Hydrogenation of Phenol over Pt/CNTs: The Effects of Pt Loading and Reaction Solvents
Abstract
:1. Introduction
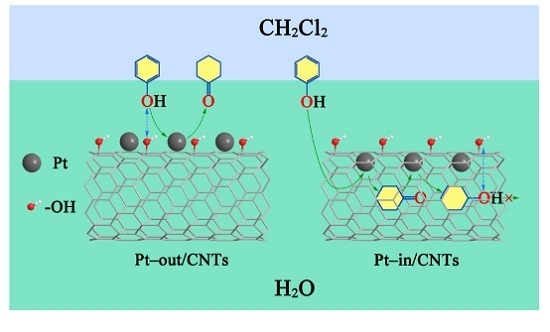
2. Results and Discussion
3. Experimental
3.1. Chemicals
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Hydrogenation Activity Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nie, R.; Miao, M.; Du, W.; Shi, J.; Liu, Y.; Hou, Z. Selective hydrogenation of C=C bond over N–doped reduced graphene oxides supported Pd catalyst. Appl. Catal. B Environ. 2016, 180, 607–613. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, B.; Ding, Y.; Wen, G.; Hamid, S.B.A.; Su, D. Disintefrative activation of Pd nanoparticles on carbon nanotubes for catalytic phenol hydrogenation. Catal. Sci. Technol. 2016, 6, 1003–1006. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Chen, L.; Ma, L.; Gao, H.; Wang, T. Direct selective hydrogenation of phenol and derivatives over polyaniline-functionalized carbon-nanotube-supported palladium. ChemPlusChem 2013, 78, 142–148. [Google Scholar] [CrossRef]
- Xiang, Y.; Kong, L.; Xie, P.; Xu, T.; Wang, J.; Li, X. Carbon nanotubes and activated carbons supported catalysts for phenol in situ hydrogenation: Hydrophobic/hydrophilic effect. Ind. Eng. Chem. Res. 2014, 53, 2197–2203. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, Q.; Cen, J.; Xiang, Y.; Li, X. Selectivity tailoring of Pd/CNTs in phenol hydrogenation by surface modification: Role of C–O oxygen species. Appl. Surf. Sci. 2015, 324, 634–639. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Long, L.; Wang, T.; Ma, L.; Wu, L.; Bai, Y.; Li, X.; Liao, S. Pt nanoparticles entrapped in titanate nanotubes (TNT) for phenol hydrogenation: The confinement effect of TNT. Chem. Commun. 2014, 50, 2794–2796. [Google Scholar] [CrossRef] [PubMed]
- Ertas, I.E.; Gulcan, M.; Bulut, A.; Yurderi, M.; Zahmakiran, M. Metal–organic framework (MIL–101) stabilized ruthenium nanoparticles: Highly efficient catalytic material in the phenol hydrogenation. Microporous Mesoporous Mater. 2016, 226, 94–103. [Google Scholar] [CrossRef]
- Kuklin, S.; Maximov, A.; Zolotukhina, A.; Karakhanov, E. New approach for highly selective hydrogenation of phenol to cyclohexanone: Combination of rhodium nanoparticles and cyclodextrins. Catal. Commun. 2016, 73, 63–68. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.; Gao, P.; Wang, L. PVP–NiB amorphous catalyst for selective hydrogenation of phenol and its derivatives. Chin. J. Catal. 2014, 35, 1793–1799. [Google Scholar] [CrossRef]
- Boullosa–Eiras, S.; Lødeng, R.; Bergem, H.; Stöcker, M.; Hannevold, L.; Blekkan, E.A. Catalytic hydrodeoxygenation (HDO) of phenol over supported molybdenum carbide, nitride, phosphide and oxide catalysts. Catal. Today 2014, 223, 44–53. [Google Scholar] [CrossRef]
- Melchionna, M.; Marchesan, S.; Prato, M.; Fornasiero, P. Carbon nanotubes and catalysis: The many facets of a successful marriage. Catal. Sci. Technol. 2015, 46, 3859–3875. [Google Scholar] [CrossRef]
- Wildgoose, G.G.; Banks, C.E.; Compton, R.G. Metal nanoparticles and related materials supported on carbon nanotubes: Methods and applications. Small 2006, 2, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Melchionna, M.; Prato, M. Functionalizing carbon nanotubes: An indispensible step towards applications. ECSJ. Solid State Sci. Technol. 2013, 2, M3040–M3045. [Google Scholar] [CrossRef]
- Matos, J.; Corma, A. Selective phenol hydrogenation in aqueous phase on Pd-based catalysts supported on hybrid TiO2-carbon materials. Appl. Catal. A Gen. 2011, 404, 103–112. [Google Scholar] [CrossRef]
- Makowski, P.; Cakan, R.D.; Antonietti, M.; Goettmann, F.; Titirici, M.M. Selective partial hydrogenation of hydroxy aromatic derivatives with palladium nanoparticles supported on hydrophilic carbon. Chem. Commun. 2008, 8, 999–1001. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ma, R.; Cao, B.; Liang, J.; Ren, Q.; Song, H. Effect of Co-B supporting methods on the hydrogenation of m-chloronitrobenzene over Co-B/CNTs amorphous alloy catalysts. Appl. Catal. A Gen. 2016, 514, 248–252. [Google Scholar] [CrossRef]
- Deng, D.; Yu, L.; Chen, X.; Wang, G.; Jin, L.; Pan, X.; Deng, J.; Sun, G.; Bao, X. Iron encapsulated within pod-like carbon nanotubes for oxygen reduction reaction. Angew. Chem. Int. Ed. 2013, 52, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Fan, Z.; Pan, X.; Bao, X. Effect of confinement in carbon nanotubes on the activity of Fischer−Tropsch iron Catalyst. J. Am. Chem. Soc. 2008, 130, 9414–9419. [Google Scholar] [CrossRef] [PubMed]
- Tavasoli, A.; Trépanier, M.; Dalai, A.K.; Abatzoglou, N. Effect of confinement in carbon nanotubes on the activity, selectivity, and lifetime of Fischer-Tropsch Co/carbon nanotube catalysts. J. Chem. Eng. Data 2010, 55, 2757–2763. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Tan, R.P.; Fazzini, P.F.; Hungria, T.; Durand, J.; Lachaize, S.; Sun, W.H.; Respaud, M.; Soulantica, K.; Serp, P. Isoprene polymerization on iron nanoparticles confined in carbon nanotubes. Chem. Eur. J. 2015, 21, 17437–17444. [Google Scholar] [CrossRef] [PubMed]
- Castillejos, E.; Debouttière, P.J.; Roiban, L.; Solhy, A.; Martinez, V.; Kihn, Y.; Ersen, O.; Philippot, K.; Chaudret, B.; Serp, P. An efficient strategy to drive nanoparticles into carbon nanotubes and the remarkable effect of confinement on their catalytic performance. Angew. Chem. Int. Ed. 2009, 48, 2529–2533. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.S.; Kwak, G.; Jun, K.W.; Hwang, J.; Lee, J. Ordered mesoporous carbon nanochannel reactors for high–performance Fischer–Tropsch synthesis. Chem. Commun. 2013, 49, 5141–5143. [Google Scholar] [CrossRef] [PubMed]
- Lemus–Yegres, L.J.; Román–Martínez, M.C.; Such–Basáñez, I.; Lecea, C.S.M. Effects of confinement in hybrid diamine–Rh complex–carbon catalysts used for hydrogenation reactions. Microporous Mesoporous Mater. 2008, 109, 305–316. [Google Scholar] [CrossRef]
- Xiao, J.; Pan, X.; Guo, S.; Ren, P.; Bao, X. Toward fundamentals of confined catalysis in carbon nanotubes. J. Am. Chem. Soc. 2015, 137, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Tessonnier, J.P.; Ersen, O.; Weinberg, G.; Pham–Huu, C.; Su, D.S.; Schlögl, R. Selective deposition of metal nanoparticles inside or outside multiwalled carbon nanotubes. ACS Nano 2009, 3, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Haneda, M.; Watanabe, T.; Kamiuchi, N.; Ozawa, M. Effect of platinum dispersion on the catalytic activity of Pt/Al2O3 for the oxidation of carbon monoxide and propene. Appl. Catal. B Environ. 2013, 142–143, 8–14. [Google Scholar] [CrossRef]
- Hu, C.; Creaser, D.; Fouladvand, S.; Grönbeck, H.; Skoglundh, M. Methyl crotonate hydrogenation over Pt: Effects of support and metal dispersion. Appl. Catal. A Gen. 2016, 511, 106–116. [Google Scholar] [CrossRef]
- Shi, L.; Chu, W.; Deng, S. Catalytic properties of Cu-Co catalysts supported on HNO3-pretreated CNTs for higher-alcohol synthesis. J. Nat. Gas Chem. 2011, 20, 48–52. [Google Scholar] [CrossRef]
- Arciniega Cano, O.; Rodríguez González, C.A.; Hernández Paz, J.F.; Amezaga Madrid, P.; García Casillas, P.E.; Martínez Hernández, A.L.; Martínez Pérez, C.A. Catalytic activity of palladium nanocubes/multiwalled carbon nanotubes structures for methyl orange dye removal. Catal. Today 2017, 282, 168–173. [Google Scholar] [CrossRef]
- Yang, L.; Lin, G.D.; Zhang, H.B. Highly efficient Pd–ZnO catalyst doubly promoted by CNTs and Sc2O3 for methanol steam reforming. Appl. Catal. A Gen. 2013, 455, 137–144. [Google Scholar] [CrossRef]
- Li, C.; Shao, Z.; Pang, M.; Williams, C.T.; Liang, C. Carbon nanotubes supported Pt catalysts for phenylacetylene hydrogenation: Effects of oxygen containing surface groups on Pt dispersion and catalytic performance. Catal. Today 2012, 186, 69–75. [Google Scholar] [CrossRef]
- Qu, P.F.; Chen, J.G.; Song, Y.H.; Liu, Z.T.; Liu, Z.W.; Li, Y.; Lu, J.; Jiang, J. Effect of Fe(III) on hydrogenation of citral over Pt supported multiwalled carbon nanotube. Catal. Commun. 2015, 68, 105–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Yang, G.; Endo, Y.; Tsubaki, N. The solvent effects during preparation of Fischer–Tropsch synthesis catalysts: Improvement of reducibility, dispersion of supported cobalt and stability of catalyst. Catal. Today 2009, 142, 85–89. [Google Scholar] [CrossRef]
- Davari, M.; Karimi, S.; Tavasoli, A.; Karimi, A. CNTs-supported cobalt catalyst in Fischer–Tropsch via CNTs functionalization. Appl. Catal. A Gen. 2014, 485, 133–142. [Google Scholar] [CrossRef]
- Peng, R.; Sun, X.; Li, S.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Shape effect of Pt/CeO2 catalysts on the catalytic oxidation of toluene. Chem. Eng. J. 2016, 306, 1234–1246. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, T.; Liu, X.; Ding, Y. Cu-promoted Pt/activated carbon catalyst for glycerol oxidation to lactic acid. J. Mol. Catal. A Chem. 2016, 424, 91–97. [Google Scholar] [CrossRef]
- Ryabenkoca, Y.; He, Q.; Miedziak, P.J.; Dummer, N.F.; Taylor, S.H.; Carley, A.F.; Morgan, D.J.; Dimitratos, N.; Willock, D.J.; Bethell, D. The selective oxidation of 1,2-propanediol to lactic acid using mild conditions and gold-based nanoparticulate catalysts. Catal. Today 2013, 203, 139–145. [Google Scholar] [CrossRef]
- Guan, Z.; Lu, S.; Li, C. Highly oxidized Pt species stabilized inside carbon nanotubes for asymmetric hydrogenation. Chin. J. Catal. 2015, 36, 1535–1542. [Google Scholar] [CrossRef]
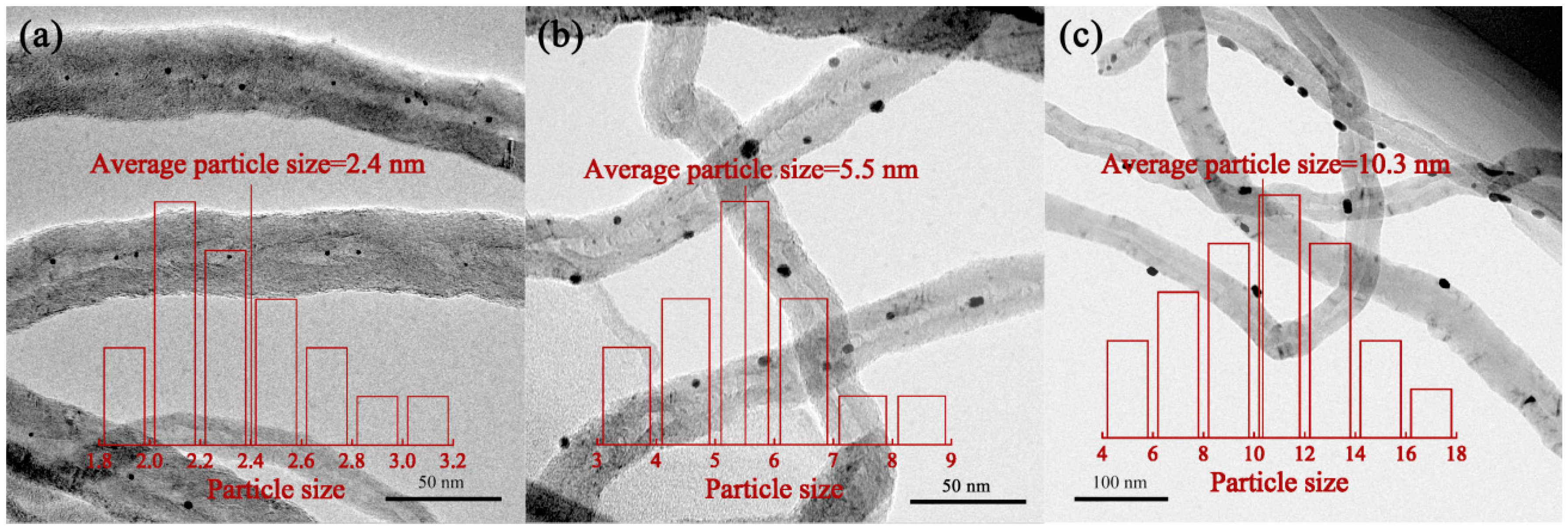
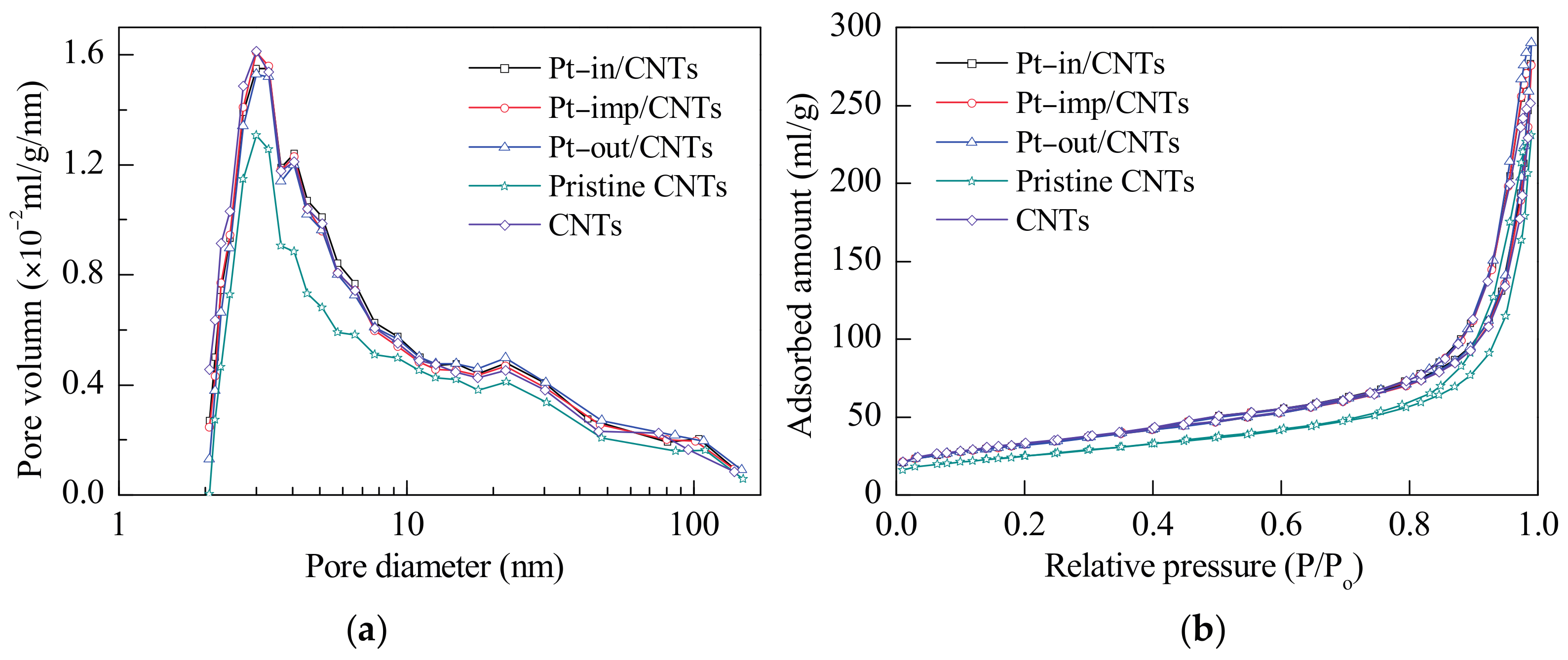
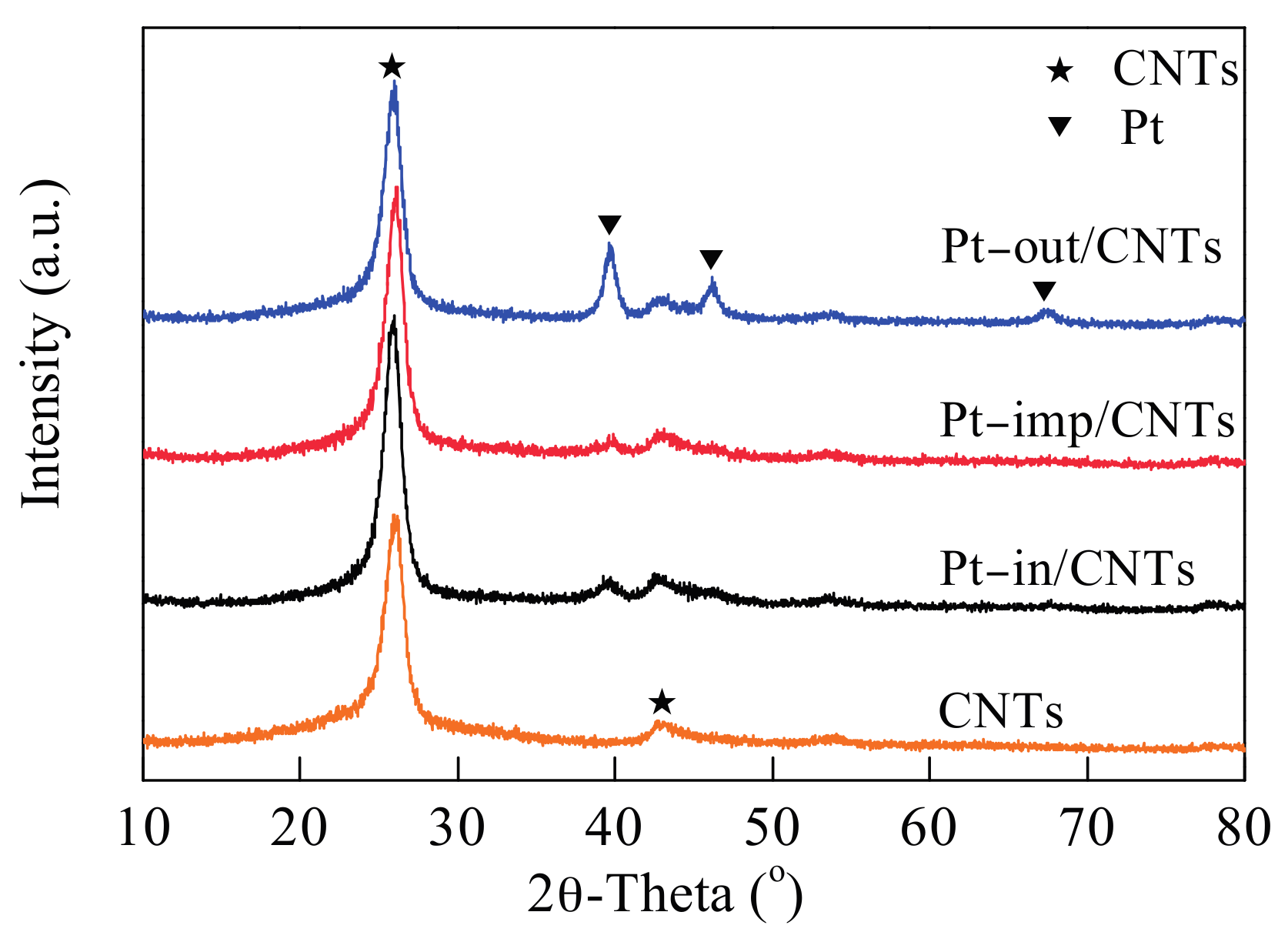
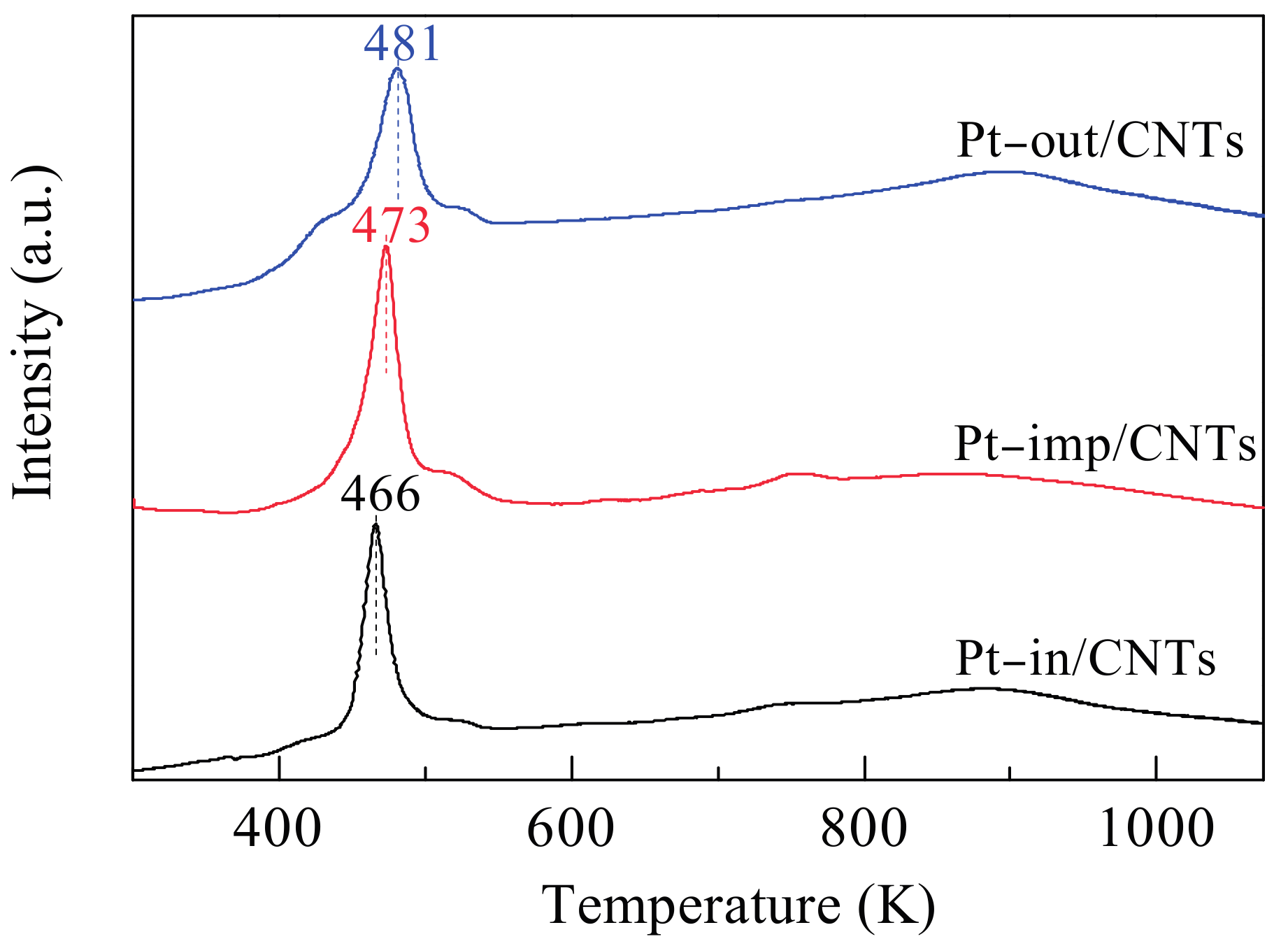

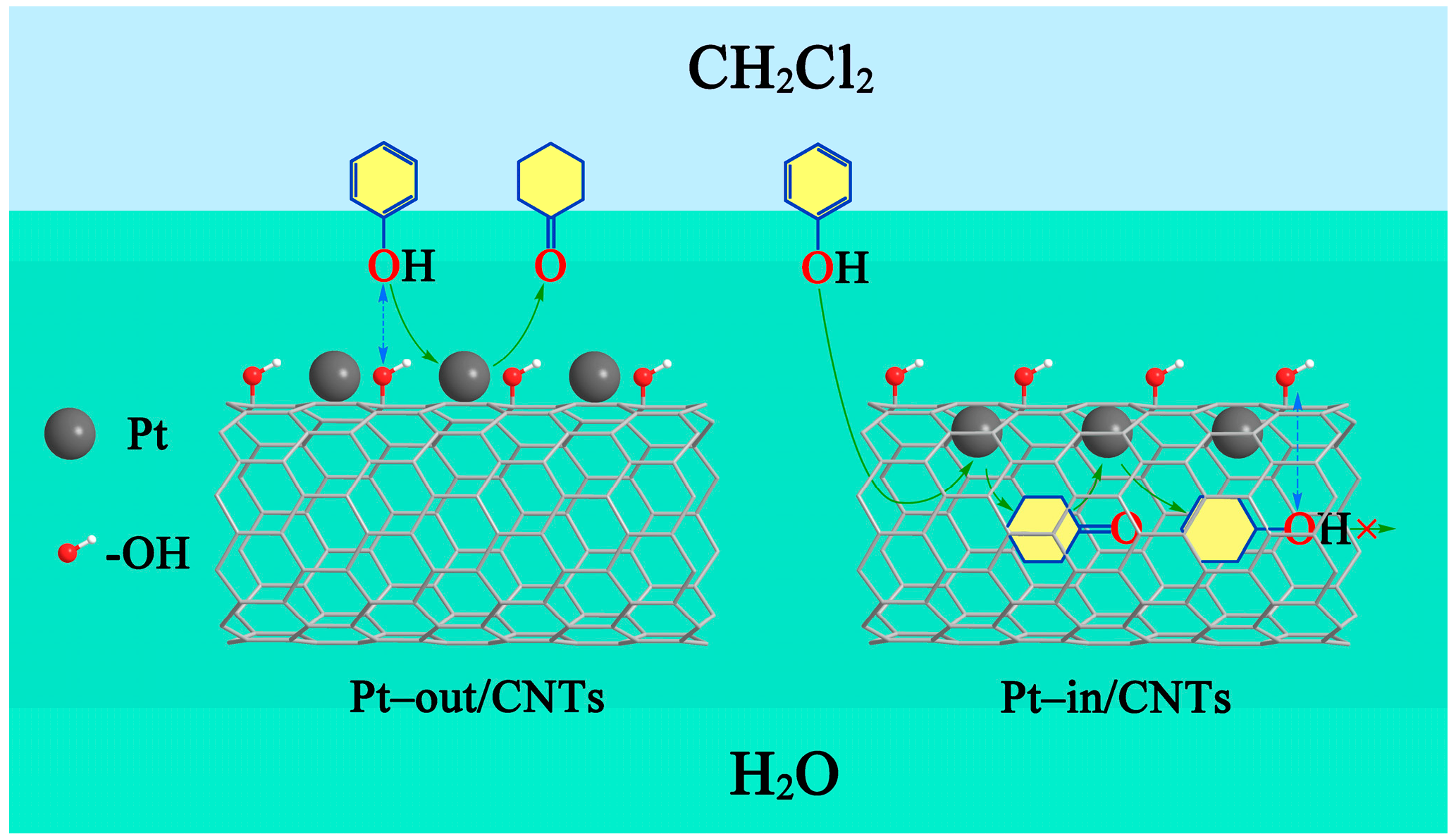
| Sample | SBET (m2/g) | Vpore (cm3/g) | Dpore (nm) | Pt Dispersion 1 | Average Particle Size of Pt (nm) | Pt0/Pt2+,4 | |
|---|---|---|---|---|---|---|---|
| CO Chemisorption 2 | TEM 3 | ||||||
| CNTs 5 | 87.2 | 0.36 | 16.1 | - | - | - | - |
| CNTs 6 | 114.3 | 0.39 | 13.4 | - | - | - | - |
| Pt–in/CNTs | 112.7 | 0.43 | 14.9 | 0.44 | 2.6 | 2.4 | 52.5/47.5 |
| Pt–imp/CNTs | 112.4 | 0.43 | 14.9 | 0.32 | 3.5 | 5.5 | 35.1/64.9 |
| Pt–out/CNTs | 111.7 | 0.45 | 15.8 | 0.17 | 6.6 | 10.3 | 31.3/68.7 |
| Catalyst | Conversion (%) | Selectivity (%) | Reaction Rate 2 | |
|---|---|---|---|---|
| Cyclohexanone | Cyclohexanol | |||
| CNTs | <1 | - | - | - |
| Pt–in/CNTs | 97.3 | 77.5 | 22.5 | 0.934 |
| Pt–imp/CNTs | 33.8 | 75.3 | 24.7 | 0.324 |
| Pt–out/CNTs | 11.6 | 72.3 | 27.7 | 0.111 |
| Catalyst | Run | Pt | Conversion | Selectivity (%) | Reaction Rate | |
|---|---|---|---|---|---|---|
| (wt %) | (%) | Cyclohexanone | Cyclohexanol | |||
| Pt–in/CNTs | 1 | 3.02 | 97.3 | 77.5 | 22.5 | 0.933 |
| 2 | - | 96.8 | 80.1 | 19.9 | 0.929 | |
| 3 | - | 96.0 | 79.9 | 20.1 | 0.921 | |
| 4 | 2.95 | 95.4 | 83.4 | 16.6 | 0.915 | |
| Pt–out/CNTs | 1 | 3.01 | 11.6 | 72.3 | 27.7 | 0.111 |
| 2 | - | 10.5 | 74.5 | 25.5 | 0.101 | |
| 3 | - | 9.0 | 74.6 | 25.4 | 0.086 | |
| 4 | 2.54 | 7.4 | 75.4 | 24.6 | 0.071 | |
| Catalyst | H2O | Conversion | Selectivity (%) | Reaction Rate | |
|---|---|---|---|---|---|
| (wt %) | (%) | Cyclohexanone | Cyclohexanol | ||
| Pt–imp/CNTs | 5 | 70.6 | 89.5 | 10.5 | 0.677 |
| 10 | 86.0 | 99.4 | 0.6 | 0.825 | |
| 15 | 89.6 | 94.3 | 5.7 | 0.860 | |
| 20 | 83.7 | 91.2 | 8.8 | 0.803 | |
| 50 | 28.1 | 78.5 | 21.5 | 0.270 | |
| 100 | 8.8 | 2.9 | 97.1 | 0.084 | |
| 100 2 | 16.4 | 17.4 | 82.6 | 0.157 | |
| 100 3 | 35.1 | 42.2 | 57.8 | 0.337 | |
| Pt–in/CNTs | 10 | 72.5 | 48.9 | 51.1 | 0.696 |
| 100 | 6.3 | 3.6 | 96.4 | 0.060 | |
| Pt–out/CNTs | 10 | 75.3 | 93.1 | 6.9 | 0.722 |
| 100 | 8.9 | 7.6 | 93.4 | 0.085 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Cao, B.; Zhu, W.; Song, H.; Wang, K.; Li, C. Hydrogenation of Phenol over Pt/CNTs: The Effects of Pt Loading and Reaction Solvents. Catalysts 2017, 7, 145. https://doi.org/10.3390/catal7050145
Li F, Cao B, Zhu W, Song H, Wang K, Li C. Hydrogenation of Phenol over Pt/CNTs: The Effects of Pt Loading and Reaction Solvents. Catalysts. 2017; 7(5):145. https://doi.org/10.3390/catal7050145
Chicago/Turabian StyleLi, Feng, Bo Cao, Wenxi Zhu, Hua Song, Keliang Wang, and Cuiqin Li. 2017. "Hydrogenation of Phenol over Pt/CNTs: The Effects of Pt Loading and Reaction Solvents" Catalysts 7, no. 5: 145. https://doi.org/10.3390/catal7050145
APA StyleLi, F., Cao, B., Zhu, W., Song, H., Wang, K., & Li, C. (2017). Hydrogenation of Phenol over Pt/CNTs: The Effects of Pt Loading and Reaction Solvents. Catalysts, 7(5), 145. https://doi.org/10.3390/catal7050145