Review on Copper and Palladium Based Catalysts for Methanol Steam Reforming to Produce Hydrogen
Abstract
:1. Introduction
2. Catalysts
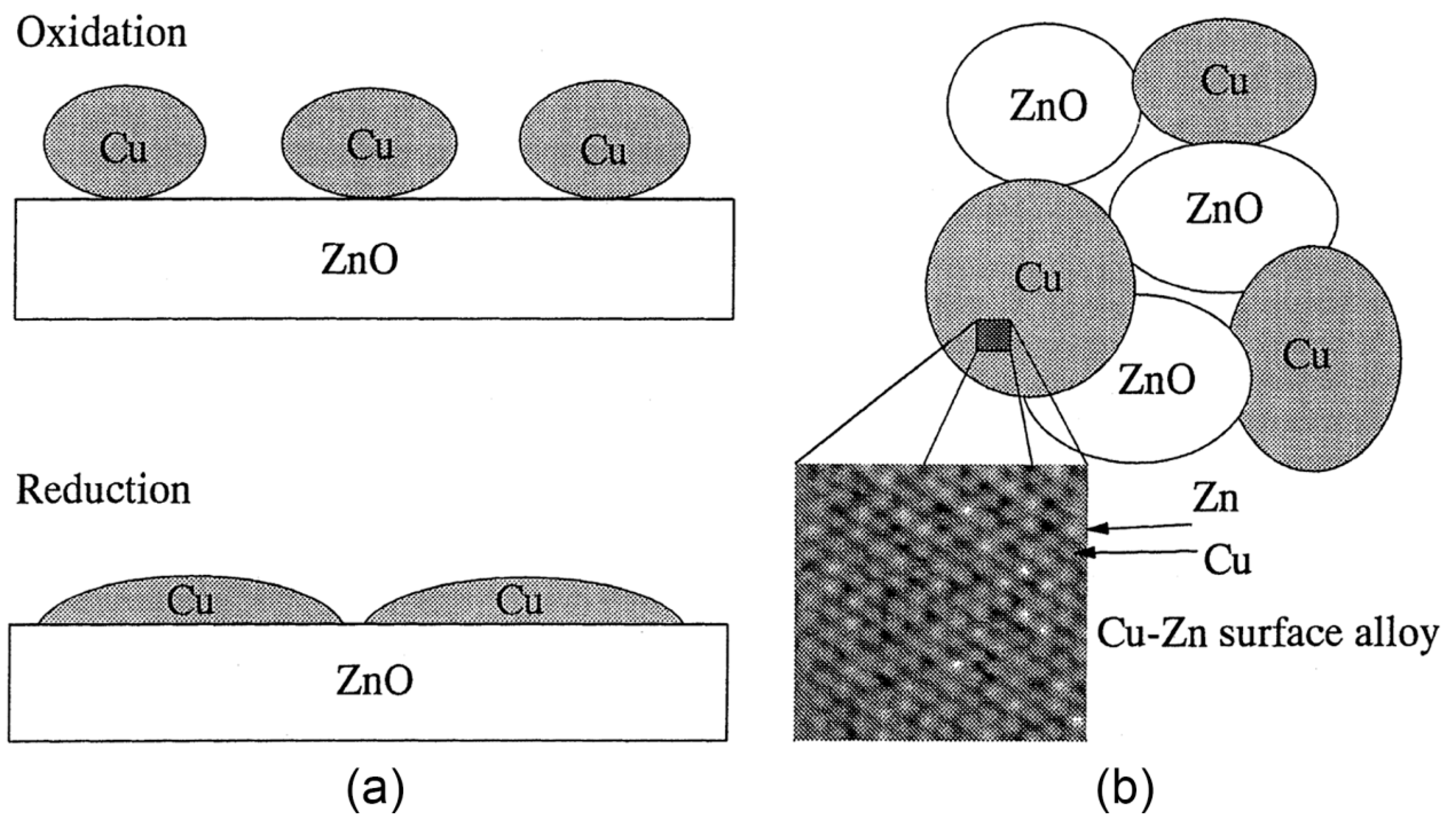
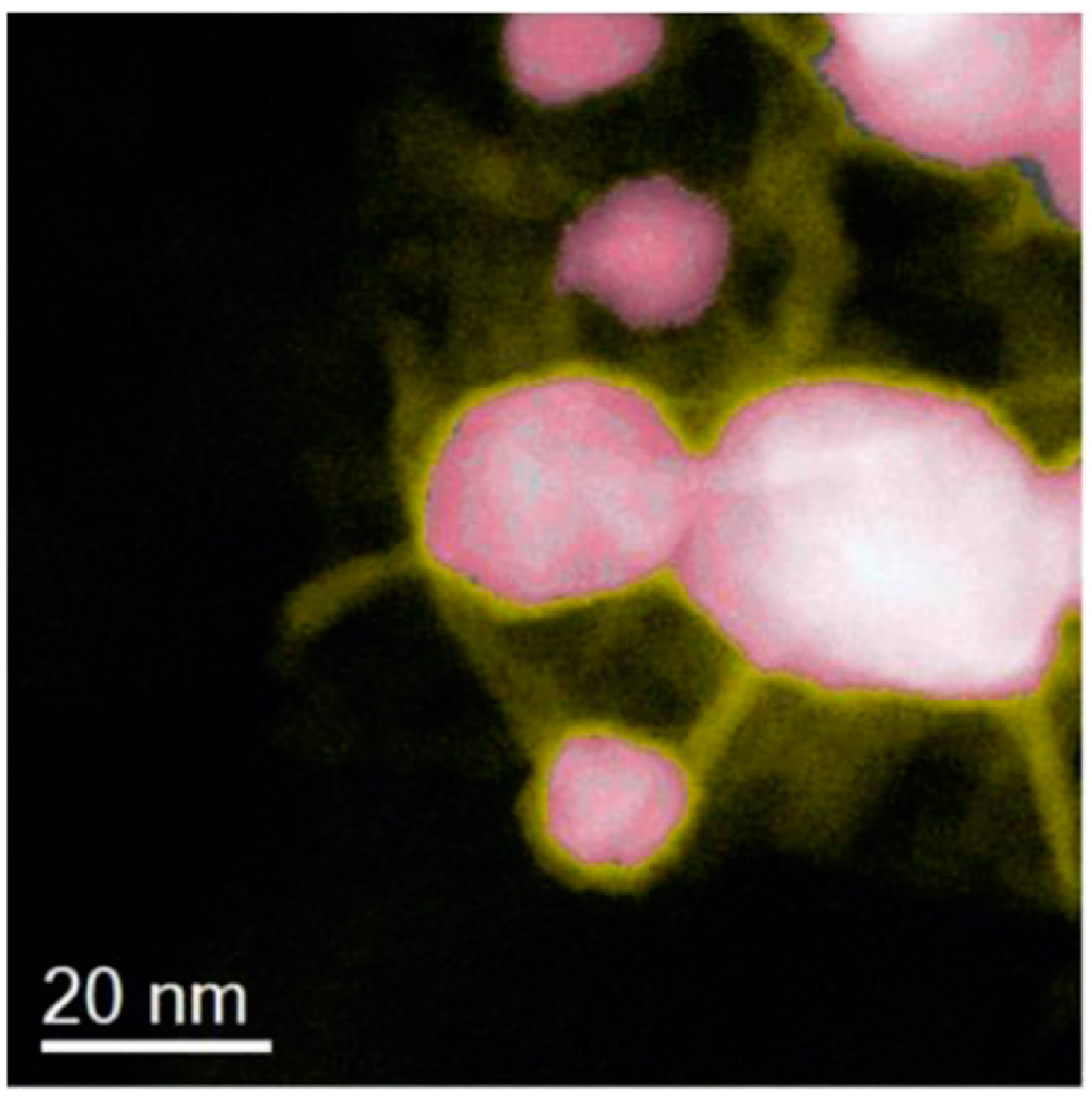
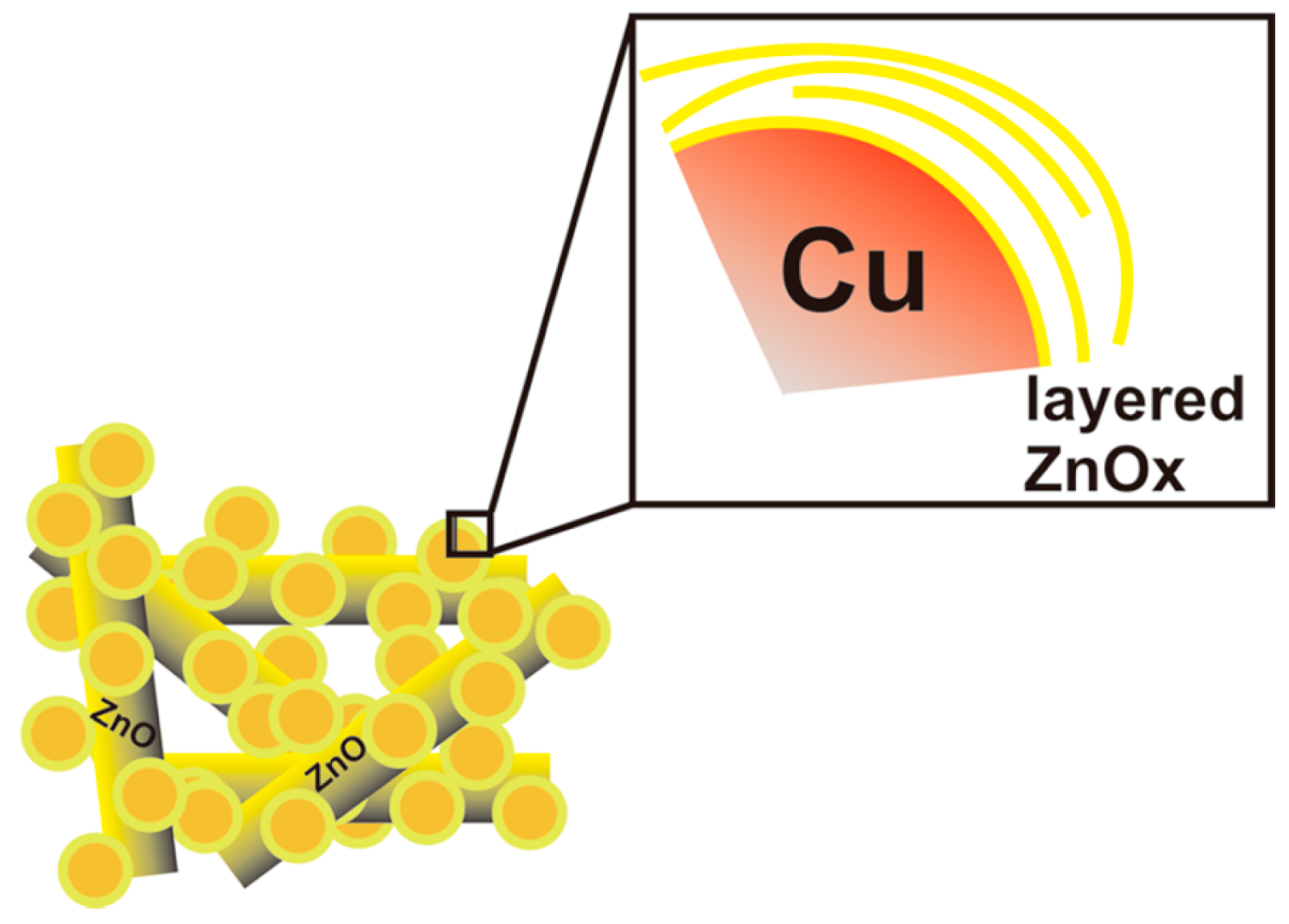
2.1. Copper-Based Catalysts
2.2. Performances of Copper-Based Catalysts
2.3. Palladium Based Catalysts
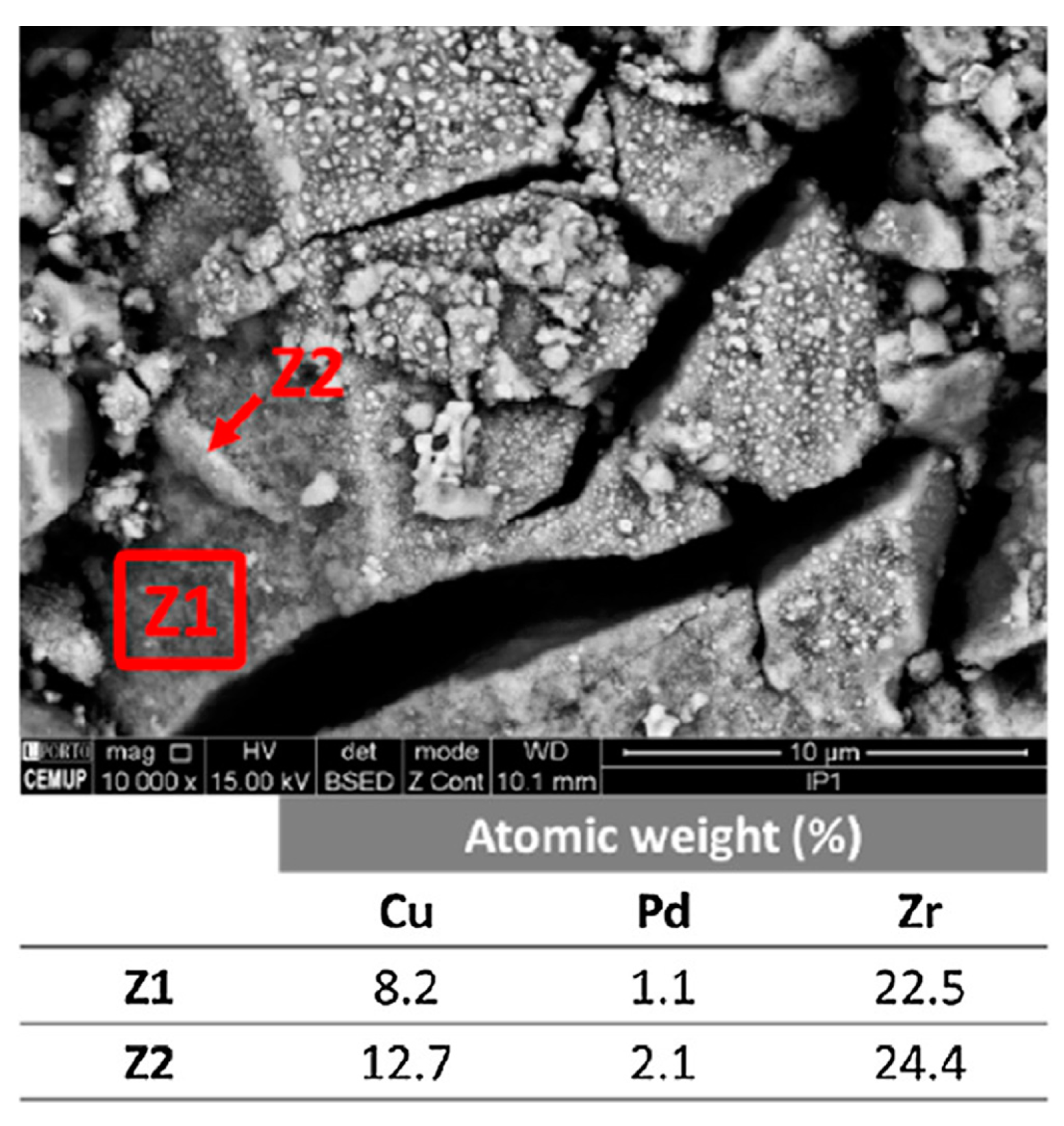
2.4. Performances of Palladium Based Catalysts
2.5. Reaction Mechanism of Methanol Steam Reforming
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, S.; Wang, X.; Xu, X.; Li, P. Hydrogen production via catalytic autothermal reforming of desulfurized Jet—A fuel. Int. J. Hydrog. Energy 2017, 42, 1932–1941. [Google Scholar] [CrossRef]
- Aicher, T.; Lenz, B.; Gschnell, F.; Groos, U.; Federici, F.; Caprile, L.; Parodi, L. Fuel processors for fuel cell APU applications. J. Power Sources 2006, 154, 503–508. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, S.; Li, P. Autothermal reforming of n-dodecane and desulfurized Jet-A fuel for producing hydrogen-rich syngas. Int. J. Hydrog. Energy 2014, 39, 19593–19602. [Google Scholar] [CrossRef]
- Choudhury, A.; Chandra, H.; Arora, A. Application of solid oxide fuel cell technology for power generation—A review. Renew. Sustain. Energy Rev. 2013, 20, 430–442. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, S.; Wang, X.; Li, P. Fuel adaptability study of a lab-scale 2.5 kWth autothermal reformer. Int. J. Hydrog. Energy 2015, 40, 6798–6808. [Google Scholar] [CrossRef]
- Iulianelli, A.; Ribeirinha, P.; Mendes, A.; Basile, A. Methanol steam reforming for hydrogen generation via conventional and membrane reactors: A review. Renew. Sustain. Energy Rev. 2014, 29, 355–368. [Google Scholar] [CrossRef]
- Yong, C.; Ooi, C.; Chai, S.; Wu, X. Review of methanol reforming-Cu-based catalysts, surface reaction mechanisms, and reaction schemes. Int. J. Hydrog. Energy 2013, 38, 9541–9552. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Xu, B. A survey of nickel-based catalysts and monolithic reformers of the onboard fuel reforming system for fuel cell APU applications. Int. J. Energy Res. 2016, 40, 1157–1177. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernandez-Garcia, M.; Martinez-Arias, A. Catalytic hydrogen production through WGS or steam reforming of alcohols over Cu, Ni and Co catalysts. Appl. Catal. A 2016, 518, 2–17. [Google Scholar] [CrossRef]
- Christensen, T. Adiabatic prereforming of hydrocarbons—An important step in syngas production. Appl. Catal. A 1996, 138, 285–309. [Google Scholar] [CrossRef]
- Kubacka, A.; Martines-Arias, A.; Fernandez-Garcia, M. Role of the interface in base-metal ceria-based catalysts for hydrogen purification and production processes. ChemCatChem 2015, 7, 3614–3624. [Google Scholar] [CrossRef]
- Alenazey, F.; Cooper, C.; Dave, C.; Elnashaie, S.; Susu, A.; Adesina, A. Coke removal from deactivated Co–Ni steam reforming catalyst using different gasifying agents: An analysis of the gas–solid reaction kinetics. Catal. Commun. 2009, 10, 406–411. [Google Scholar] [CrossRef]
- Kolb, G. Review: Microstructured reactors for distributed and renewable production of fuels and electrical energy. Chem. Eng. Process. 2013, 65, 1–44. [Google Scholar] [CrossRef]
- Lindstrom, B.; Petterson, L. Deactivation of copper-based catalysts for fuel cell applications. Catal. Lett. 2001, 74, 27–30. [Google Scholar] [CrossRef]
- Rameshan, C.; Stadlmayr, W.; Penner, S.; Lorenz, H.; Memmel, N.; Havecker, M.; Blume, R.; Teschner, D.; Rocha, T.; Zemlyanov, D.; et al. Hydrogen production by methanol steam reforming on copper boosted by zinc-assisted water activation. Angew. Chem. Int. Ed. 2012, 51, 3002–3006. [Google Scholar] [CrossRef] [PubMed]
- Kasatkin, I.; Kurr, P.; Kniep, B.; Trunschke, A.; Schlogl, R. Role of lattice strain and defects in copper particles on the activity of Cu/ZnO/Al2O3 catalysts for methanol synthesis. Angew. Chem. Int. Ed. 2007, 119, 7465–7468. [Google Scholar] [CrossRef]
- Burch, R.; Golunski, E.; Spencer, M. The role of copper and zinc oxide in methanol synthesis catalysts. J. Chem. Soc. Faraday Trans. 1990, 86, 2683–2691. [Google Scholar] [CrossRef]
- Bowker, M.; Hadden, R.; Houghton, H.; Hyland, J.; Waugh, K. The mechanism of methanol synthesis on copper/zinc oxide/alumina catalysts. J. Catal. 1988, 109, 263–273. [Google Scholar] [CrossRef]
- Clausen, B.; Schiøtz, J.; Gråbæk, L.; Ovesen, C.; Jacobsen, K.; Nørskov, J.; Topsøe, H. Wetting/non-wetting phenomena during catalysis: Evidence from in situ on-line EXAFS studies of Cu-based catalysts. Top. Catal. 1994, 1, 367–376. [Google Scholar] [CrossRef]
- Grunwaldt, J.D.; Molenbroek, A.; Topsoe, N.Y.; Topsoe, H.; Clausen, B. In Situ investigations of structural changes in Cu/ZnO catalysts. J. Catal. 2000, 194, 452–460. [Google Scholar] [CrossRef]
- Yurieva, T.; Plyasova, L.; Makarova, O.; Krieger, T. Mechanisms for hydrogenation of acetone to isopropanol and of carbon oxides to methanol over copper-containing oxide catalysts. J. Mol. Catal. 1996, 113, 455–468. [Google Scholar] [CrossRef]
- Nakamura, J.; Choi, Y.; Fujitani, T. On the issue of the active site and the role of ZnO in Cu/ZnO methanol synthesis catalysts. Top. Catal. 2003, 22, 277–285. [Google Scholar] [CrossRef]
- Alejo, L.; Lago, R.; Pena, M.; Fierro, J. Partial oxidation of methanol to produce hydrogen over Cu-Zn-based catalysts. Appl. Catal. A 1997, 162, 281–297. [Google Scholar] [CrossRef]
- Lunkenbein, T.; Schumann, J.; Behrens, M.; Schlogl, R.; Willinger, M. Formation of a ZnO overlayers in industrial Cu/ZnO/Al2O3 catalysts induced by strong metal-support interactions. Angew. Chem. Int. Ed. 2015, 54, 4544–4548. [Google Scholar] [CrossRef] [PubMed]
- Fichtl, M.; Schumann, J.; Kasatkin, I.; Jacobsen, N.; Behrens, M.; Schlogl, R.; Muhler, M.; Hinrichsen, O. Counting of oxygen defects versus metal surface sites in methanol synthesis catalysts by different probe molecules. Angew. Chem. Int. Ed. 2014, 53, 7043–7047. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Hou, X.; Liu, Y.; Qing, S.; Gao, Z. Cu-Al spinel oxide as an efficient catalyst for methanol steam reforming. Angew. Chem. Int. Ed. 2014, 53, 11886–11889. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Guo, J.; Liang, D.; Hou, Z.; Fei, J.; Zheng, X. Production of syngas via autothermal reforming of methane in a fluidized-bed reactor over the combined CeO2-ZrO2/SiO2 supported Ni catalysts. Int. J. Hydrog. Energy 2008, 33, 5493–5500. [Google Scholar] [CrossRef]
- Liu, Y.; Hayakawa, T.; Suzuki, K.; Hamakawa, S. Production of hydrogen by steam reforming of methanol over Cu/CeO2 catalysts derived from Ce1−xCuxO2−x precursors. Catal. Commun. 2001, 2, 195–200. [Google Scholar] [CrossRef]
- Patel, S.; Pant, K. Activity and stability enhancement of copper-alumina catalysts using cerium and zinc promoters for the selective production of hydrogen via steam reforming of methanol. J. Power Sources 2006, 159, 139–143. [Google Scholar] [CrossRef]
- Men, Y.; Gnaser, H.; Zapf, R.; Hessel, V.; Ziegler, C. Parallel screening of Cu/CeO2/γ-Al2O3 catalysts for steam reforming of methanol in a 10-channel micro-structured reactor. Catal. Commun. 2004, 5, 671–675. [Google Scholar] [CrossRef]
- Men, Y.; Gnaser, H.; Ziegler, C.; Zapf, R.; Hessel, V.; Kolb, G. Characterization of Cu/CeO2/γ-Al2O3 thin film catalysts by thermal desorption spectroscopy. Catal. Lett. 2005, 105, 35–40. [Google Scholar] [CrossRef]
- Men, Y.; Gnaser, H.; Zapf, R.; Hessel, V.; Ziegler, C.; Kolb, G. Steam reforming of methanol over Cu/CeO2/γ-Al2O3 catalysts in a microchannel reactor. Appl. Catal. A 2004, 277, 83–90. [Google Scholar] [CrossRef]
- Wu, G.; Mao, D.; Lu, G.; Cao, Y.; Fan, K. The role of the promoters in Cu based catalysts for methanol steam reforming. Catal. Lett. 2009, 130, 177–184. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, K.; Kim, T.; Ko, C.; Park, H.; Song, I. Hydrogen production by steam reforming of methanol in a micro-channel reactor coated with Cu/ZnO/ZrO2/Al2O3 catalyst. J. Power Sources 2006, 159, 1296–1299. [Google Scholar] [CrossRef]
- Huang, G.; Liaw, B.; Jhang, C.; Chen, Y. Steam reforming of methanol over CuO/ZnO/CeO2/ZrO2/Al2O3 catalysts. Appl. Catal. A 2009, 358, 7–12. [Google Scholar] [CrossRef]
- Pan, L.; Wang, S. Modeling of a compact plate-fin reformer for methanol steam reforming in fuel cell systems. Chem. Eng. J. 2005, 108, 51–58. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, L.; Ni, C.; Sun, T.; Zhao, S.; Wang, S.; Wang, A.; Hu, Y. CeO2-ZrO2-promoted CuO/ZnO catalyst for methanol steam reforming. Int. J. Hydrog. Energy 2013, 38, 4397–4406. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, L.; Ni, C.; Sun, T.; Wang, S.; Hu, Y.; Wang, A.; Zhao, S. Effects of precipitation aging time on the performance of CuO/ZnO/CeO2-ZrO2 for methanol steam reforming. J. Fuel Chem. Technol. 2013, 41, 883–888. [Google Scholar] [CrossRef]
- Baneshi, J.; Haghighi, M.; Jodeiri, N.; Abdollahifar, M.; Ajamein, H. Homogeneous precipitation synthesis of CuO-ZrO2-CeO2-Al2O3 nanocatalyst used in hydrogen production via methanol steam reforming for fuel cell applications. Energy Convers. Manag. 2014, 87, 928–937. [Google Scholar] [CrossRef]
- Zeng, D.; Pan, M.; Wang, L.; Tang, Y. Fabrication and characteristics of cube-post microreactors for methanol steam reforming. Appl. Energy 2012, 91, 208–213. [Google Scholar] [CrossRef]
- Pan, M.; Wu, Q.; Jiang, L.; Zeng, D. Effect of microchannel structure on the reaction performance of methanol steam reforming. Appl. Energy 2015, 154, 416–427. [Google Scholar] [CrossRef]
- Jones, S.; Neal, L.; Everett, M.; Hoflund, G.; Hagelin-Weaver, H. Characterization of ZrO2-promoted Cu/ZnO/nano-Al2O3 methanol steam reforming catalysts. Appl. Surf. Sci. 2010, 256, 7345–7353. [Google Scholar] [CrossRef]
- Chang, C.; Chang, C.; Chiang, S.; Liaw, B.; Chen, Y. Oxidative steam reforming of methanol over CuO/ZnO/CeO2/ZrO2/Al2O3 catalysts. Int. J. Hydrog. Energy 2010, 35, 7675–7683. [Google Scholar] [CrossRef]
- Ahmadi, F.; Haghighi, M.; Ajamein, H. Sonochemically coprecipitation synthesis of CuO/ZnO/ZrO2/Al2O3 nanocatalyst for fuel cell grade hydrogen production via steam methanol reforming. J. Mol. Catal. A 2016, 421, 196–208. [Google Scholar] [CrossRef]
- Iwasa, N.; Kudo, S.; Takahashi, H.; Masuda, S.; Takezawa, N. Highly selective supported Pd catalysts for steam reforming of methanol. Catal. Lett. 1993, 19, 211–216. [Google Scholar] [CrossRef]
- Iwasa, N.; Masuda, S.; Ogawa, N.; Takezawa, N. Steam reforming of methanol over Pd/ZnO: Effect of the formation of PdZn alloys upon the reaction. Appl. Catal. A 1995, 125, 145–157. [Google Scholar] [CrossRef]
- Chin, Y.; Dagle, R.; Hu, J.; Dohnalkova, A.; Wang, Y. Steam reforming of methanol over highly active Pd/ZnO catalyst. Catal. Today 2002, 77, 79–88. [Google Scholar] [CrossRef]
- Chin, Y.; Wang, Y.; Dagle, R.; Li, X. Methanol steam reforming over Pd/ZnO: Catalyst preparation and pretreatment studies. Fuel Process Technol. 2003, 83, 193–201. [Google Scholar] [CrossRef]
- Karim, A.; Conant, T.; Datye, A. The role of PdZn alloy formation and particle size on the selectivity for steam reforming of methanol. J. Catal. 2006, 243, 420–427. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, J.; Dagle, V.; Halevi, B.; Datye, A.; Wang, Y. Influence of ZnO facets on Pd/ZnO catalysts for methanol steam reforming. ACS Catal. 2014, 4, 2379–2386. [Google Scholar] [CrossRef]
- Liu, S.; Takahashi, K.; Uematsu, K.; Ayabe, M. Hydrogen production by oxidative methanol reforming on Pd/ZnO. Appl. Catal. A 2005, 283, 125–135. [Google Scholar] [CrossRef]
- Liu, S.; Takahashi, K.; Ayabe, M. Hydrogen production by oxidative methanol reforming on Pd/ZnO catalyst: Effects of Pd loading. Catal. Today 2003, 87, 247–253. [Google Scholar] [CrossRef]
- Pfeifer, P.; Schubert, K.; Liauw, M.; Emig, G. PdZn catalysts prepared by washcoating microstructured reactors. Appl. Catal. A 2004, 270, 165–175. [Google Scholar] [CrossRef]
- Liu, S.; Takahashi, K.; Fuchigami, K.; Uematsu, K. Hydrogen production by oxidative methanol reforming on Pd/ZnO: Catalyst deactivation. Appl. Catal. A 2006, 299, 58–65. [Google Scholar] [CrossRef]
- Ota, A.; Kunkes, E.; Kasatkin, I.; Groppo, E.; Ferri, D.; Poceiro, B.; Yerga, R.; Behrens, M. Comparative study of hydrotalcite-derived supported Pd2Ga and PdZn intermetallic nanoparticles as methanol synthesis and methanol steam reforming catalysts. J. Catal. 2012, 293, 27–38. [Google Scholar] [CrossRef]
- Conant, T.; Karim, A.; Lebarbier, V.; Wang, Y.; Girgsdies, F.; Schlogl, R.; Datye, A. Stability of bimetallic Pd-Zn catalysts for the steam reforming of methanol. J. Catal. 2008, 257, 64–70. [Google Scholar] [CrossRef]
- Iwasa, N.; Mayanagi, T.; Nomura, W.; Arai, M.; Takezawa, N. Effect of Zn addition to supported Pd catalysts in the steam reforming of methanol. Appl. Catal. A 2003, 248, 153–160. [Google Scholar] [CrossRef]
- Ranganathan, E.; Bej, S.; Thompson, L. Methanol steam reforming over Pd/ZnO and Pd/CeO2 catalysts. Appl. Catal. A 2005, 289, 153–162. [Google Scholar] [CrossRef]
- Iwasa, N.; Takezawa, N. New supported Pd and Pt alloy catalysts for steam reforming and dehydrogenation of methanol. Top. Catal. 2003, 22, 215–224. [Google Scholar] [CrossRef]
- Collins, S.; Delgado, J.; Mira, C.; Calvino, J.; Bernal, S.; Chiavassa, D.; Baltanas, M.; Bonivardi, A. The role of Pd-Ga bimetallic particles in the bifunctional mechanism of selective methanol synthesis via CO2 hydrogenation on a Pd/Ga2O3 catalyst. J. Catal. 2012, 292, 90–98. [Google Scholar] [CrossRef]
- Mayr, L.; Lorenz, H.; Armbruster, M.; Villaseca, S.; Luo, Y.; Cardoso, R.; Burkhardt, U.; Zemlyanov, D.; Haevecker, M.; Blume, R.; et al. The catalytic properties of thin film Pd-rich GaPd2 in methanol steam reforming. J. Catal. 2014, 309, 231–240. [Google Scholar] [CrossRef]
- Men, Y.; Kolb, G.; Zapf, R.; O’Connell, M.; Ziogas, A. Methanol steam reforming over bimetallic Pd-In/Al2O3 catalysts in a microstructured reactor. Appl. Catal. A 2010, 380, 15–20. [Google Scholar] [CrossRef]
- Föttinger, K. The effect of CO on intermetallic PdZn/ZnO and Pd2Ga/Ga2O3 methanol steam reforming catalysts: A comparative study. Catal. Today 2013, 208, 106–112. [Google Scholar] [CrossRef]
- Azenha, C.; Mateos-Pedrero, C.; Queiros, S.; Concepcion, P.; Mendes, A. Innovative ZrO2-supported CuPd catalysts for the selective production of hydrogen from methanol steam reforming. Appl. Catal. B 2017, 203, 400–407. [Google Scholar] [CrossRef]
- Kopfle, N.; Mayr, L.; Schmidmair, D.; Bernardi, J.; Knop-Gericke, A.; Havecker, M.; Klotzer, B.; Penner, S. A Comparative Discussion of the Catalytic Activity and CO2-Selectivity of Cu-Zr and Pd-Zr (Intermetallic) Compounds in Methanol Steam Reforming. Catalysts 2017, 7, 53. [Google Scholar] [CrossRef]
- Echave, F.; Sanz, O.; Montes, M. Washcoating of micro-channel reactors with PdZnO catalyst for methanol steam reforming. Appl. Catal. A 2014, 474, 159–167. [Google Scholar] [CrossRef]
- Sanz, O.; Velasco, I.; Reyero, I.; Legorburu, I.; Arzamendi, G.; Gandia, L.; Montes, M. Effect of the thermal conductivity of metallic monoliths on methanol steam reforming. Catal. Today 2016, 273, 131–139. [Google Scholar] [CrossRef]
- Xia, G.; Holladay, J.; Dagle, R.; Jones, E.; Wang, Y. Development of highly active Pd-ZnO/Al2O3 catalysts for microscale fuel processor applications. Chem. Eng. Technol. 2005, 28, 515–519. [Google Scholar] [CrossRef]
- Barrios, C.; Bosco, M.; Baltanas, M.; Bonivardi, A. Hydrogen production by methanol steam reforming: Catalytic performance of supported-Pd on zinc-cerium oxides’ nanocomposites. Appl. Catal. B 2015, 179, 262–275. [Google Scholar] [CrossRef]
- Kuc, J.; Weidenkaff, A.; Matam, S. Methanol steam reforming on perovskite-type oxides LaCo1−x−yPdxZnyO3±δ: Effect of Pd/Zn on CO2 Selectivity. Top. Catal. 2015, 58, 905–909. [Google Scholar] [CrossRef]
- Santacesaria, E.; Carra, S. Kinetics of a catalytic steam reforming of methanol in a CSTR reactor. Appl. Catal. 1983, 5, 345–358. [Google Scholar] [CrossRef]
- Breen, J.; Meunier, F.; Ross, J. Mechanistic aspects of the steam reforming of methanol over a CuO/ZnO/ZrO2/Al2O3 catalyst. Chem. Commun. 1999, 22, 2247–2248. [Google Scholar] [CrossRef]
- Agrell, J.; Bigersson, H.; Boutonnet, M. Steam reforming of methanol over Cu/ZnO/Al2O3 catalysts: A kinetic analysis and trategies for suppression of CO formation. J. Power Sources 2002, 106, 249–257. [Google Scholar] [CrossRef]
- Peppley, B.; Amphlett, J.; Kearns, L.; Mann, R. Methanol-steam reforming on Cu/ZnO/Al2O3. Part 1. The reaction network. Appl. Catal. A 1999, 179, 21–30. [Google Scholar] [CrossRef]
- Peppley, B.; Amphlett, J.; Kearns, L.; Mann, R. Methanol-steam reforming on Cu/ZnO/Al2O3. Part 2. A comprehensive kinetic model. Appl. Catal. A 1999, 179, 31–49. [Google Scholar] [CrossRef]
- Jiang, C.; Trimm, D.; Wainwright, M. Kinetic mechanism for the reaction between methanol and water over a Cu-ZnO-Al2O3 catalyst. Appl. Catal. A 1993, 97, 145–158. [Google Scholar] [CrossRef]
- Jiang, C.; Trimm, D.; Wainwright, M. Kinetic study of stream reforming of methanol over copper-based catalysts. Appl. Catal. A 1993, 93, 245–255. [Google Scholar] [CrossRef]
- Breen, J.; Ross, J. Methanol reforming for fuel-cell applications: Development of zirconia-containing Cu-Zn-Al catalysts. Catal. Today 1999, 51, 521–533. [Google Scholar] [CrossRef]
- Cao, C.; Xia, G.; Holladay, J.; Jones, E.; Wang, Y. Kinetic studies of methanol steam reforming over Pd/ZnO catalyst using a microchannel reactor. Appl. Catal. A 2004, 262, 19–29. [Google Scholar] [CrossRef]
- Uriz, I.; Arzamendi, G.; Dieguez, P.; Echave, F.; Sanz, O.; Montes, M.; Gandia, L. CFD analysis of the effects of the flow distribution and heat losses on the steam reforming of methanol in catalytic (Pd/ZnO) microreactors. Chem. Eng. J. 2014, 238, 37–44. [Google Scholar] [CrossRef]
- Frank, B.; Jentoft, F.; Soerijanto, H.; Krohnert, J.; Schlogl, R.; Schomacker, R. Steam reforming of methanol over copper-containing catalysts: Influence of support material on microkinetics. J. Catal. 2007, 246, 177–192. [Google Scholar] [CrossRef]
- Mastalir, A.; Frank, B.; Szizybalski, A.; Soerijanto, H.; Deshpande, A.; Niederberger, M.; Schomacker, R.; Schlogl, R.; Ressler, T. Steam reforming of methanol over Cu/ZrO2/CeO2 catalysts: A kinetic study. J. Catal. 2005, 230, 464–475. [Google Scholar] [CrossRef]
- Harold, M.; Nair, B.; Kolios, G. Hydrogen generation in a Pd membrane fuel processor: Assessment of methanol-based reaction systems. Chem. Eng. Sci. 2003, 58, 2551–2571. [Google Scholar] [CrossRef]
- Morillo, A.; Freund, A.; Merten, C. Concept and design of a novel compact reactor for autothermal steam reforming with integrated evaporation and CO cleanup. Ind. Eng. Chem. Res. 2004, 43, 4624–4634. [Google Scholar] [CrossRef]
- Gallucci, F.; Paturzo, L.; Basile, A. Hydrogen recovery from methanol steam reforming in a dense membrane reactor: Simulation study. Ind. Eng. Chem. Res. 2004, 43, 2420–2432. [Google Scholar] [CrossRef]
- Hsueh, C.; Chu, H.; Yan, W.; Leu, G.; Tsai, J. Three-dimensional analysis of a plate methanol steam micro-reformer and a methanol catalytic combustor with different flow channel designs. Int. J. Hydrog. Energy 2011, 36, 13575–13586. [Google Scholar] [CrossRef]
- Tadbir, M.; Akbari, M. Methanol steam reforming in a planar wash coated microreactor integrated with a micro-combustor. Int. J. Hydrog. Energy 2011, 36, 12822–12832. [Google Scholar] [CrossRef]
- Chein, R.; Chen, Y.; Chung, J. Thermal resistance effect on methanol-steam reforming performance in micro-scale reformers. Int. J. Hydrog. Energy 2012, 37, 250–262. [Google Scholar] [CrossRef]
- Suh, J.; Lee, M.; Greif, R.; Grigoropoulos, C. A study of steam methanol reformer in a micro-reactor. J. Power Sources 2007, 173, 458–466. [Google Scholar] [CrossRef]
- Purnama, H.; Ressler, T.; Jentoft, R.; Soerijanto, H.; Schlogl, R.; Schomacker, R. CO formation/selectivity for steam reforming of methanol with a commercial CuO/ZnO/Al2O3 catalysts. Appl. Catal. A 2004, 259, 83–94. [Google Scholar] [CrossRef]
- Chen, W.; Lin, M.; Jiang, T.; Chen, M. Modeling and simulation of hydrogen generation from high temperature and low temperature water gas shift reactions. Int. J. Hydrog. Energy 2008, 33, 6644–6656. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Shuai, K.; Xu, B. Review on Copper and Palladium Based Catalysts for Methanol Steam Reforming to Produce Hydrogen. Catalysts 2017, 7, 183. https://doi.org/10.3390/catal7060183
Xu X, Shuai K, Xu B. Review on Copper and Palladium Based Catalysts for Methanol Steam Reforming to Produce Hydrogen. Catalysts. 2017; 7(6):183. https://doi.org/10.3390/catal7060183
Chicago/Turabian StyleXu, Xinhai, Kaipeng Shuai, and Ben Xu. 2017. "Review on Copper and Palladium Based Catalysts for Methanol Steam Reforming to Produce Hydrogen" Catalysts 7, no. 6: 183. https://doi.org/10.3390/catal7060183





