45. [Google Scholar]
- Heller, E.; Lautenschläger, W.; Holzgrabe, U. Microwave-enhanced hydrogenations at medium pressure using a newly constructed reactor. Tetrahedron Lett. 2005, 46, 1247–1249. [Google Scholar] [CrossRef]
- Vanier, G.S. Simple and efficient microwave-assisted hydrogenation reactions at moderate temperature and pressure. Synlett 2007, 131–135. [Google Scholar] [CrossRef]
- Piras, L.; Genesio, E.; Ghiron, C.; Taddei, M. Microwave-assisted hydrogenation of pyridines. Synlett 2008, 1125–1128. [Google Scholar]
- Irfan, M.; Petricci, E.; Glassnov, T.N.; Taddei, M.; Kappe, C.O. Continuous flow hydrogenation of functionalized pyridines. Eur. J. Org. Chem. 2009, 1327–1334. [Google Scholar] [CrossRef]
- Brennführer, A.; Neumann, H.; Beller, M. Palladium-Catalyzed Carbonylation Reactions of Aryl Halides and Related Compounds. Angew. Chemie Int. Ed. 2009, 48, 4114–4133. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, J.; Balducci, E.; Zaza, S.; Petricci, E.; Taddei, M. Microwave-Assisted Carbonylation and Cyclocarbonylation of Aryl Iodides under Ligand Free Heterogeneous Catalysis. J. Org. Chem. 2010, 75, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, M.; Cravotto, G.; Georgakopoulos, S.; Heropoulos, G.; Martina, K.; Tagliapietra, S. Pd/C-catalyzed aerobic oxidative esterification of alcohols and aldehydes: A highly efficient microwave-assisted green protocol. Beilstein J. Org. Chem. 2014, 10, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.K.; Manhas, M.S.; Ghosh, M.; Shah, M.; Raju, V.S.; Bari, S.S.; Newaz, S.N.; Banik, B.K.; Chaudhary, A.G.; Barakat, K.J. Microwave-induced organic reaction enhancement chemistry. 2. Simplified techniques. J. Org. Chem. 1991, 56, 6968–6970. [Google Scholar] [CrossRef]
- Bose, A.K.; Banik, B.K.; Barakat, K.J.; Manhas, M.S. Simplified rapid hydrogenation under microwave irradiation: selective transformationof beta-lactams. Synlett 1993, 575–576. [Google Scholar] [CrossRef]
- Bose, A.K.; Banik, B.K.; Mathur, C.; Wagle, D.R.; Manhas, M.S. Polyhydroxy Amino Acid Derivatives via β-Lactams Using Enantiospecific Approaches and Microwave Techniques. Tetrahedron 2000, 56, 5603–5619. [Google Scholar] [CrossRef]
- Smidovnik, A.; Kobe, J.; Leskovsek, S.; Koloini, T. Kinetics of catalytic transfer hydrogenation of soybean oil. J. Am. Oil Chem. Soc. 1994, 71, 507–511. [Google Scholar] [CrossRef]
- Dayal, B.; Ertel, N.H.; Rapole, K.R.; Asgaonkar, A.; Salen, G. Rapid hydrogenation of unsaturated sterols and bile alcohols using microwaves. Steroids 1997, 62, 451–454. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Sinha, A.K. Solid-supported green synthesis of substituted hydrocinnamic esters by focused microwave irradiation. Helv. Chim. Acta 2006, 89, 483–495. [Google Scholar] [CrossRef]
- Mcerlean, C.S.P.; Moody, C.J. First Synthesis of tridecyl-1, 4-benzoquinone, an Unusual Quinone Isolated from Embelia ribes. J. Org. Chem. 2007, 72, 10298–10301. [Google Scholar] [CrossRef] [PubMed]
- Collina, S.; Loddo, G.; Urbano, M.; Linati, L.; Callegari, A.; Ortuso, F.; Alcaro, S.; Laggner, C.; Langer, T.; Prezzavento, O.; Ronsisvalle, G.; Azzolina, O. Design, synthesis, and SAR analysis of novel selective sigma1 ligands. Bioorg. Med. Chem. 2007, 15, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Fustero, S.; Sánchez-Roselló, M.; Aceña, J.L.; Fernández, B.; Asensio, A.; Sanz-Cervera, J.F.; Del Pozo, C. Cross-metathesis reactions as an efficient tool in the synthesis of fluorinated cyclic β-amino acids. J. Org. Chem. 2009, 74, 3414–3423. [Google Scholar] [CrossRef] [PubMed]
- Cini, E.; Bifulco, G.; Menchi, G.; Rodriquez, M.; Taddei, M. Synthesis of enantiopure 7-substituted azepane-2-carboxylic acids as templates for conformationally constrained peptidomimetics. European J. Org. Chem. 2012, 2133–2141. [Google Scholar] [CrossRef]
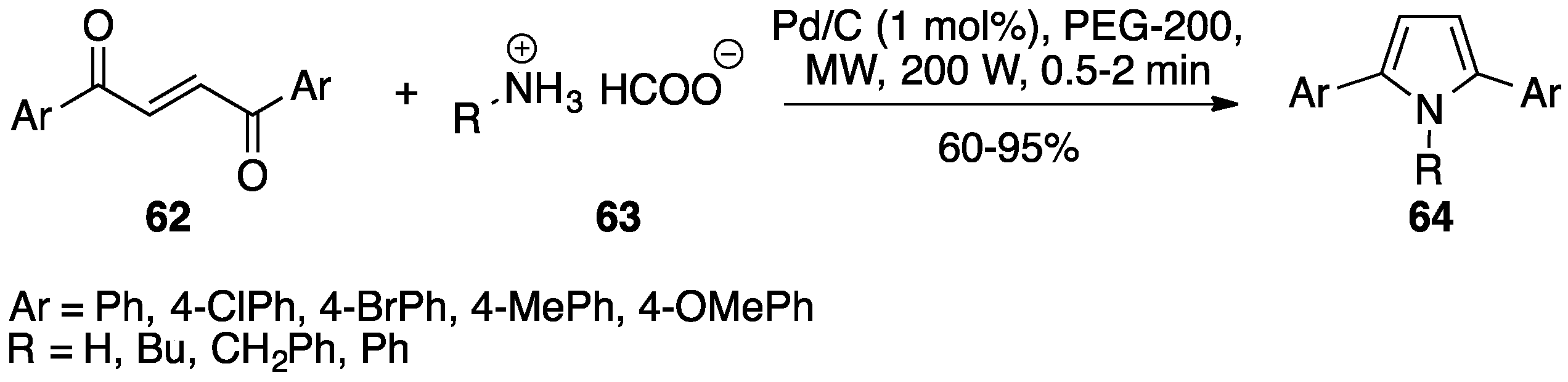
- Rao, H.S.P.; Jothilingam, S.; Scheeren, H.W. Microwave mediated facile one-pot synthesis of polyarylpyrroles from but-2-ene- and but-2-yne-1,4-diones. Tetrahedron 2004, 60, 1625–1630. [Google Scholar] [CrossRef]
- Testard, A.; Logé, C.; Léger, B.; Robert, J.M.; Lozach, O.; Blairvacq, M.; Meijer, L.; Thiéry, V.; Besson, T. Thiazolo[5,4-f]quinazolin-9-ones, inhibitors of glycogen synthase kinase-3. Bioorganic Med. Chem. Lett. 2006, 16, 3419–3423. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, F.R.; Berecibar, A.; Wrigglesworth, R.; Besson, T. Efficient synthesis of thiazoloquinazolinone derivatives. Tetrahedron Lett. 2003, 44, 4455–4458. [Google Scholar] [CrossRef]
- Stiasni, N.; Kappe, C.O. A tandem intramolecular Michael-addition/elimination sequence in dihydropyrimidone to quinoline rearrangements. Arkivoc 2002, 2002, 71–79. [Google Scholar]
- Datta, G.K.; Von Schenck, H.; Hallberg, A.; Larhed, M. Selective terminal heck arylation of vinyl ethers with aryl chlorides: A combined experimental-computational approach including synthesis of betaxolol. J. Org. Chem. 2006, 71, 3896–3903. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.J.; Salunke, D.B.; Sun, C.M. Multistep microwave-assisted divergent synthesis of indolo-fused pyrazino-/diazepinoquinoxalinones on PEG support. Org. Lett. 2010, 12, 2174–2177. [Google Scholar] [CrossRef] [PubMed]
- Dhole, S.; Selvaraju, M.; Maiti, B.; Chanda, K.; Sun, C.-M. Microwave Controlled Reductive Cyclization: A Selective Synthesis of Novel Benzimidazole-alkyloxypyrrolo[1,2- a ]quinoxalinones. ACS Comb. Sci. 2015, 17, 310–316. [Google Scholar] [CrossRef] [PubMed]
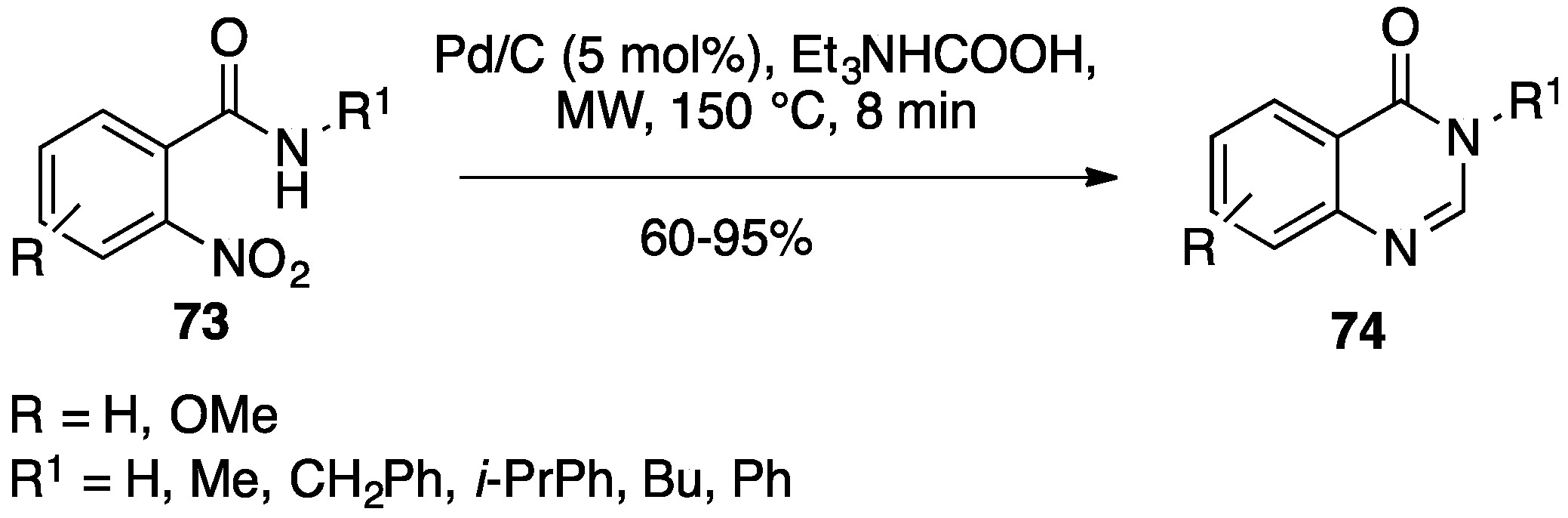
- Zhu, K.; Hao, J.; Zhang, C.; Zhang, J.; Feng, Y.; Qin, H. Diversified facile synthesis of benzimidazoles, quinazolin–4(3H)–ones and 1,4-benzodiazepine-2,5-diones via palladium-catalyzed transfer hydrogenation/condensation cascade of nitro arenes under microwave irradiation. RSC Adv. 2015, 5, 11132–11135. [Google Scholar] [CrossRef]
- Flyrén, K.; Bergquist, L.O.; Castro, V.M.; Fotsch, C.; Johansson, L.; St. Jean, D.J.; Sutin, L.; Williams, M. Piperidine amides as 11β-hydroxysteroid dehydrogenase type 1 inhibitors. Bioorganic Med. Chem. Lett. 2007, 17, 3421–3425. [Google Scholar] [CrossRef] [PubMed]
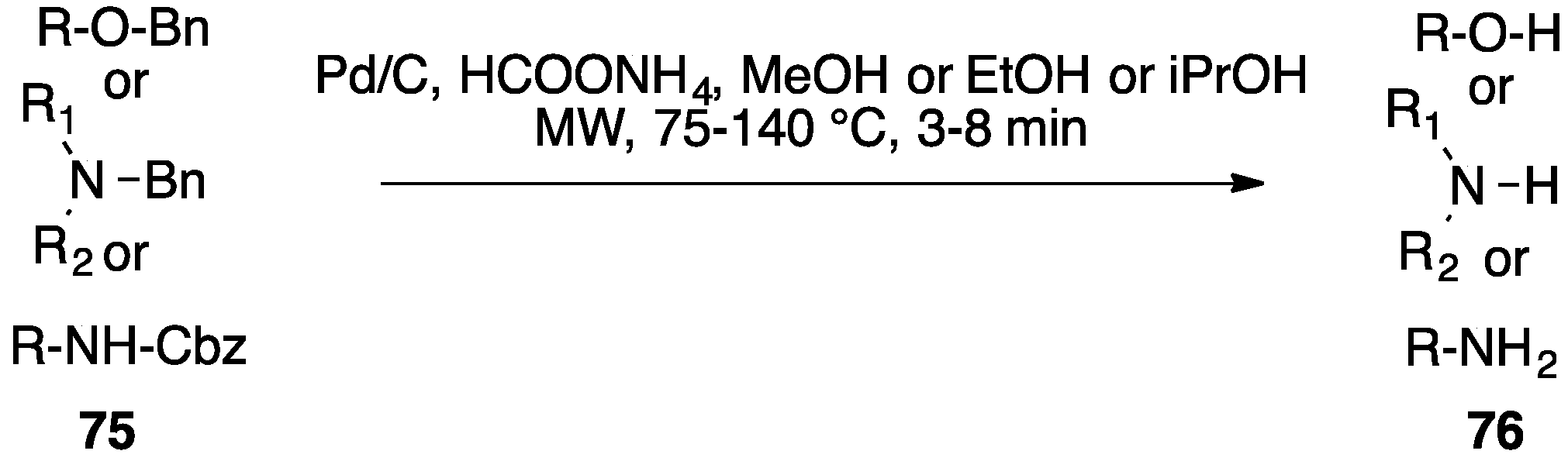
- Daga, M.C.; Taddei, M.; Varchi, G. Rapid microwave-assisted deprotection of N–Cbz and N–Bn derivatives. Tetrahedron Lett. 2001, 42, 5191–5194. [Google Scholar] [CrossRef]
- Esposito, A.; Piras, P.P.; Ramazzotti, D.; Taddei, M. First Stereocontrolled Synthesis of (S)-Cleonin and Related Cyclopropyl-Substituted Amino Acids. Org. Lett. 2001, 3, 3273–3275. [Google Scholar] [CrossRef] [PubMed]
- Desai, B.; Dallinger, D.; Kappe, C.O. Microwave-assisted solution phase synthesis of dihydropyrimidine C5 amides and esters. Tetrahedron 2006, 62, 4651–4664. [Google Scholar] [CrossRef]
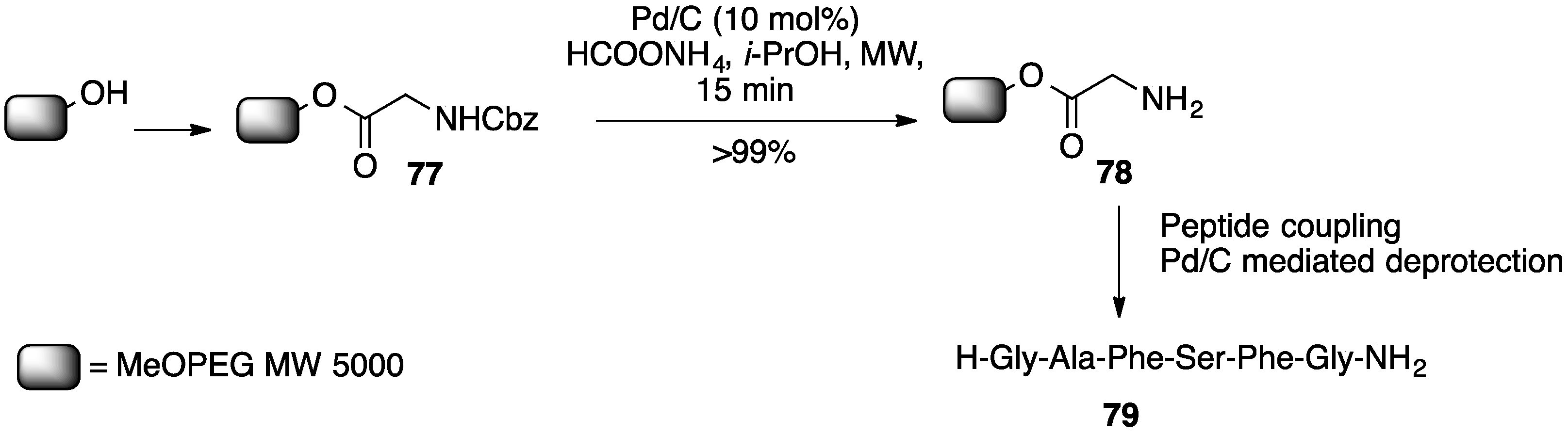
- Porcheddu, A.; Ruda, G.F.; Sega, A.; Taddei, M. A new, rapid, general procedure for the synthesis of organic molecules supported on methoxy–polyethylene glycol (MeOPEG) under microwave irradiation conditions. Eur. J. Org. Chem. 2003, 907–912. [Google Scholar] [CrossRef]
- Quai, M.; Repetto, C.; Barbaglia, W.; Cereda, E. Fast deprotection of phenoxy benzyl ethers in transfer hydrogenation assisted by microwave. Tetrahedron Lett. 2007, 48, 1241–1245. [Google Scholar] [CrossRef]
- Paul, N.K.; Dietrich, L.; Jha, A. Convenient Synthesis of 1-Arylmethyl-2-naphthols. Synth. Commun. 2007, 37, 877–888. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, R.; Wang, Q.; Wu, C.; Yu, Y.; Zhao, F. Selective reduction of phenol derivatives to cyclohexanones in water under microwave irradiation. New J. Chem. 2012, 36, 1085. [Google Scholar] [CrossRef]
- Jumde, V.R.; Petricci, E.; Petrucci, C.; Santillo, N.; Taddei, M.; Vaccaro, L. Domino Hydrogenation–Reductive Amination of Phenols, a Simple Process To Access Substituted Cyclohexylamines. Org. Lett. 2015, 17, 3990–3993. [Google Scholar] [CrossRef] [PubMed]
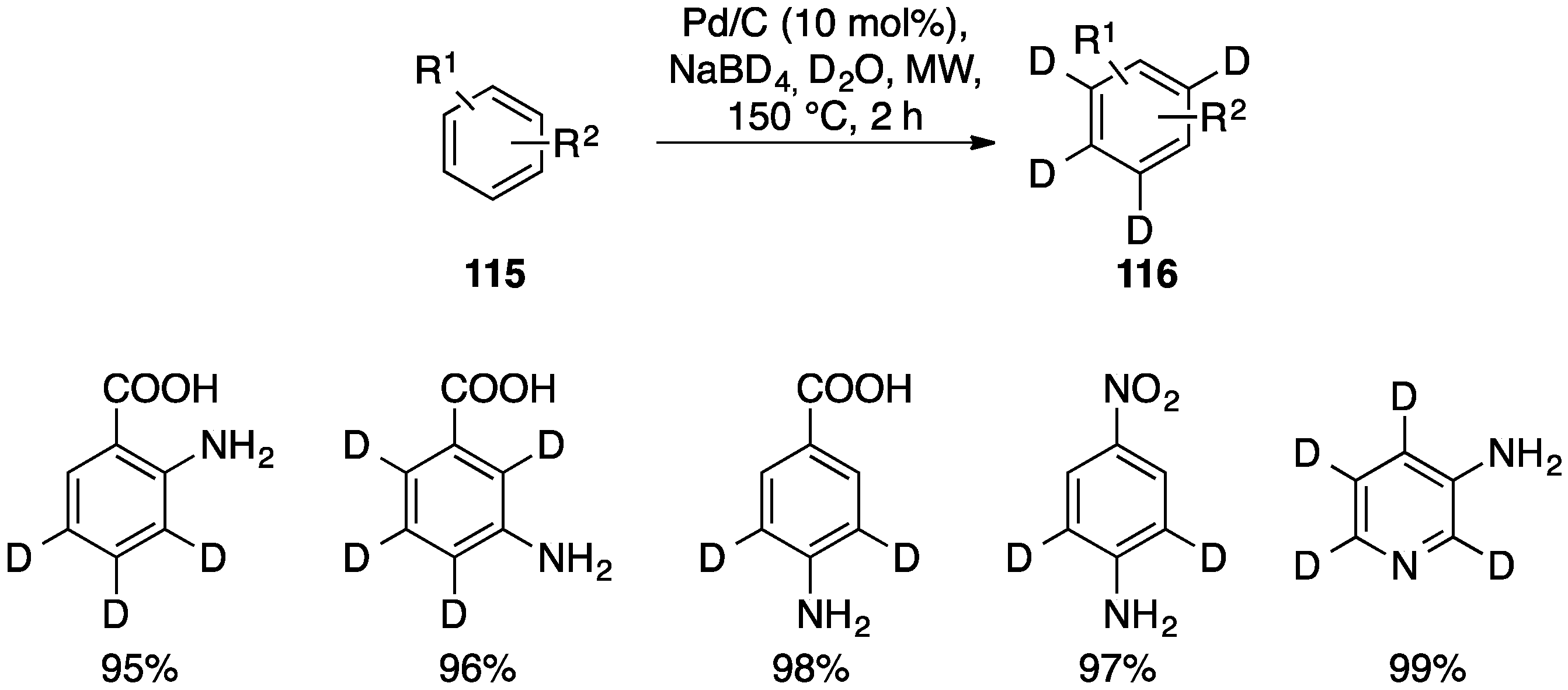
- Jones, J.R.; Lockley, W.J.S.; Lu, S.Y.; Thompson, S.P. Microwave-enhanced aromatic dehalogenation studies: A rapid deuterium-labelling procedure. Tetrahedron Lett. 2001, 42, 331–332. [Google Scholar] [CrossRef]
- Tavor, D.; Popov, S.; Dlugy, C.; Wolfson, A. Catalytic transfer-hydrogenations of olefins in glycerol. Org. Commun. 2010, 70–75. [Google Scholar]
- Amarasekara, A.S.; Hasan, M.A. Pd/C catalyzed conversion of levulinic acid to γ-valerolactone using alcohol as a hydrogen donor under microwave conditions. Catal. Commun. 2015, 60, 5–7. [Google Scholar] [CrossRef]
- Vaccaro, L.; Ferlin, F.; Santoro, S.; Luciani, L.; Ackermann, L. Biomass-derived solvents as effective media for cross-coupling reactions and C–H functionalization processes. Green Chem. 2017. [CrossRef]
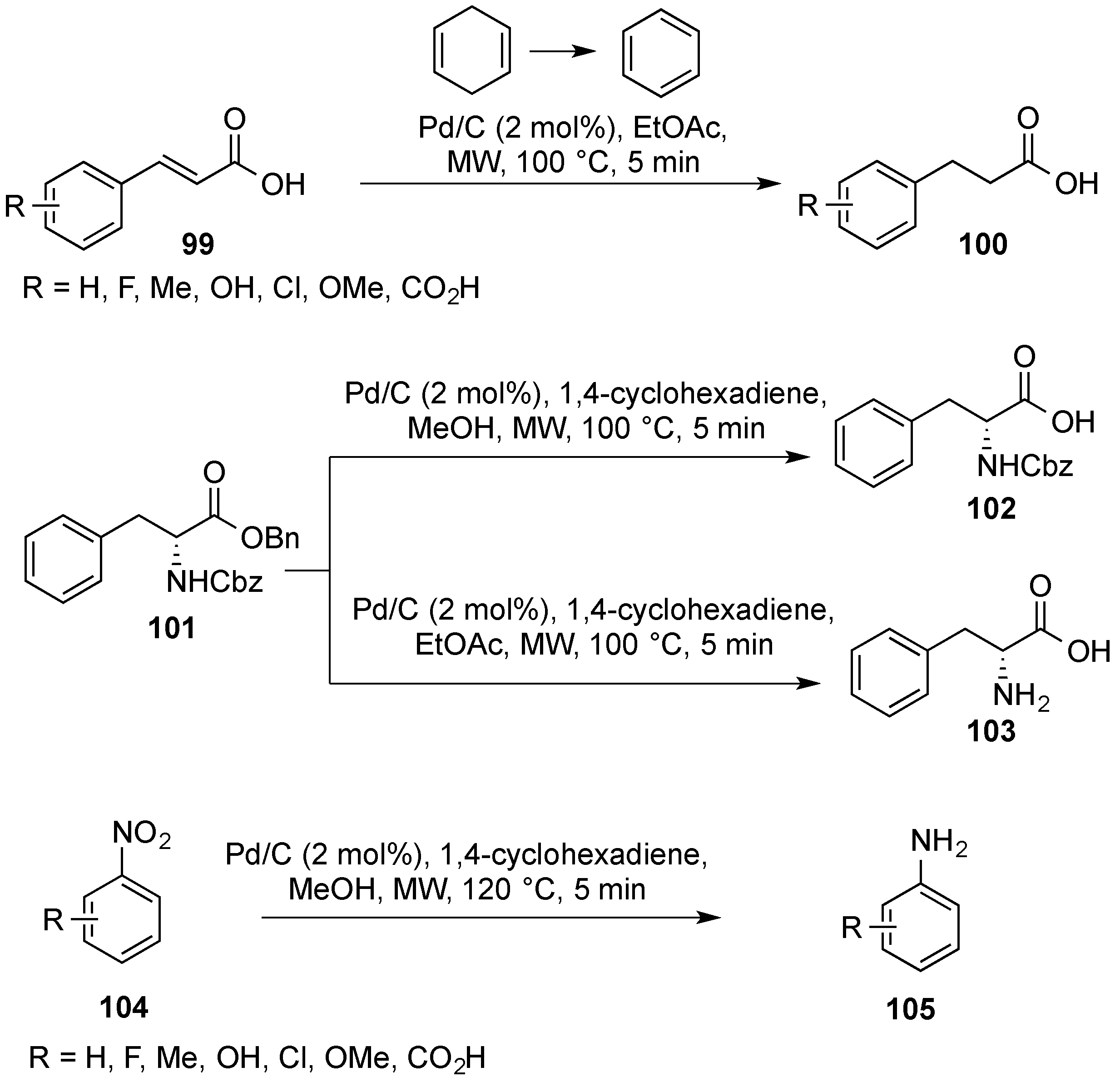
- Quinn, J.F.; Razzano, D.A.; Golden, K.C.; Gregg, B.T. 1,4-Cyclohexadiene with Pd/C as a rapid, safe transfer hydrogenation system with microwave heating. Tetrahedron Lett. 2008, 49, 6137–6140. [Google Scholar] [CrossRef]
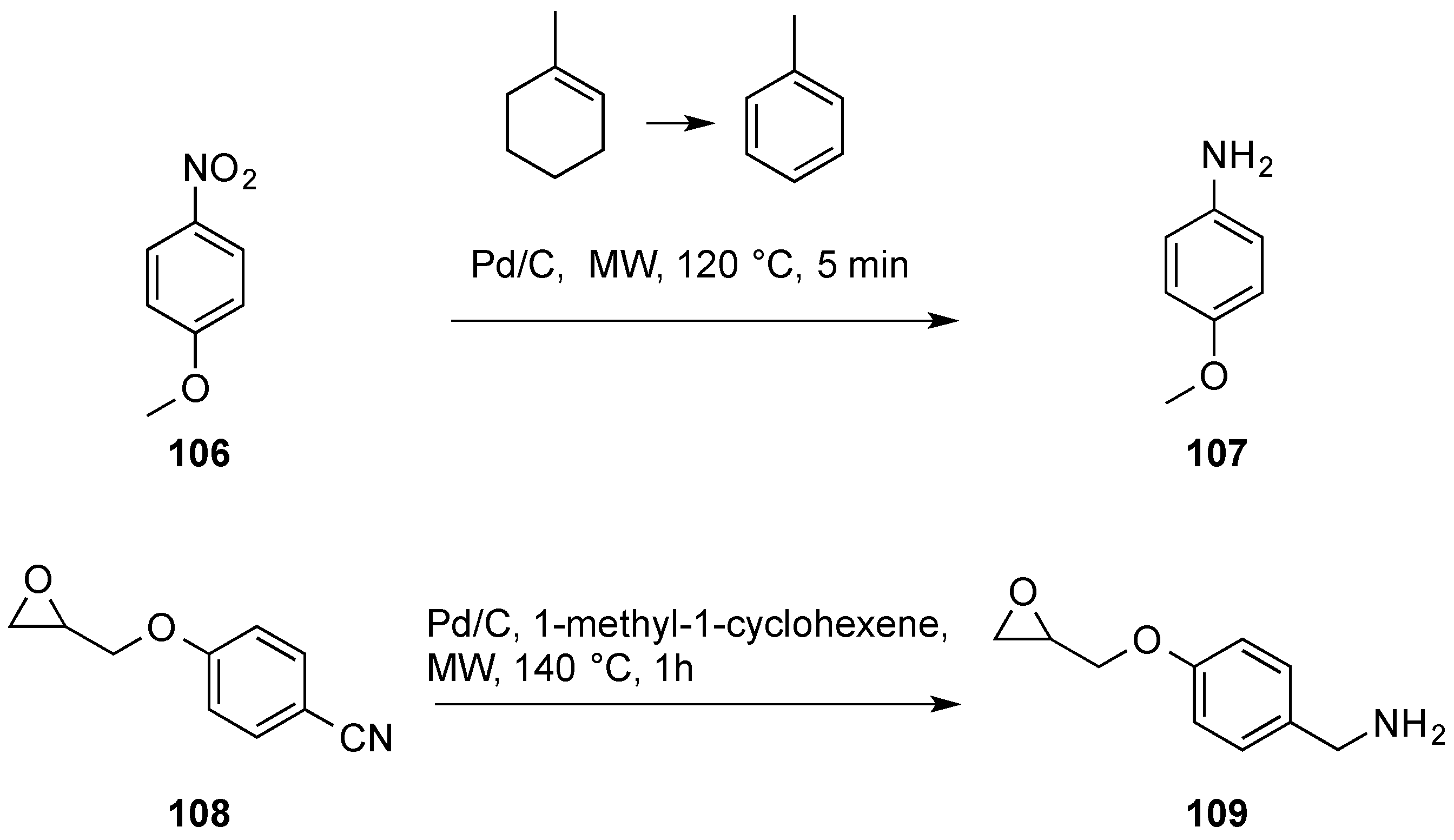
- Quinn, J.F.; Bryant, C.E.; Golden, K.C.; Gregg, B.T. Rapid reduction of heteroaromatic nitro groups using catalytic transfer hydrogenation with microwave heating. Tetrahedron Lett. 2010, 51, 786–789. [Google Scholar] [CrossRef]
- Theis, J.; Ritter, H. Formation of epoxide-amine oligo-adducts as OH-functionalized initiators for the ring-opening polymerization of ??-caprolactone. Beilstein J. Org. Chem. 2010, 6, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Theis, J.; Ritter, H.; Klee, J.E. Microwave-assisted one-pot synthesis of hyperbranched epoxide-amine adducts. Macromol. Rapid Commun. 2009, 30, 1424–1427. [Google Scholar] [CrossRef] [PubMed]
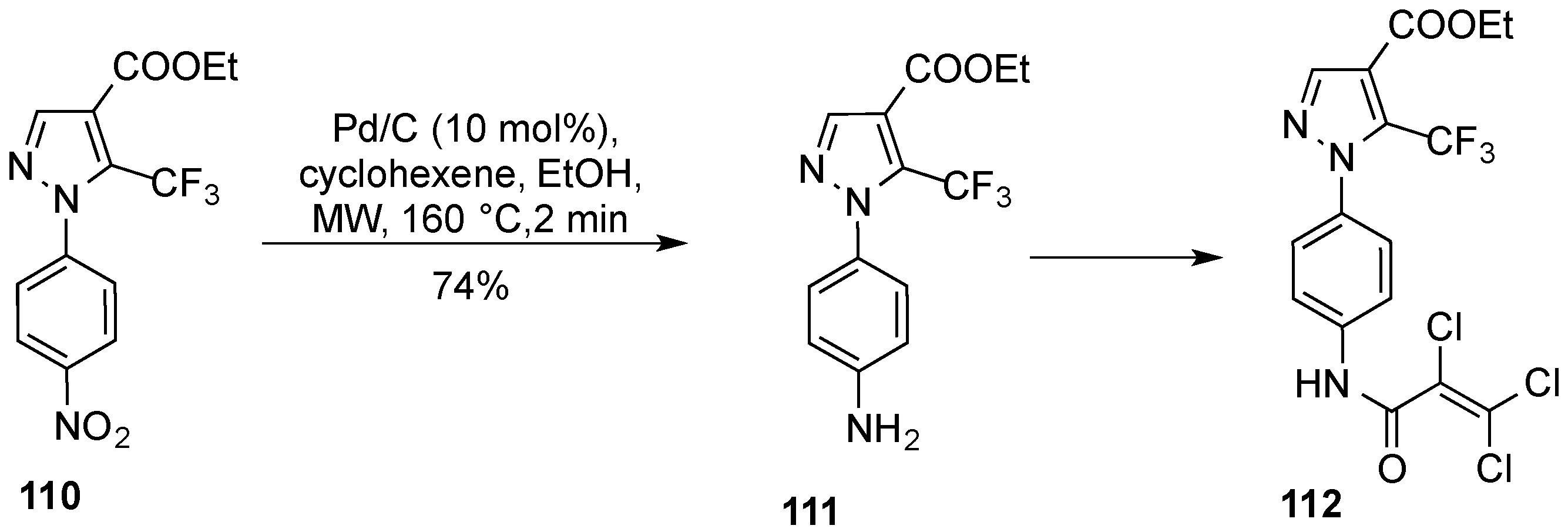
- Glasnov, T.N.; Groschner, K.; Kappe, C.O. High-speed microwave-assisted synthesis of the trifluoromethylpyrazol- derived canonical transient receptor potential (TRPC) channel inhibitor Pyr3. ChemMedChem 2009, 4, 1816–1818. [Google Scholar] [CrossRef] [PubMed]
- Felpin, F.X.; Fouquet, E. A useful, reliable and safer protocol for hydrogenation and the hydrogenolysis of O-benzyl groups: The in situ preparation of an active Pd0/C catalyst with well-defined properties. Chem. A Eur. J. 2010, 16, 12440–12445. [Google Scholar] [CrossRef] [PubMed]
- Derdau, V.; Atzrodt, J.; Zimmermann, J.; Kroll, C.; Brückner, F. Hydrogen-deuterium exchange reactions of aromatic compounds and heterocycles by NaBD4-activated rhodium, platinum and palladium catalysts. Chem. A Eur. J. 2009, 15, 10397–10404. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, S.-I. Synthetic Aspects of Metal-Catalyzed Oxidations of Amines and Related Reactions. Angew. Chem. Int. Ed. Engl. 1995, 34, 2443–2465. [Google Scholar] [CrossRef]
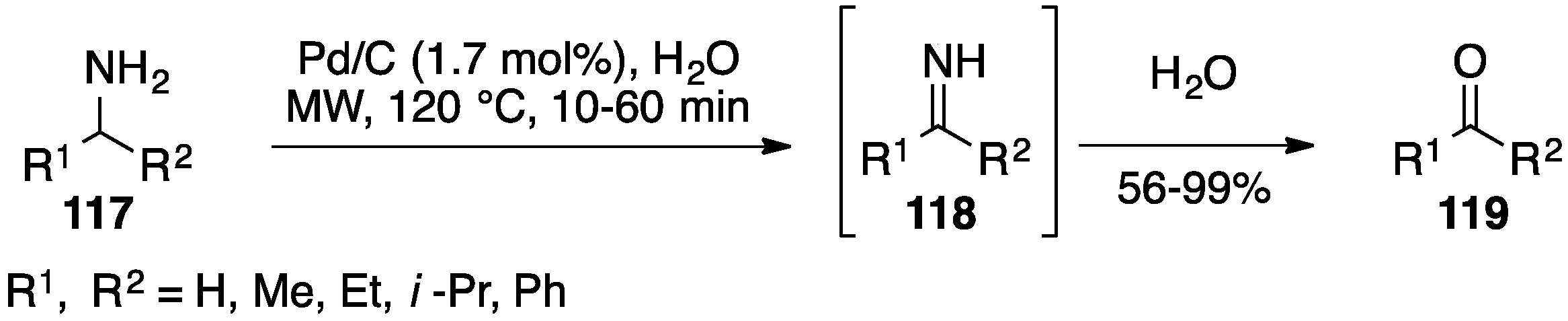
- Miyazawa, A.; Tanaka, K.; Sakakura, T.; Tashiro, M.; Tashiro, H.; Prakash, G.K.S.; Olah, G.A. Microwave-assisted direct transformation of amines to ketones using water as an oxygen source. Chem. Commun. 2005, 2104–2106. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, A.; Saitou, K.; Tanaka, K.; Gädda, T.M.; Tashiro, M.; Prakash, G.K.S.; Olah, G.A. Reaction of primary amines with Pt/C catalyst in water under microwave irradiation: A convenient synthesis of secondary amines from primary amines. Tetrahedron Lett. 2006, 47, 1437–1439. [Google Scholar] [CrossRef]
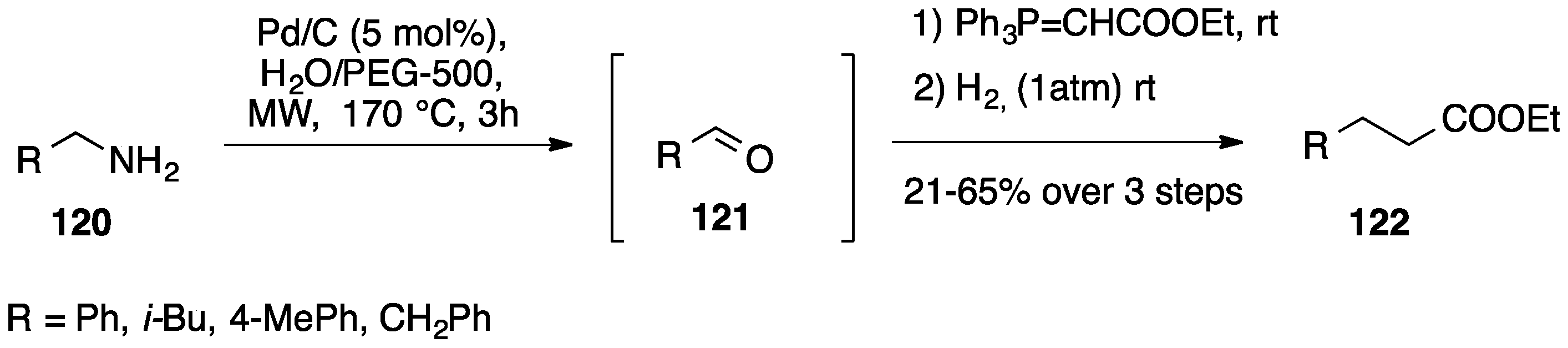
- Chandrasekhar, S.; Pavan Kumar Reddy, G.; Nagesh, C.; Raji Reddy, C. A novel one-pot conversion of amines to homologated esters in poly(ethylene glycol). Tetrahedron Lett. 2007, 48, 1269–1271. [Google Scholar] [CrossRef]
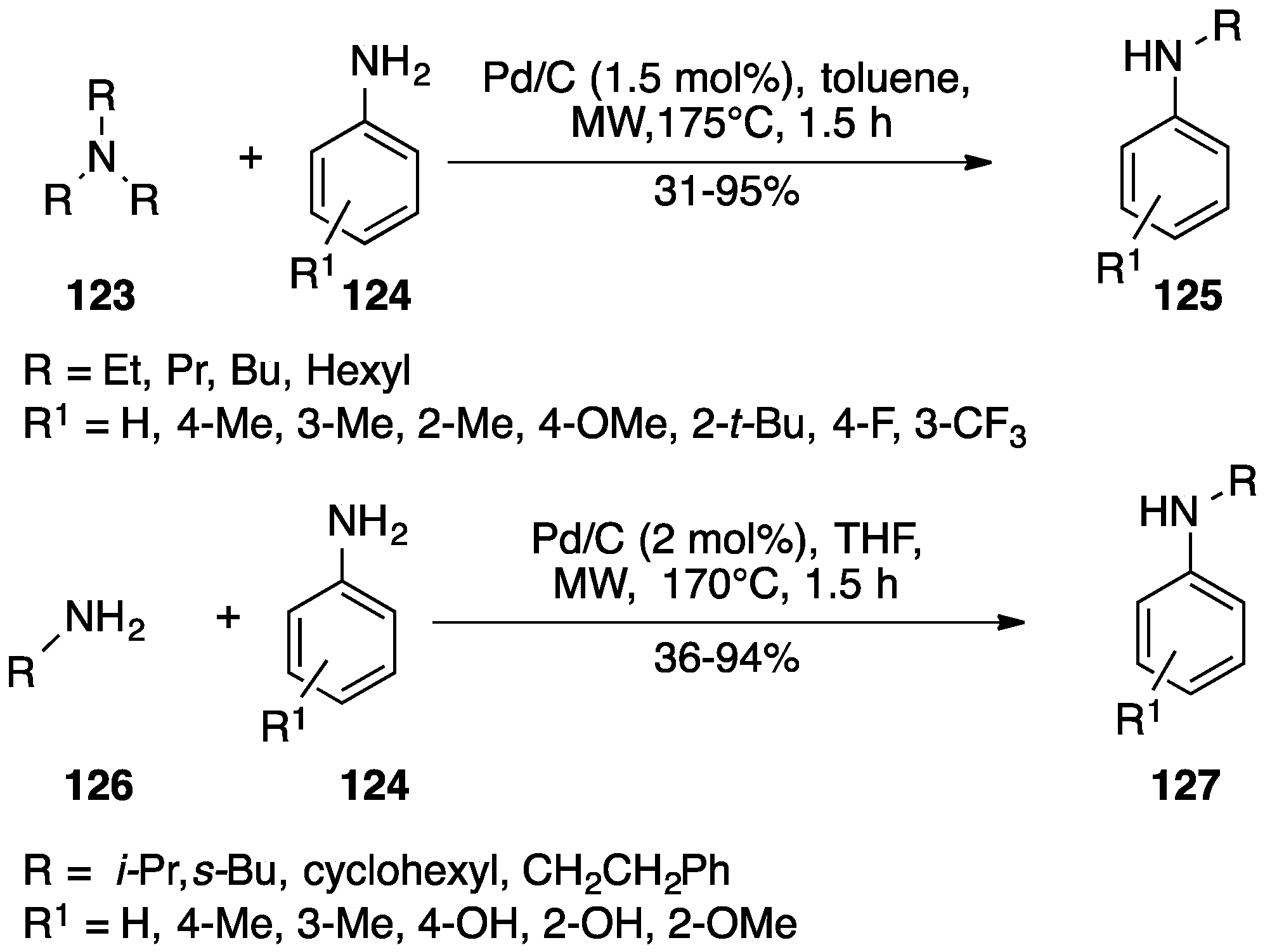
- Lubinu, M.C.; De Luca, L.; Giacomelli, G.; Porcheddu, A. Microwave-promoted selective mono-n-alkylation of anilines with tertiary amines by heterogeneous catalysis. Chem. A Eur. J. 2011, 17, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Linciano, P.; Pizzetti, M.; Porcheddu, A. Use of Primary Amines for the Selective N-Alkylation of Anilines by a Reusable Heterogeneous Catalyst. Synlett 2013, 2249–2254. [Google Scholar]
- De Luca, L.; Porcheddu, A. Microwave-assisted synthesis of polysubstituted benzimidazoles by heterogeneous Pd-catalyzed oxidative C–H activation of tertiary amines. Eur. J. Org. Chem. 2011, 5791–5795. [Google Scholar] [CrossRef]
- Pizzetti, M.; De Luca, E.; Petricci, E.; Porcheddu, A.; Taddei, M. A General Approach to Substituted Benzimidazoles and Benzoxazoles via Heterogeneous Palladium-Catalyzed Hydrogen-Transfer with Primary Amines. Adv. Synth. Catal. 2012, 354, 2453–2464. [Google Scholar] [CrossRef]
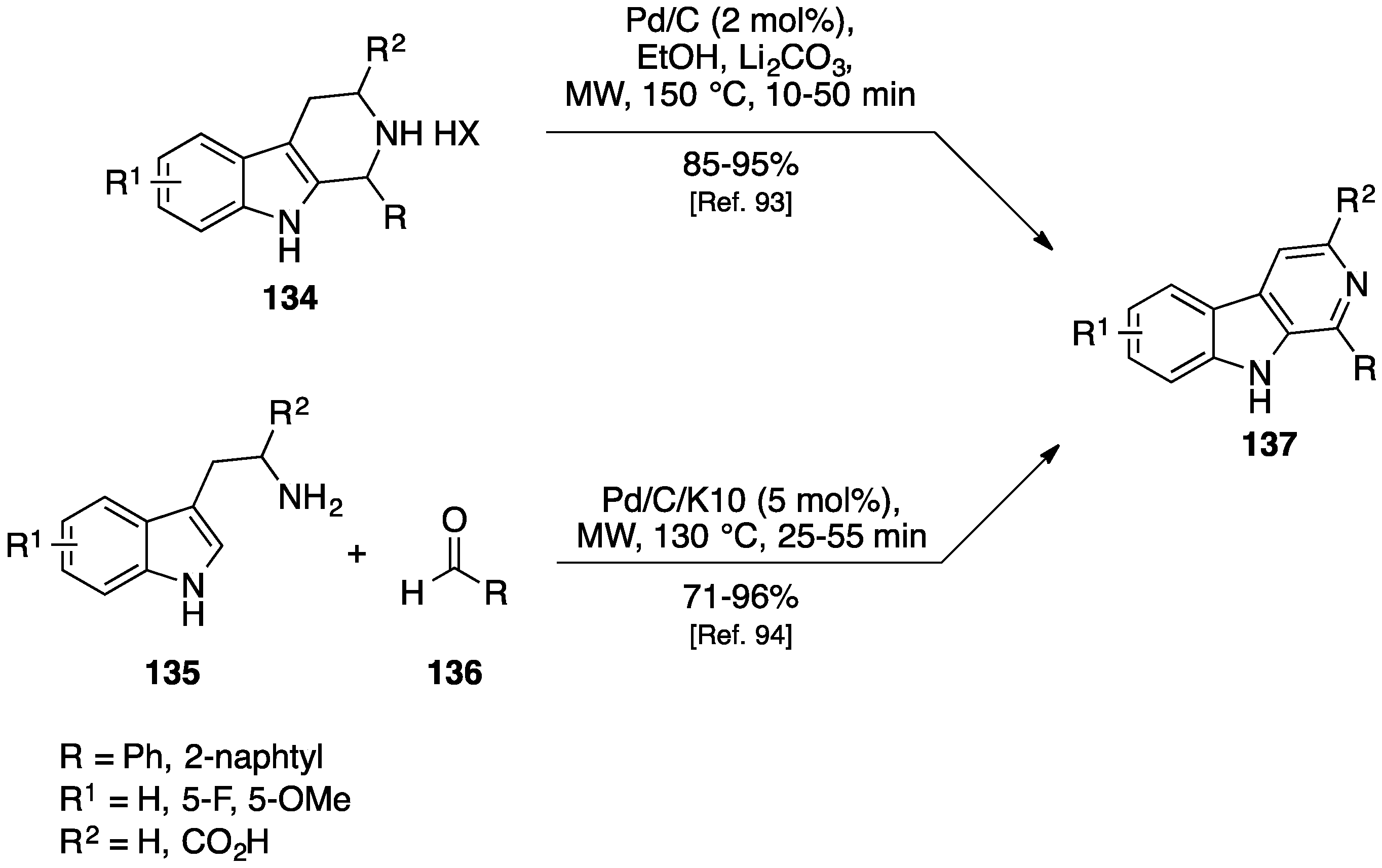
- Eagon, S.; Anderson, M.O. Microwave-Assisted Synthesis of Tetrahydro-β-carbolines and β-Carbolines. Eur. J. Org. Chem. 2014, 1653–1665. [Google Scholar] [CrossRef]
- Kulkarni, A.; Abid, M.; Török, B.; Huang, X. A direct synthesis of β-carbolines via a three-step one-pot domino approach with a bifunctional Pd/C/K-10 catalyst. Tetrahedron Lett. 2009, 50, 1791–1794. [Google Scholar] [CrossRef]
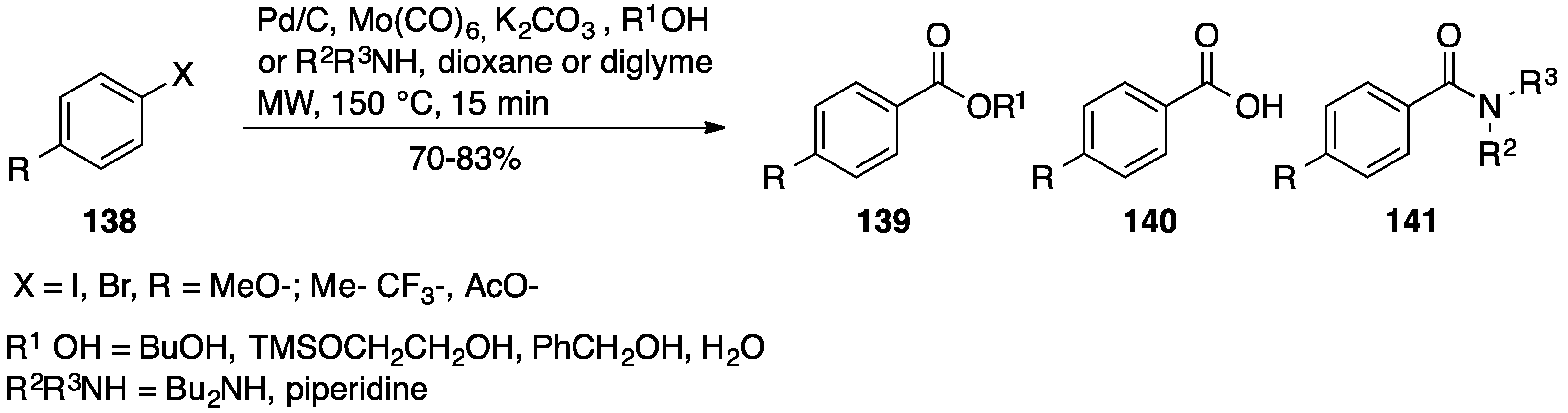
- Georgsson, J.; Hallberg, A.; Larhed, M. Rapid Palladium-Catalyzed Synthesis of Esters from Aryl Halides Utilizing Mo(CO)6 as a Solid Carbon Monoxide Source. Drug Discov. Today 2003, 350–352. [Google Scholar]
- Kaiser, N.F.K.; Hallberg, A.; Larhed, M. In situ generation of carbon monoxide from solid molybdenum hexacarbonyl. A convenient and fast route to palladium-catalyzed carbonylation reactions. J. Comb. Chem. 2002, 4, 109–111. [Google Scholar] [CrossRef] [PubMed]
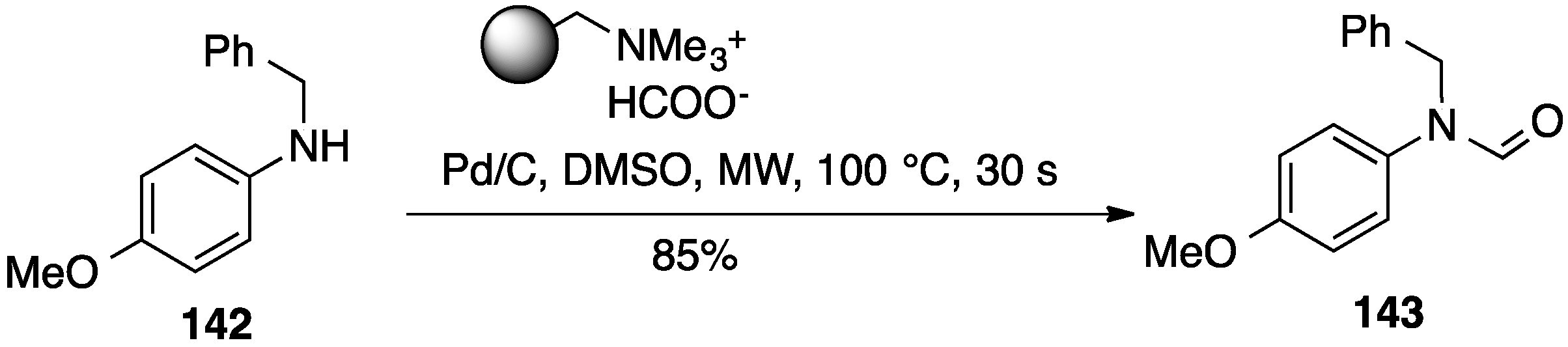
- Desai, B.; Danks, T.N.; Wagner, G. Thermal and microwave-assisted N-formylation using solid-supported reagents. Tetrahedron Lett. 2005, 46, 955–957. [Google Scholar] [CrossRef]
- Zhu, G.; Qiu, X.; Zhao, Y.; Qian, Y.; Pang, Y.; Ouyang, X. Depolymerization of lignin by microwave-assisted methylation of benzylic alcohols. Bioresour. Technol. 2016, 218, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, W.; You, Z.; Wang, Z.; Luo, Y.; Gao, L.; Yin, C.; Peng, R.; Lan, L. A new type of power energy for accelerating chemical reactions: the nature of a microwave-driving force for accelerating chemical reactions. Sci. Rep. 2016, 6, 25149. [Google Scholar] [CrossRef] [PubMed]
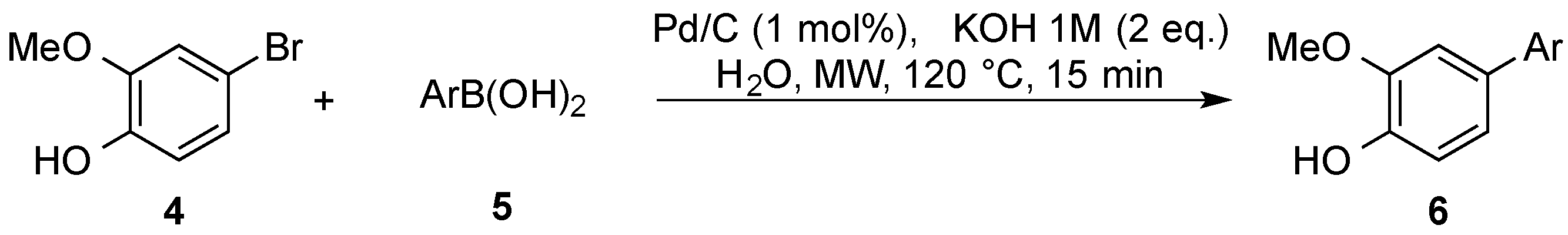
| Entry | ArB(OH)2 5 | Yield |
|---|---|---|
| 1 | PhB(OH)2 | 83% |
| 2 | 2-MeC6H4B(OH)2 | 91% |
| 3 | 3-MeC6H4B(OH)2 | 90% |
| 4 | 4-MeC6H4B(OH)2 | 89% |
| 5 | 1-naphthyl B(OH)2 | 58% |
| 6 | 2- naphthyl B(OH)2 | 48% |
| 7 | 4-MeOC6H4B(OH)2 | 31% |
| 8 | 4-ClC6H4B(OH)2 | 35% |
| 9 | 4-MeO2CC6H4B(OH)2 | 10% |
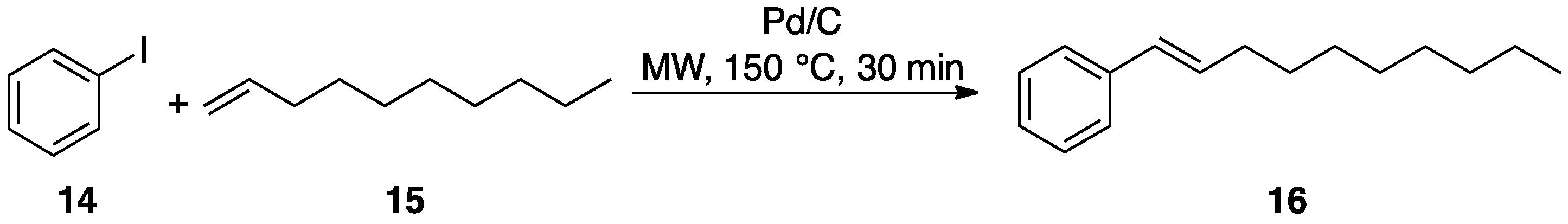
| Catalyst Table | Time (min) | Conversion |
|---|---|---|
| 5% Pd/C | 10.5 | 54% |
| 5% Pd/MgO | 10.0 | 39% |
| 5% Pd/γA12O3 | 10.0 | 59% |
| 0.3% Pd/SiO2-A12O3 | 6.0 | 53% |

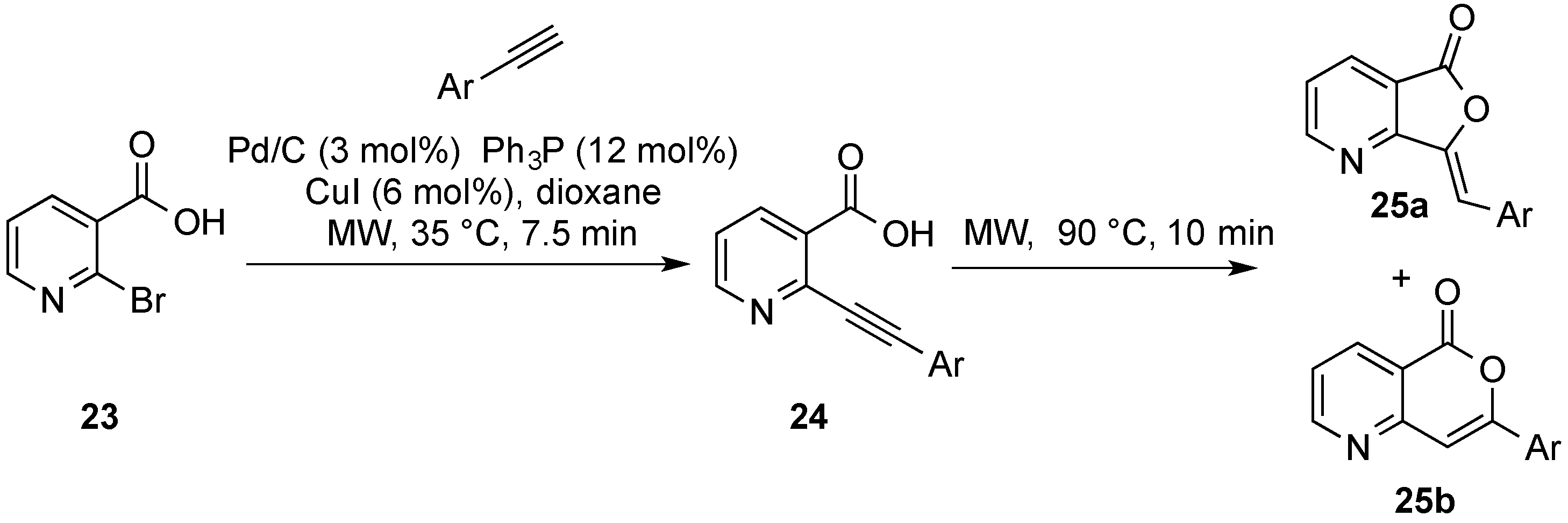
| Entry | Ar(Het) | 25a (%) | 25b (%) | Entry | Ar(Het) | 25a (%) | 25b (%) |
|---|---|---|---|---|---|---|---|
| 1 | Ph | 40 | 18 | 5 | 3-F-C6H4 | 38 | 18 |
| 2 | 3-MeOC6H4 | 45 | 16 | 6 | 2-F-C6H4 | 45 | 11 |
| 3 | 4-MeOC6H4 | 43 | 23 | 7 | 4-Me2NC6H4 | 24 | 16 |
| 4 | 2-MeOC6H4 | 21 | 41 | 8 | 3-Thienyl | 52 | - |
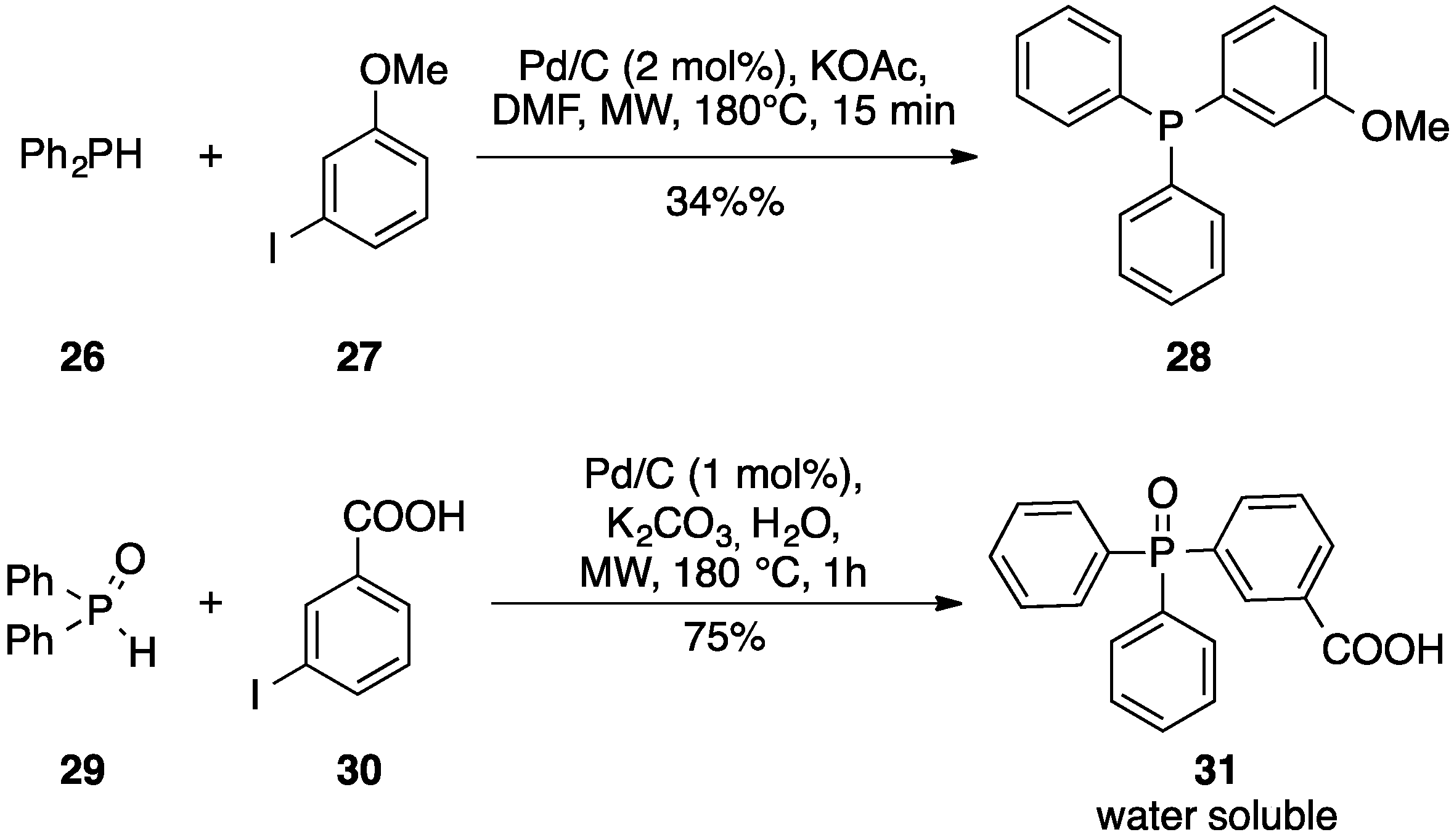
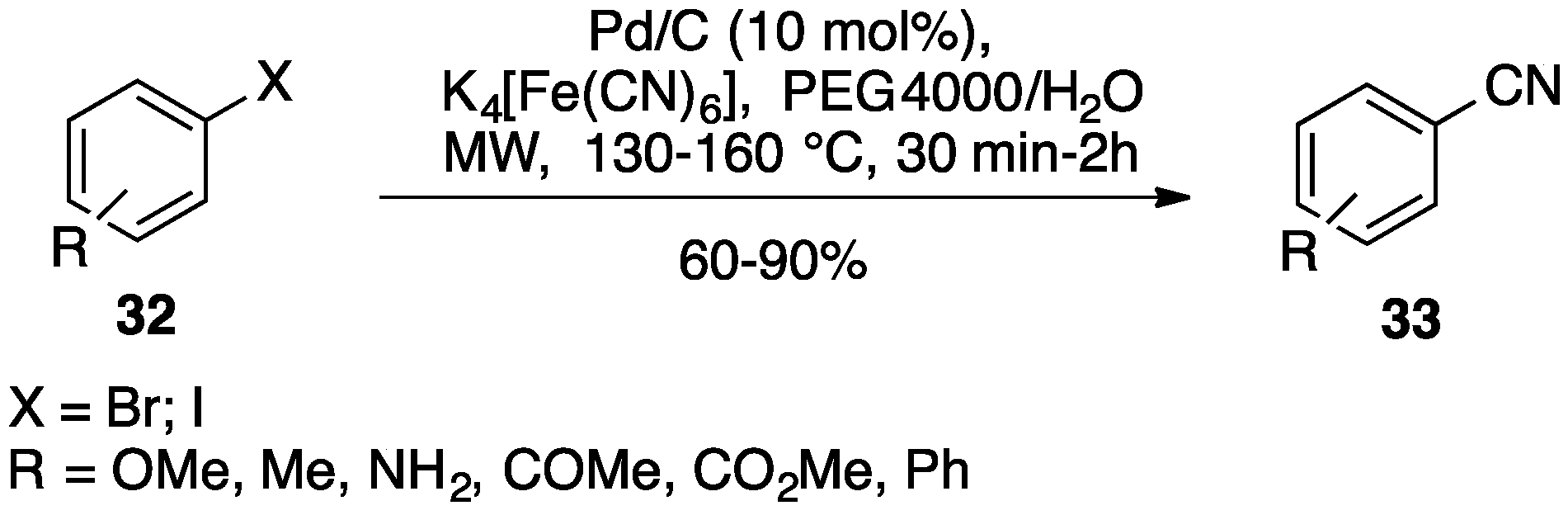
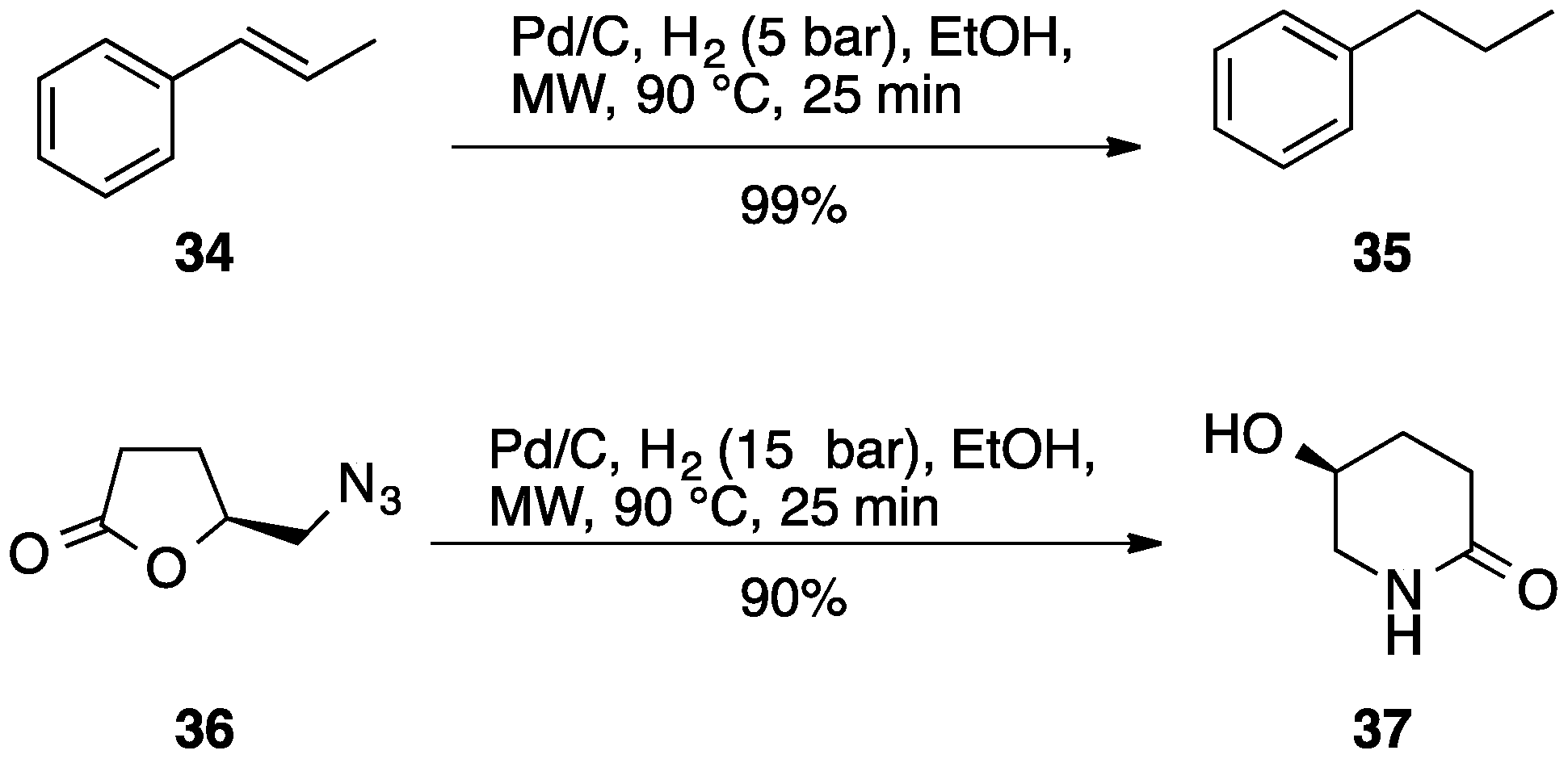
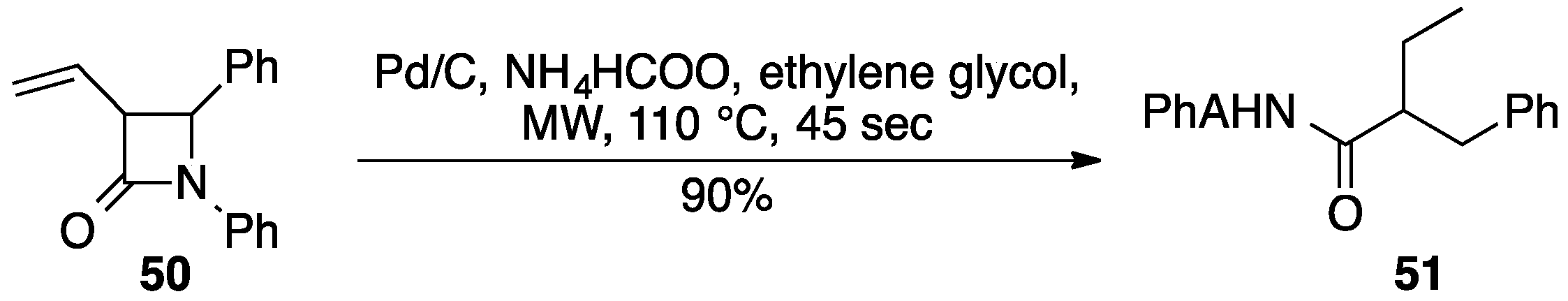




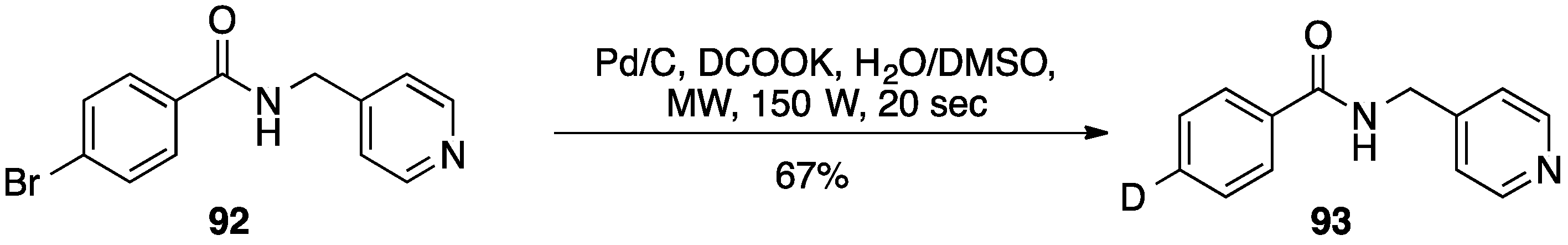

| Entry | Aryl Halide | Boronic Acid | US Yield % | MW Yield % | Comb.US/MW Yield, % |
|---|---|---|---|---|---|
| 1 | 3-Br-anisole | Phenylboronic acid | 54 | 64 | 88 |
| 2 | 2-I-thiophene | Phenylboronic acid | 40 | 37 | 59 |
| 3 | 4-Cl-nitrobenzene | Phenylboronic acid | 22 | 30 | 57 |
| 4 | - | Thianthrene-1-boronic acid a | 48 | 55 | 69 |
| 5 | - | 4-t-Buty-l-boronic acid a | 68 | 74 | 86 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cini, E.; Petricci, E.; Taddei, M. Pd/C Catalysis under Microwave Dielectric Heating. Catalysts 2017, 7, 89. https://doi.org/10.3390/catal7030089
Cini E, Petricci E, Taddei M. Pd/C Catalysis under Microwave Dielectric Heating. Catalysts. 2017; 7(3):89. https://doi.org/10.3390/catal7030089
Chicago/Turabian StyleCini, Elena, Elena Petricci, and Maurizio Taddei. 2017. "Pd/C Catalysis under Microwave Dielectric Heating" Catalysts 7, no. 3: 89. https://doi.org/10.3390/catal7030089