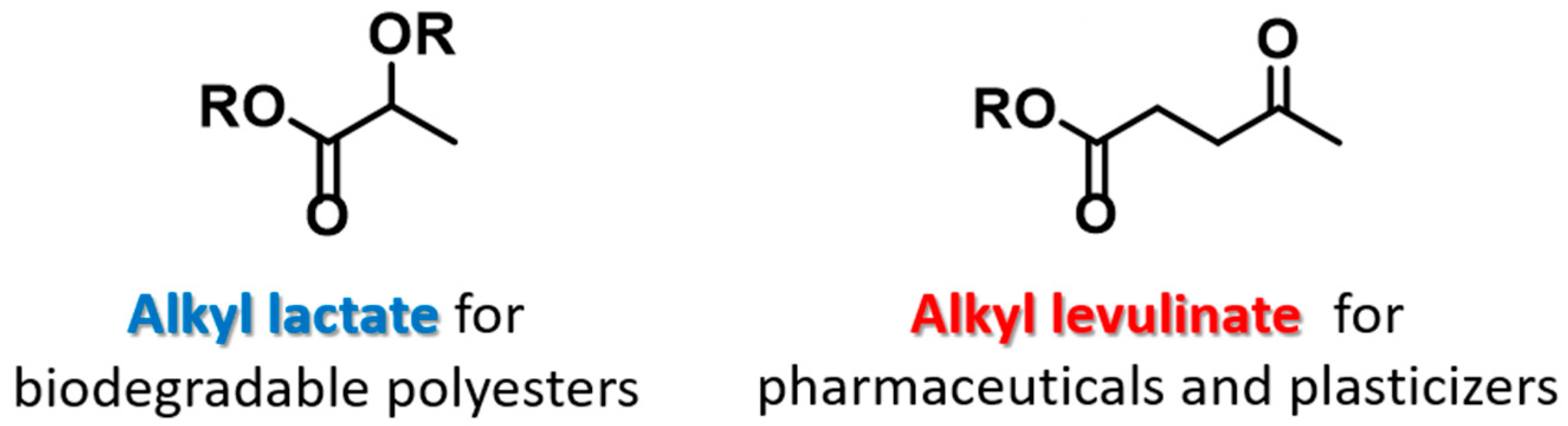
Figure 1 shows that the molecular structures and applications of alkyl lactate and alkyl levulinate. Two molecules of lactic acid can be dehydrated to yield the lactone lactide, and in the presence of catalysts, this lactide can polymerize to give polylactide (PLA), a biodegradable polyester [
23]. Indeed, PLA is an excellent example of a plastic that is not derived from petrochemicals. Lactic acid is also employed in pharmaceutical technologies to produce water-soluble lactates from otherwise-insoluble active ingredients. In addition, it finds further use in topical preparations and cosmetics as an acidity regulator and for its disinfectant and keratolytic properties. In contrast, levulinic acid is employed as a precursor for pharmaceuticals, plasticizers, and various other additives, and is recognized as a building block or starting material for a wide number of compounds. Indeed, this family contributes to various large volume chemical markets, commonly being applied in biofuels such as γ-valerolactone, 2-methyl-tetrahydrofuran, and ethyl levulinate as shown in References [
24,
25,
26,
27,
28].
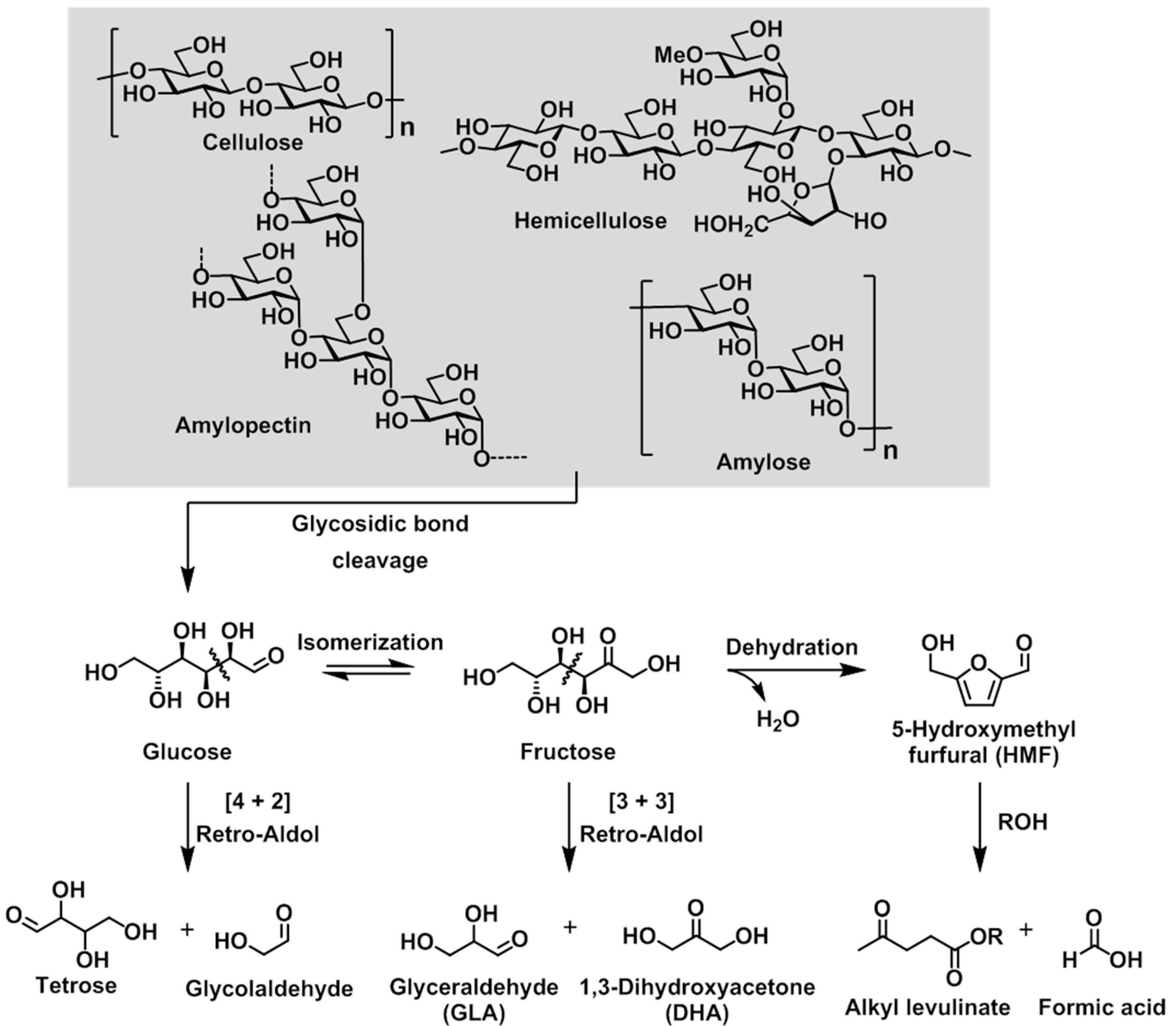
The degradation pathway of polysaccharides such as cellulose, hemicellulose, amylose, and amylopectin is shown in
Scheme 1. Initially, cleavage of the glycosidic linkage in all polysaccharides leads to the production of a common main component, namely the glucose monomer. Each molecule of glucose can then be decomposed to give a tetrose (C4) unit and a glycolaldehyde (C2) unit via a retro-aldol reaction. Alternatively, the isomerization of glucose to yield fructose is thermodynamically preferable, and the corresponding retro-aldol reaction of fructose affords trioses (C3), including 1,3-dihydroxyacetone (DHA) and glyceraldehyde (GLA). A separate dehydration reaction of fructose leads to the formation of furfural derivatives, which can react with alcohols to form alkyl levulinate. In general, the product selectivity is strongly dependent on the characteristics of the catalyst, i.e., the balance between its Lewis and Brønsted acidities.
2.1. Production of Alkyl Lactate from Carbohydrates Using Homogeneous Catalysts
In 2005, Hayashi et al. reported the one-pot synthesis of methyl lactate from DHA and GLA, which were obtained via fermentation and the retro-aldol reaction of glucose [
29]. In this cascade transformation, sequential dehydration, a 1,2-hydride shift, and the addition of methanol take place to yield the desired product. The use of tin halides led to high product yields, while other Lewis acid catalysts (Cr, Zr, Al, etc.) exhibited low activities in this reaction system (
Table 2). To explain this unique behavior of the homogeneous tin metal catalysts, we employed density functional theory calculations to examine the coupling reaction between DHA and formaldehyde, in which tin catalysts also accelerated the aldol condensation [
30]. The calculation results indicated that the strong interaction between the tin catalyst and the reaction intermediates possessing carbonyl moieties can reduce the transition state energy, therefore accelerating the reaction.
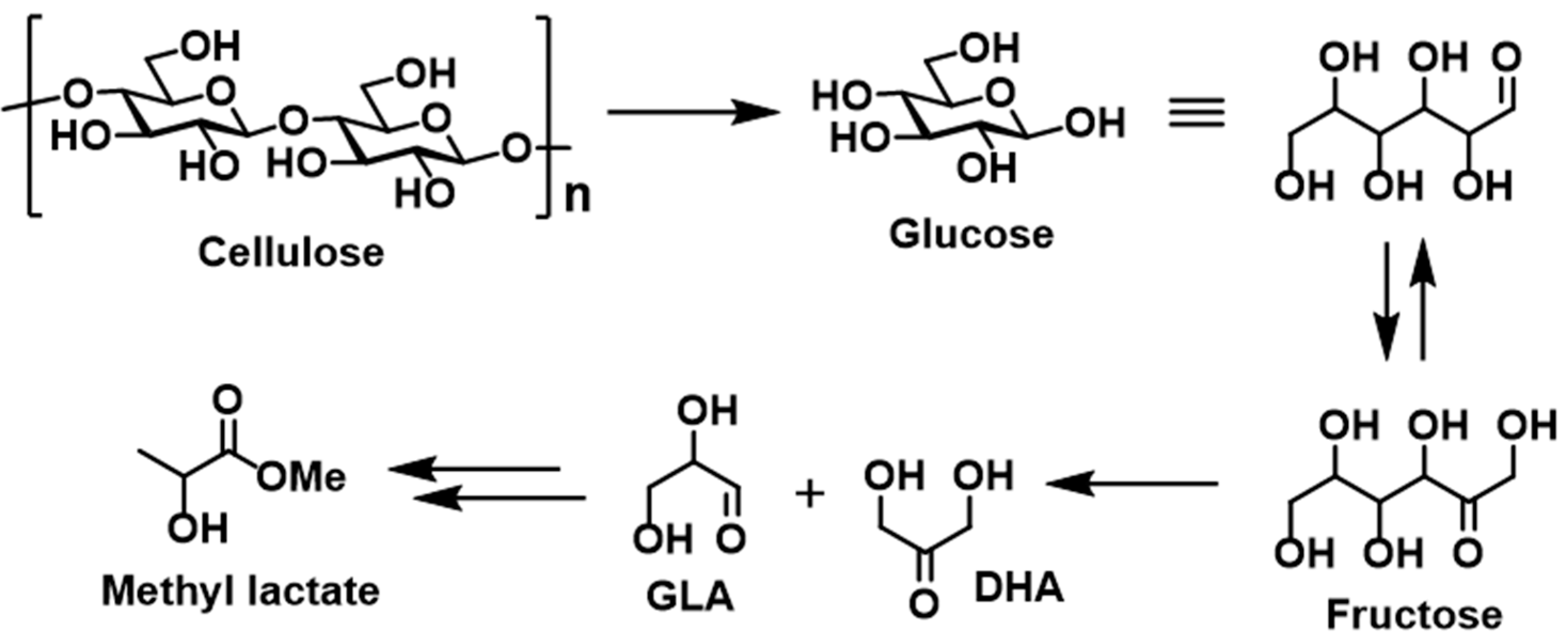
In 2017, Dusselier et al. reported a synthetic process to produce methyl lactate from cellulose (
Scheme 2) [
31]. Following the initial decomposition of cellulose to give the glucose monomer, a subsequent isomerization of glucose followed by a retro-aldol reaction of fructose afforded the desired product. Interestingly, the use of Sn(OTf)
2 (Tf = triflate) accelerated the retro-aldol reaction. In addition, the balance between Sn as a Lewis acid and TfOH as a Brønsted acid has a strong influence on product selectivity, therefore indicating that tuning of the Lewis and Brønsted acidic ratio is crucial to achieving the desired degradation of the polysaccharides and of glucose.
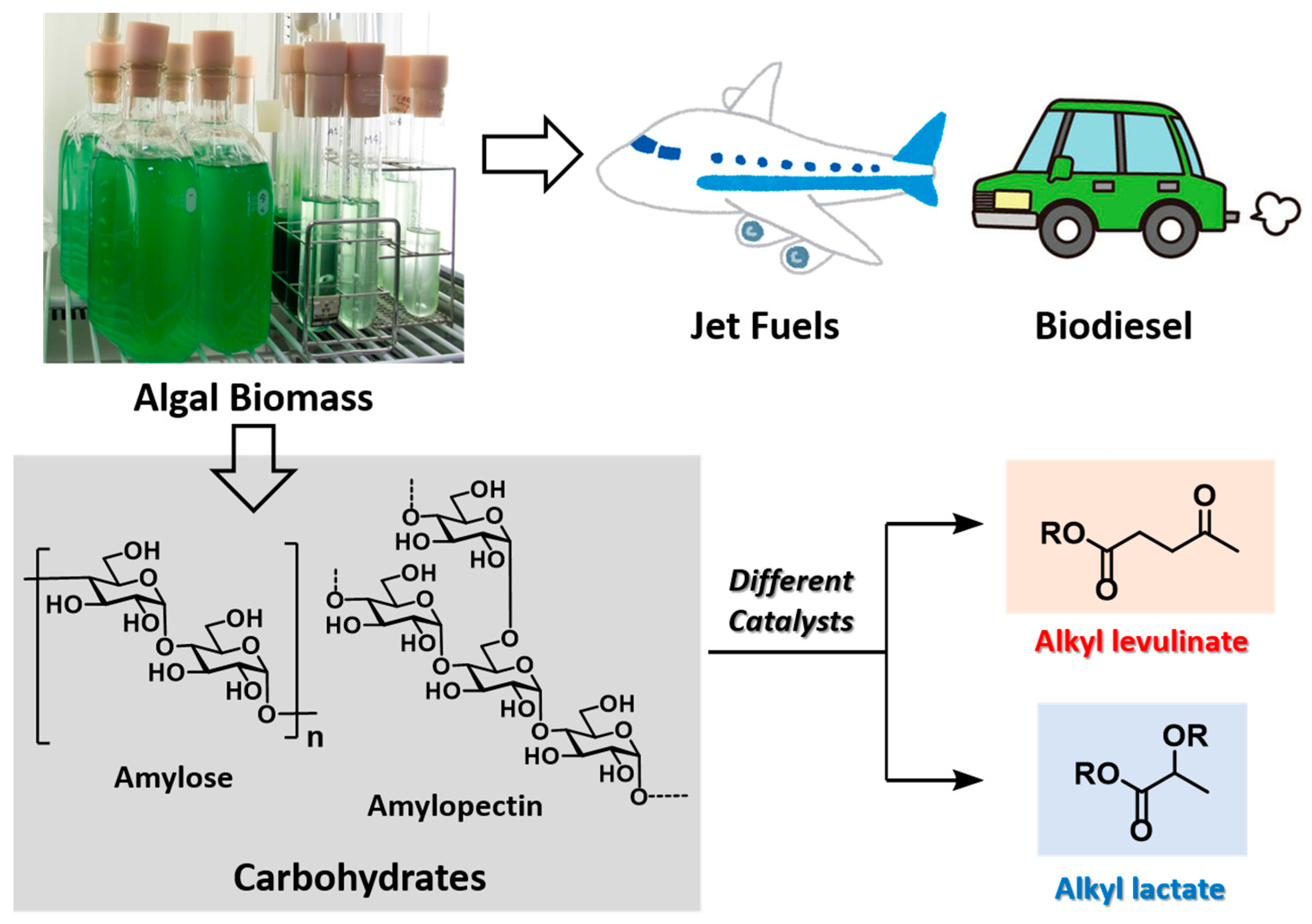
2.3. Chemical Production Using Algal Residue as a Carbon Source
In 2017, we achieved the successful transformation of algal residue into methyl levulinate and methyl lactate [
33]. Following oil abstraction from
Cyanidioschyzon merolae 10D, the algal residue contained amylose and amylopectin as starch polysaccharides, which were then used to produce the target chemicals (
Figure 2).
Initially, we optimized the reaction conditions required for the conversion of authentic starch into either methyl levulinate or methyl lactate (
Table 3). According to a previous report [
31] on the degradation of cellulose, the use of Sn(OTf)
2 led to the production of methyl levulinate and methyl lactate in comparable yields. In addition, as a strong Brønsted acid is required for the synthesis of methyl levulinate (see
Scheme 3), the use of Sn(OTf)
2 was examined, and in this case, only methyl levulinate was obtained in a high yield (entry 1). Furthermore, a high yield of methyl levulinate was achieved using only TfOH, indicating that TfOH is crucial both for the degradation of polysaccharides and for the sequential dehydration reactions (entry 2). In contrast, to selectively synthesize methyl lactate, a balance between the ratio of Lewis and Brønsted acids is important, as mentioned above. Based on these considerations, we examined the effect of different tin halides on the transformation, including SnCl
4·5H
2O, SnBr
4, and SnI
4. Although the use of SnCl
4·5H
2O did not result in the desired product (entry 3), the use of SnBr
4 and SnI
4 gave methyl lactate in satisfactory yields (entries 4 and 5). Other metal halides were not active in this transformation. We also found that the product selectivity was reversible in the presence of HBr, which confirms that the use of tin metal as a Lewis acid is essential in the retro-aldol reaction of fructose.
According to previous reports, SnCl
4 is the most effective tin halide catalyst for the production of methyl lactate from 3- [
29] or 6-carbon [
34] sugars. This can be explained by examining the electronegativity (Χ
p) of the different halogen atoms (see
Table 4) and the pK
a values of the hydrogen halides (see
Table 5). However, in our case, the use of SnCl
4 failed to result in the desired methyl lactate (
Table 3, entry 3). Although the strong Lewis acidity of Sn in SnCl
4 appears to accelerate the retro-aldol reaction, the dehydration, and the 1,2-hydride shift, the glycosidic linkages are not cleaved smoothly due to the low pK
a of HCl. In contrast, as the strong Brønsted acid HBr and HI accelerate the cleavage of the glycosidic linkages, the use of SnBr
4 and SnI
4 produced methyl lactate in moderate yields (i.e., 27% and 20% yields, respectively, see
Table 3, entries 4 and 5).
Based on these results obtaining for the authentic starch sample, the corresponding reactions using algae were investigated. The cultivation conditions and pretreatment method employed are described in the literature [
33] along with details regarding quantification of the neutral sugars. Preparation of the algae for use as a reaction substrate was carried out as indicated in
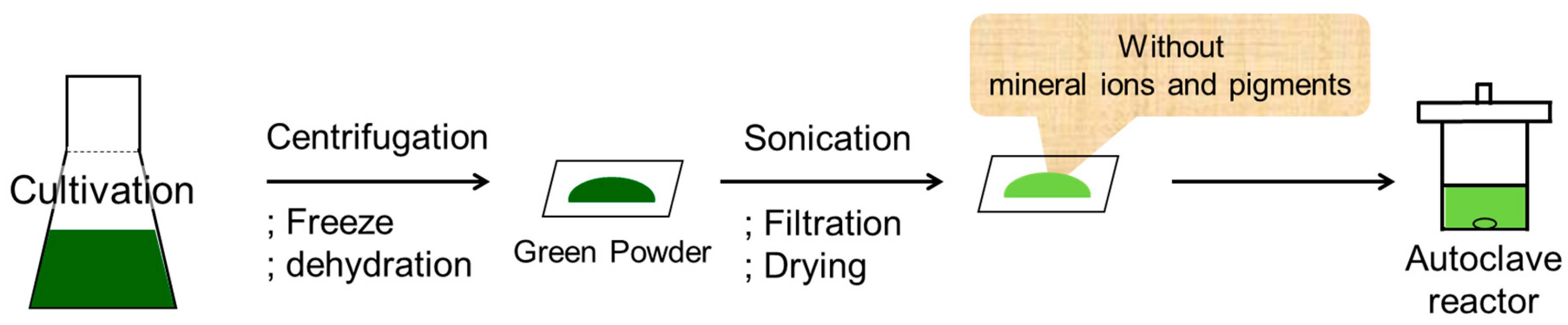
Figure 3. After cultivation of the algae, the centrifugation, freezing, and dehydration steps resulted in a green powder. This powder was then suspended in methanol and subjected to sonication to yield the reaction substrate containing both oils and carbohydrates. However, when the reaction was attempted using this suspension, no traces of the desired products were obtained. We therefore hypothesized that the mineral ions and pigments present in the green powder reduced the catalytic activity, likely through catalyst poisoning. Thus, the sonicated powder suspension was filtered, washed with methanol, and dried in vacuo prior to application in the reaction.
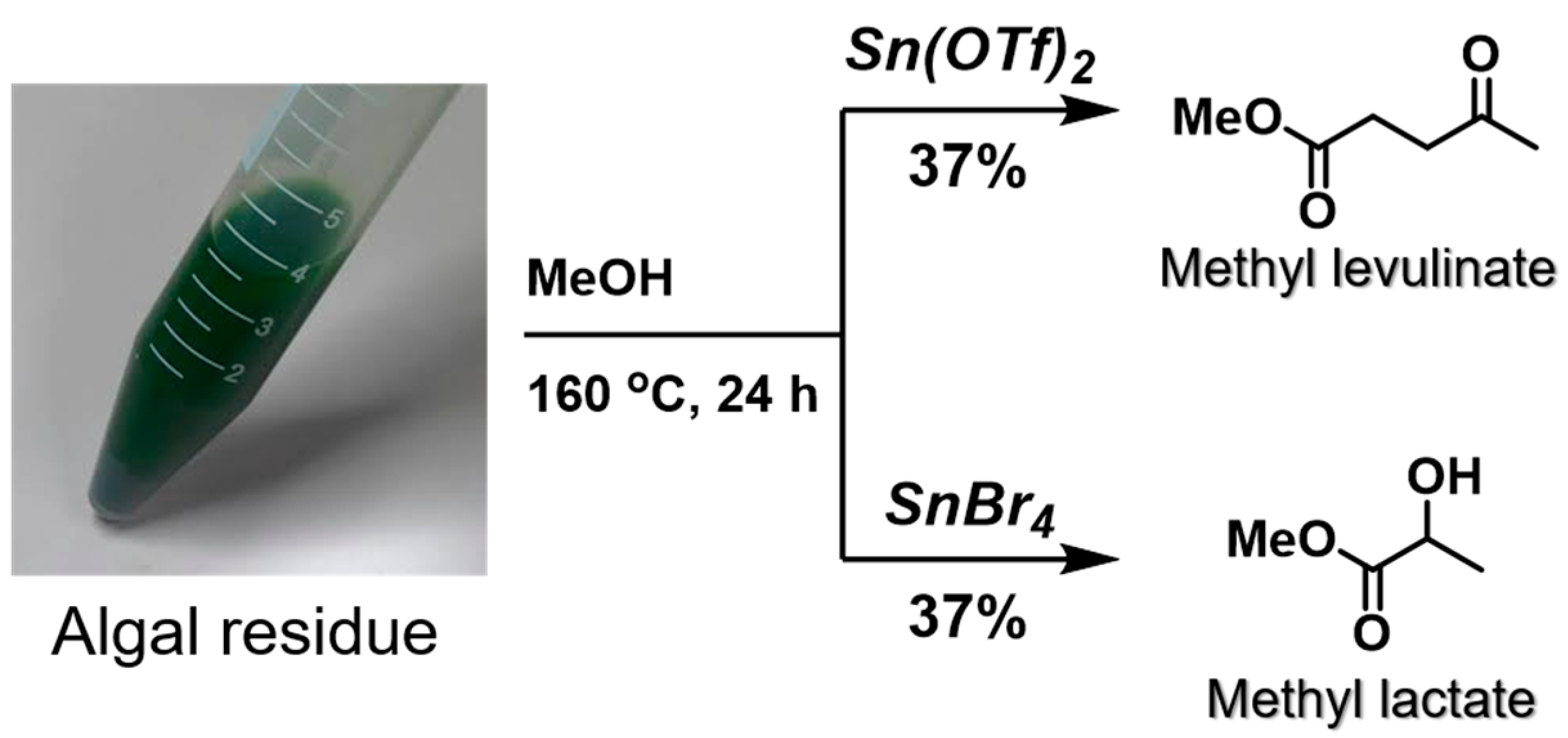
The resulting dried sample was then employed to examine the degradation of the algal carbohydrates (
Scheme 4). Initially, Sn(OTf)
2 was applied as the catalyst under ths same conditions employed for the authentic starch sampe, and the desired products (i.e., methyl levulinate and methyl lactate) were obtained in 37% and 9% yields, respectively. In contrast, the use of SnBr
4 produced respective yields of 6% and 37%, with methyl lactate being the major product, as predicted. Hence, the introduction of an optimized pretreatment method to remove the mineral ions and pigments was successful in retaining the catalyst activity and producting the desired products. However, the goal of this study was to produce chemicals of interest using the algal residue following abstraction of the oil components. Therefore, the sonication step was carried out in a mixed solvent (CHCl
3/MeOH) to separate the oil components from the algal residue. As indicated in
Scheme 4, the product yields obtained from the algal residue were comparable to those obtained without the oil extraction stage. These results confirm that the homogeneous catalysts could also be employed for reactions involving the algal residues.