Co-Immobilization of Superoxide Dismutase with Catalase on Soft Microparticles Formed by Self-Assembly of Amphiphilic Poly(Aspartic Acid)
Abstract
:1. Introduction
2. Results and Discussion
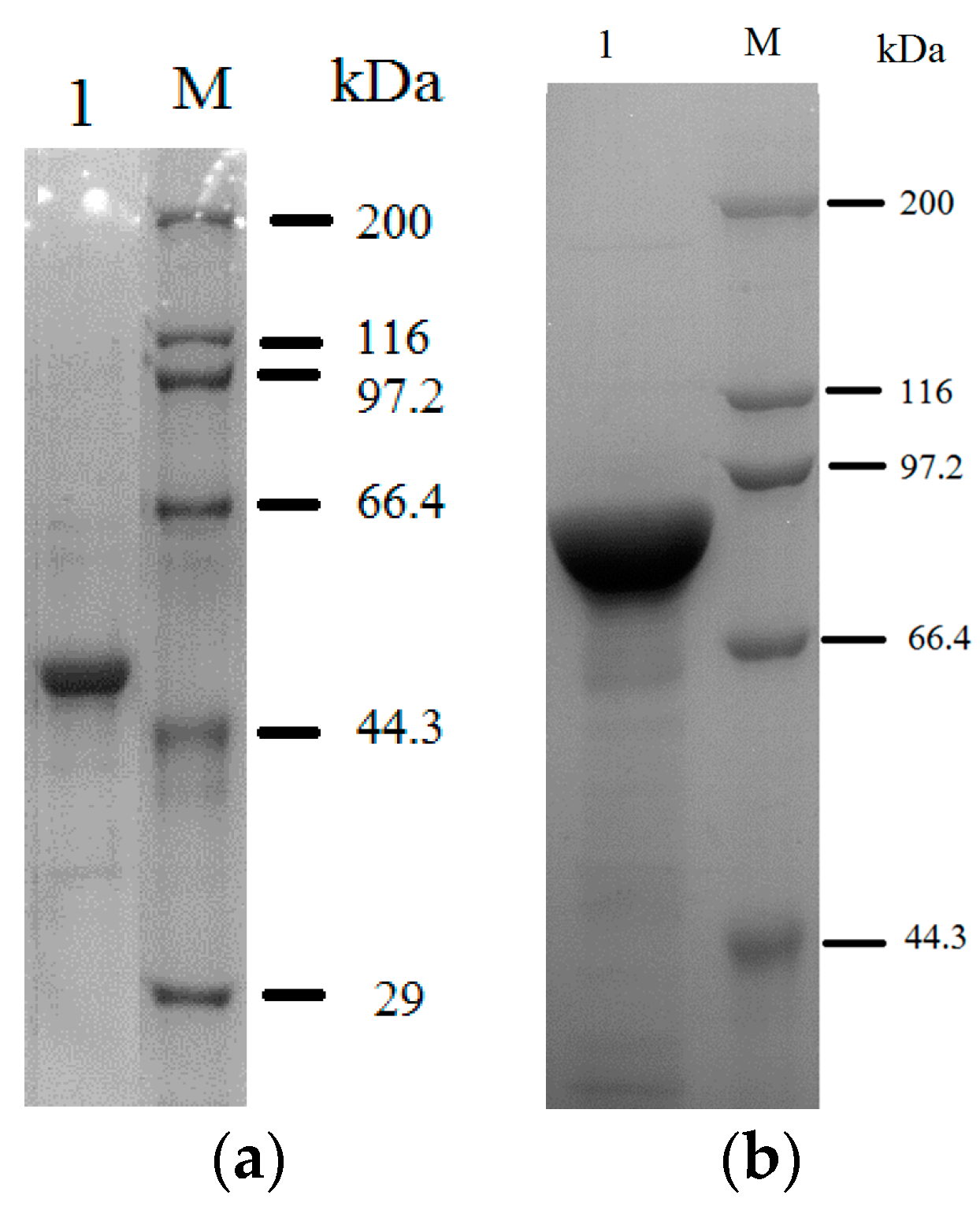
2.1. Purification of the Fusion Enzyme
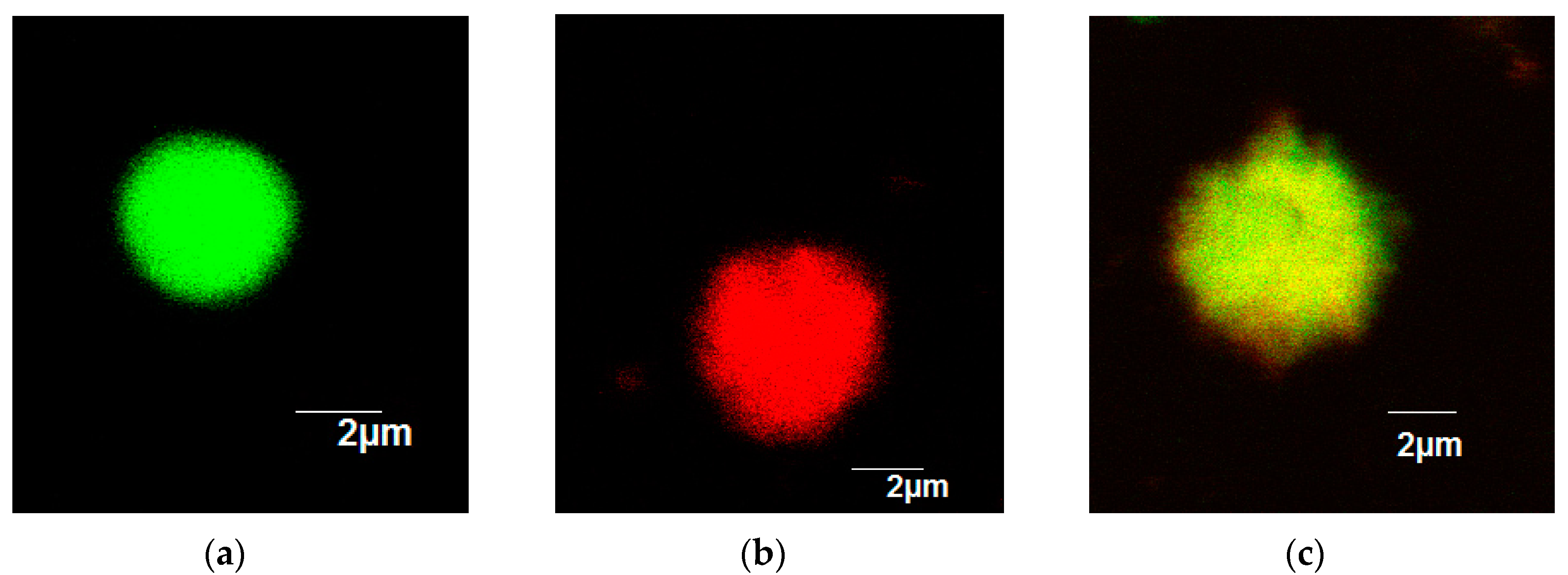
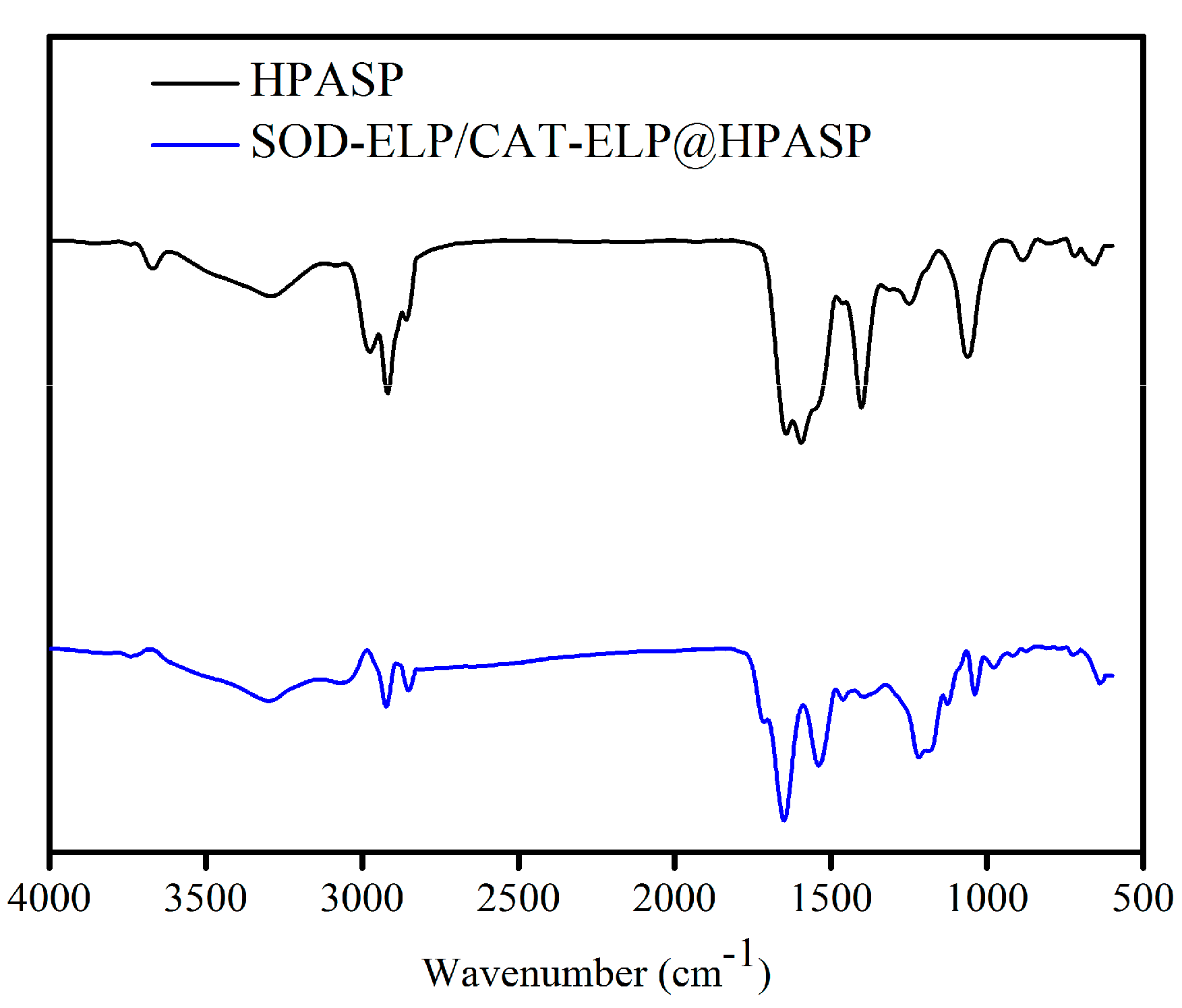
2.2. Immobilization of SOD-ELP and CAT-ELP on HPASP
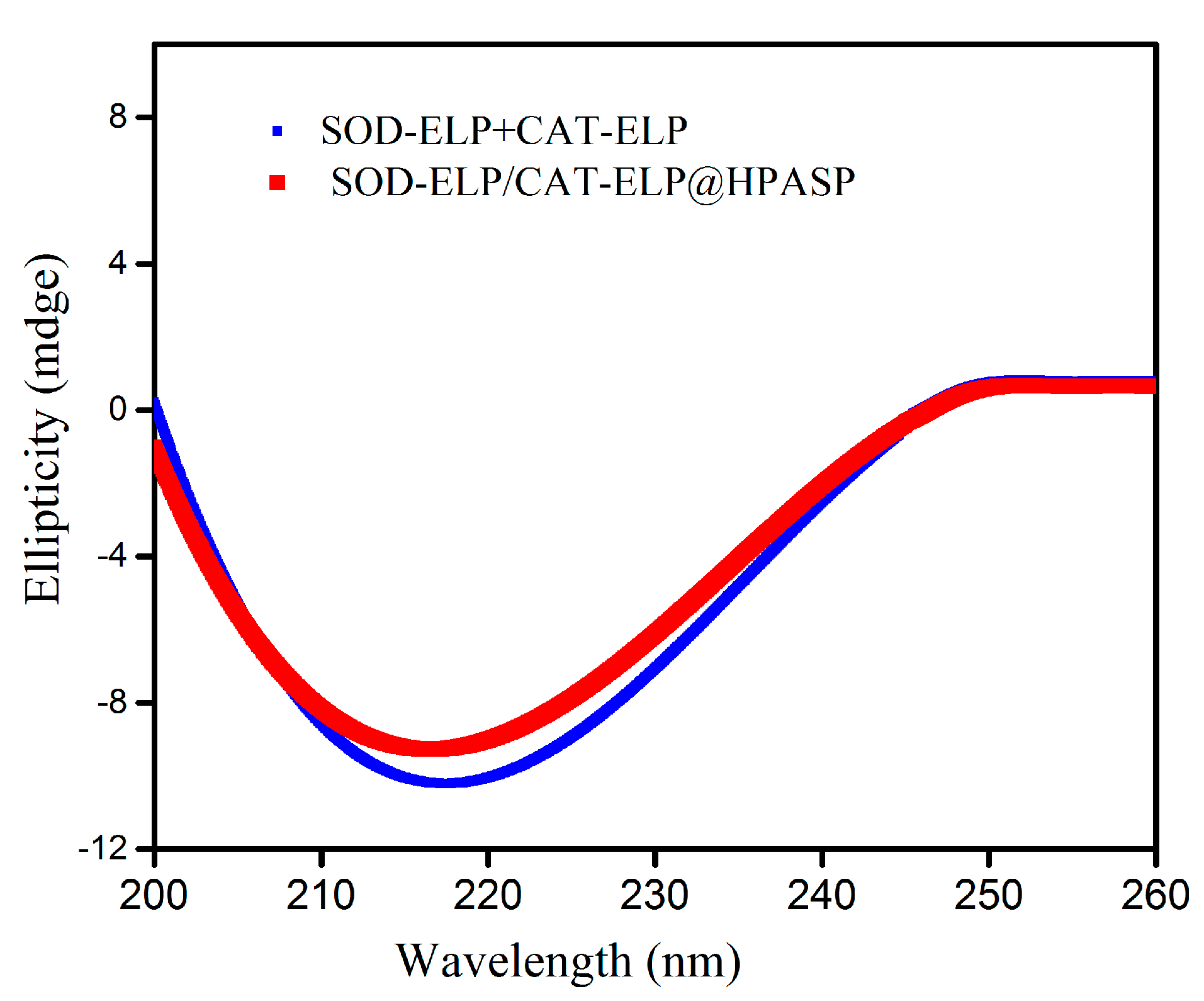
2.3. Secondary Structures of Enzymes Monitored by Circular Dichroism Spectra
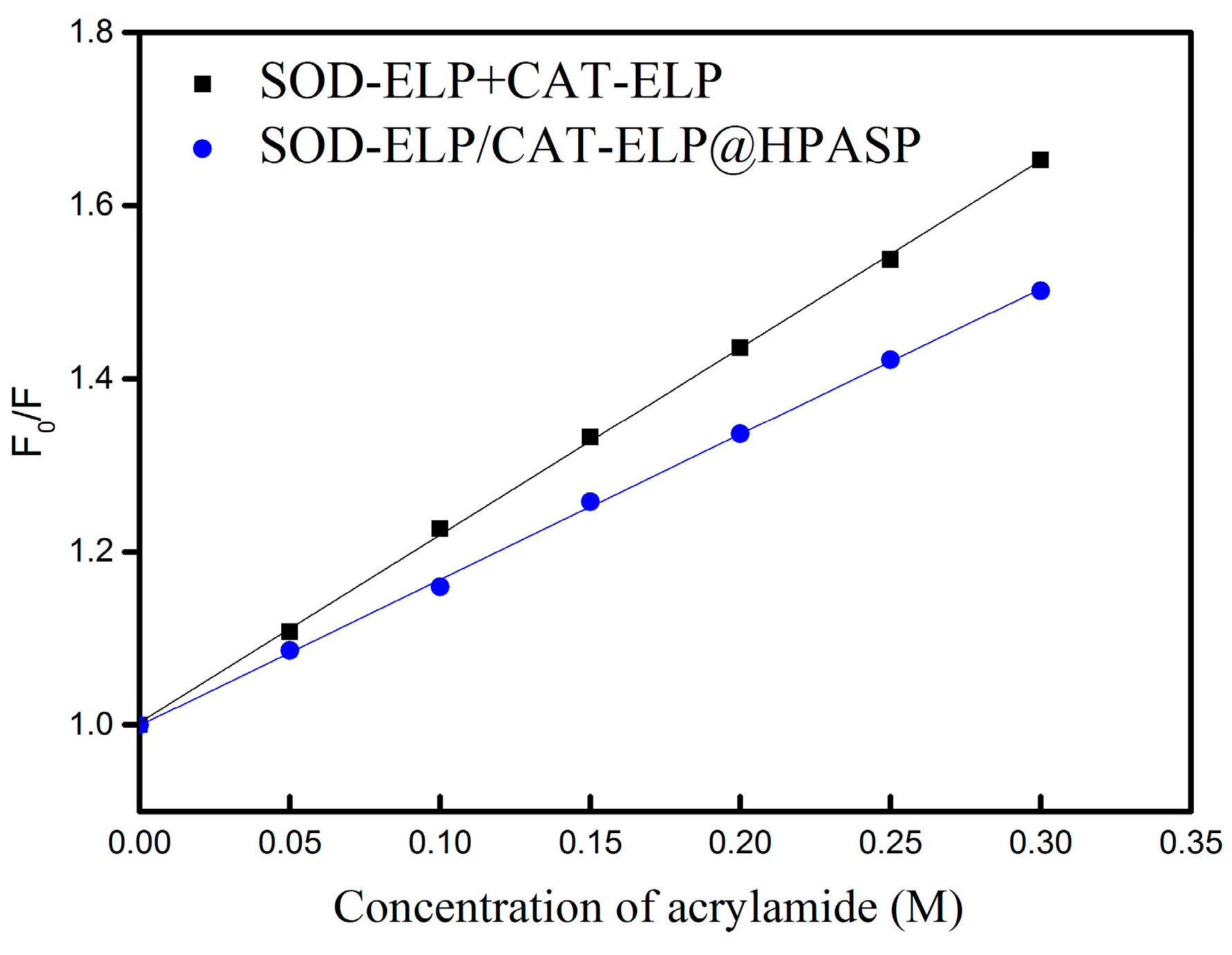
2.4. Stability of the Immobilized Enzymes against Denaturing by Urea
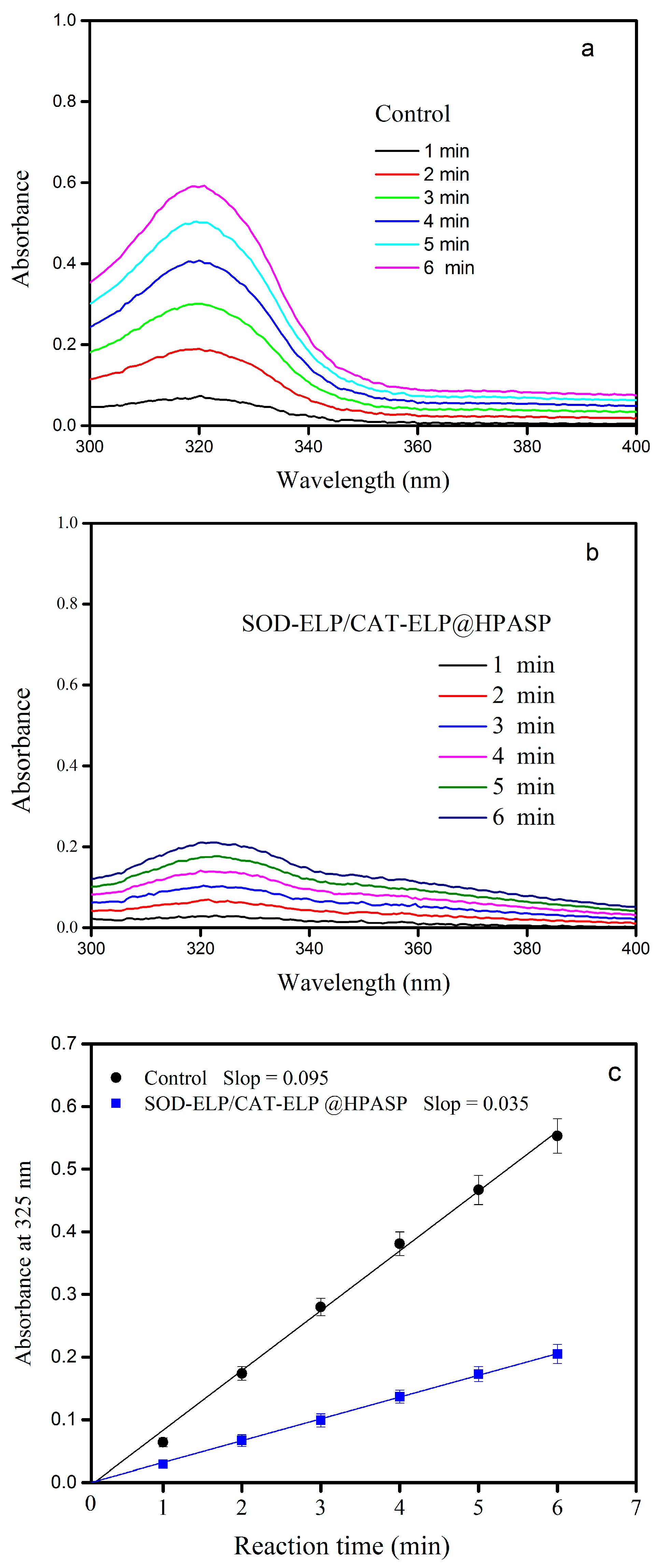
2.5. Enzymatic Activity
3. Experimental Section
3.1. Materials
3.2. Protein Expression and Purification
3.3. Co-Immobilization of SOD-ELP and CAT-ELP on HPASP
3.4. Characterization
3.5. Fluorescence Measurements
3.6. Enzyme Activity Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hu, P.; Tirelli, N. Scavenging ROS: Superoxide dismutase/catalase mimetics by the use of an oxidation-sensitive nanocarrier/enzyme conjugate. Bioconj. Chem. 2012, 23, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tao, H.; Lin, Y.; Hu, Y.; An, H.; Zhang, D.; Feng, S.; Hu, H.; Wang, R.; Li, X.; et al. A superoxide dismutase/catalase mimetic nanomedicine for targeted therapy of inflammatory bowel disease. Biomaterials 2016, 105, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Paclick, K.P.; Laroux, F.S.; Fuseler, J.; Wolf, R.E.; Gray, L.; Hoffman, J.; Grisham, M.B. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic. Biol. Med. 2002, 33, 311–322. [Google Scholar]
- Tainer, J.A.; Getzoff, E.D.; Richardson, J.S.; Richardson, DC. Structure and mechanism of copper, zinc superoxide dismutase. Nature 1983, 306, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.J.; Shin, D.S.; Getzoff, E.D.; Tainer, J.A. The structural biochemistry of thesuperoxide dismutases. Biochim. Biophys. Acta 2010, 1804, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Snow-Lisy, D.C.; Sabanegh, E.S.; Samplaski, M.K.; Morris, V.B.; Labhasetwar, V. Superoxide dismutase-loaded biodegradable nanoparticles targeted with a follicle-stimulating hormone peptide protect Sertoli cells from oxidative stress. Fertil. Steril. 2014, 101, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Lacatusu, I.; Badea, N.; Badea, G.; Brasoveanu, L.; Stan, R.; Ott, C.; Oprea, O.; Meghea, A. Ivy leaves extract based-lipid nanocarriers and their bioefficacy on antioxidant and antitumor activities. RSC Adv. 2016, 6, 77243–77255. [Google Scholar] [CrossRef]
- Caddeoa, C.; Manconi, M.; Fadda, A.M.; Lai, F.; Lampis, S.; Diez-Sales, O.; Sinico, C. Nanocarriers for antioxidant resveratrol: Formulation approach, vesicle self-assembly and stability evaluation. Colloids Surf. B Biointerfaces 2013, 111, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Gopinath, P. Antioxidant nanozyme: A facile synthesis and evaluation of the reactive oxygen species scavenging potential of nanoceria encapsulated albumin nanoparticles. J. Mater. Chem. B 2015, 3, 4843–4852. [Google Scholar] [CrossRef]
- Rosenbaugh, E.G.; Roat, J.W.; Yang, R.F.; Manickam, D.S.; Yin, J.X.; Schultz, H.D.; Bronich, T.K.; Batrakova, E.V.; Kabanov, A.V.; Zucker, I.H.; et al. The attenuation of central angiotensin II-dependent pressor response and intra-neuronal signaling by intracarotid injection of nanoformulated copper/zinc superoxide dismutase. Biomaterials 2010, 31, 5218–5226. [Google Scholar] [CrossRef] [PubMed]
- Manickam, D.S.; Brynskikh, A.M.; Kopanic, J.L.; Sorgen, P.L.; Klyachko, N.L.; Batrakova, E.V.; Bronich, T.K.; Kabanov, A.V. Well-defined cross-linked antioxidant nanozymes for treatment of ischemic brain injury. J. Control. Release 2012, 162, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Perriotte-Olson, C.; Adi, N.; Manickam, D.S.; Westwood, R.A.; Desouza, C.V.; Natarajan, G.; Crook, A.; Kabanov, A.V.; Saraswathi, V. Nanoformulated Copper/zinc Superoxide Dismutase Reduces Adipose Inflammation in Obesity. Obesity 2015, 24, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Corvo, M.L.; Jorge, J.C.; van’t Hof, R.; Cruz, M.E.; Crommelinc, D.J.; Stormc, G. Superoxide dismutase entrapped in long-circulating liposomes: Formulation design and therapeutic activity in rat adjuvant arthritis. Biochim. Biophys. Acta 2002, 1564, 227–236. [Google Scholar] [CrossRef]
- Corvo, M.L.; Martins, M.B.; Francisco, A.P.; Morais, J.G.; Cruz, M.E. Liposomal formulations of Cu, Zn-superoxide dismutase: Physico-chemical characterization and activity assessment in an inflammation model. J. Control. Release 1997, 43, 1–8. [Google Scholar] [CrossRef]
- Regnault, C.; Roch-Arveiller, M.; Tissot, M.; Sarfati, G.; Giroud, J.P.; Postaire, E.; Hazebroucq, G. Effect of encapsulation on the anti-inflammatory properties of superoxide dismutase after oral administration. Clin. Chim. Acta 1995, 240, 117–127. [Google Scholar] [CrossRef]
- Rengel, R.G.; Barišić, K.; Pavelić, Z.; Grubišić, T.Z.; Čepelak, G.I.; Filipović-Grčić, J. High efficiency entrapment of superoxide dismutase into mucoadhesive chitosan-coated liposomes. Eur. J. Pharm. Sci. 2002, 15, 441–448. [Google Scholar] [CrossRef]
- Veronese, F.M.; Largajolli, R.; Boccu, E.; Benassi, C.A.; Schiavon, O. Surface modification of proteins-Activation of monomethoxy-polyethylene glycols by phenylchloroformates and modification of ribonuclease and superoxide-dismutase. Appl. Biochem. Biotechnol. 1985, 11, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Minor, R.L.; White, C.W.; Repine, J.E.; Rosen, G.M.; Freeman, B.A. Superoxide-dismutase and catalase conjugated to polyethylene-glycol increases endothelial enzymeactivity and oxidant resistance. J. Biol. Chem. 1988, 263, 6884–6892. [Google Scholar] [PubMed]
- Beauchamp, C.O.; Gonias, S.L.; Menapace, D.P.; Pizzo, S.V. A new procedure for the synthesis of polyethylene glycolprotein adducts—Effects on function, receptor recognition, and clearance of superoxide-dismutase, lactoferrin, and alpha-2-macroglobulin. Anal. Biochem. 1983, 131, 25–33. [Google Scholar] [CrossRef]
- Muizelaar, J.P.; Marmarou, A.; Young, H.F.; Choi, S.C.; Wolf, A.; Schneider, R.L.; Kontos, H.A. Improving the outcome of severe head-injury with the oxygen radical scavenger polyethylene glycol-conjugated superoxide-dismutase—A phase-II trial. J. Neurosurg. 1993, 78, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Caliceti, P.; Schiavon, O.; Morpurgo, M.; Veronese, F.M.; Sartore, L.; Ranucci, E.; Ferruti, P. Physicochemical and biological properties of monofunctional hydroxy terminating poly(nvinylpyrrolidone) conjugated superoxide-dismutase. J. Bioact. Compat. Polym. 1995, 10, 103–120. [Google Scholar] [CrossRef]
- Yi, X.A.; Zimmerman, M.C.; Yang, R.F.; Tong, J.; Vingoradow, S.; Kabanov, A.V. Pluronic-modified superoxide dismutase 1 attenuates angiotensin ii-induced increase in intracellular superoxide in neurons. Free Radic. Biol. Med. 2010, 49, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Pastor, I.; Esquembre, R.; Micol, V.; Mallavia, R.; Mateo, C.R. A ready-to-use fluorimetric biosensor for superoxide radical using superoxide dismutase and peroxidase immobilized in sol-gel glasses. Anal. Biochem. 2004, 334, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Giovagnoli, S.; Luca, G.; Casaburi, I.; Paolo, B.; Macchiarulo, G.; Ricci, M.; Calvitti, M.; Basta, G.; Calafiore, R.; Rossi, C. Long-term delivery of superoxide dismutase and catalase entrapped in poly(lactide-co-glycolide) microspheres: In Vitro effects on isolated neonatal porcine pancreatic cell clusters. J. Control. Release 2005, 107, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.E.; Chilkoti, A. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: Examples from the elastin-like polypeptide system. Biomacromolecules 2002, 3, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Kolling, O. Re-examination of the effect of aprotic solvents upon the fundamental vibrational peak of the carbonyl group in 1,1,3,3-tetramethylurea. Appl. Spectrosc. 1999, 53, 29–32. [Google Scholar] [CrossRef]
- Daud, F.N.; Ahmad, A.; Badri, K.H. An investigation on the properties of palm-based polyurethane solid polymer electrolyte. Int. J. Polym. Sci. 2014, 2014, 1–5. [Google Scholar] [CrossRef]
- Arroyo, M.; Menéndez, M.; García, J.L.; Campillo, N.; Hormigo, D.; de la Mata, M.; Castillón, P.; Acebal, C. The role of cofactor binding in tryptophan accessibility and conformational stability of His-tagged D-amino acid oxidase from Trigonopsis variabilis. Biochim. Biophys. Acta 2007, 1774, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.R.; Palmer, L.R.; Szabo, A.G. Acrylamide quenching of the intrinsic fluorescence of tryptophan residues genetically engineered into the soluble colicin E1 channel peptide. Structural characterization of the insertion-competent state. Biochemistry 1993, 32, 6974–6981. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Liu, M.; Chen, S.; Chen, X.; Wang, J. New insight into molecular interactions of imidazolium ionic liquids with bovine serum albumin. J. Phys. Chem. B 2011, 115, 12306–12314. [Google Scholar] [CrossRef] [PubMed]
- Georlette, D.; Blaise, V.; Bouillenne, F.; Damien, F.; Thorbjarnardottir, S.H.; Depiereux, E.; Gerday, C.; Uversky, V.H.; Feller, G. Adenylation-dependent conformation and unfolding pathways of the NAD+-dependent DNA ligase from the thermophile Thermus scotoductus. Biophys. J. 2004, 86, 1089–1104. [Google Scholar] [CrossRef]
- Li, X. Improved pyrogallol autoxidation method: A reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Zhao, J.; Sun, J.; Feng, W. Specific Ligation of Two Multimeric Enzymes with Native Peptides and Immobilization with Controlled Molar Ratio. Bioconj. Chem. 2017, 28, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, S.; Li, R.; Wang, W.; Feng, W.; Ji, P. Co-Immobilization of Superoxide Dismutase with Catalase on Soft Microparticles Formed by Self-Assembly of Amphiphilic Poly(Aspartic Acid). Catalysts 2017, 7, 217. https://doi.org/10.3390/catal7070217
Mao S, Li R, Wang W, Feng W, Ji P. Co-Immobilization of Superoxide Dismutase with Catalase on Soft Microparticles Formed by Self-Assembly of Amphiphilic Poly(Aspartic Acid). Catalysts. 2017; 7(7):217. https://doi.org/10.3390/catal7070217
Chicago/Turabian StyleMao, Siyu, Rong Li, Wenchen Wang, Wei Feng, and Peijun Ji. 2017. "Co-Immobilization of Superoxide Dismutase with Catalase on Soft Microparticles Formed by Self-Assembly of Amphiphilic Poly(Aspartic Acid)" Catalysts 7, no. 7: 217. https://doi.org/10.3390/catal7070217



