Pulse Microcalorimetry Study of Methane Dry Reforming Reaction on Ni/Ceria-Zirconia Catalyst
Abstract
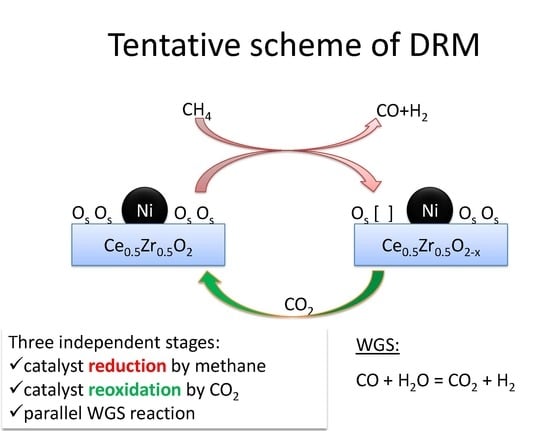
:1. Introduction
2. Results
2.1. Catalyst Characterization
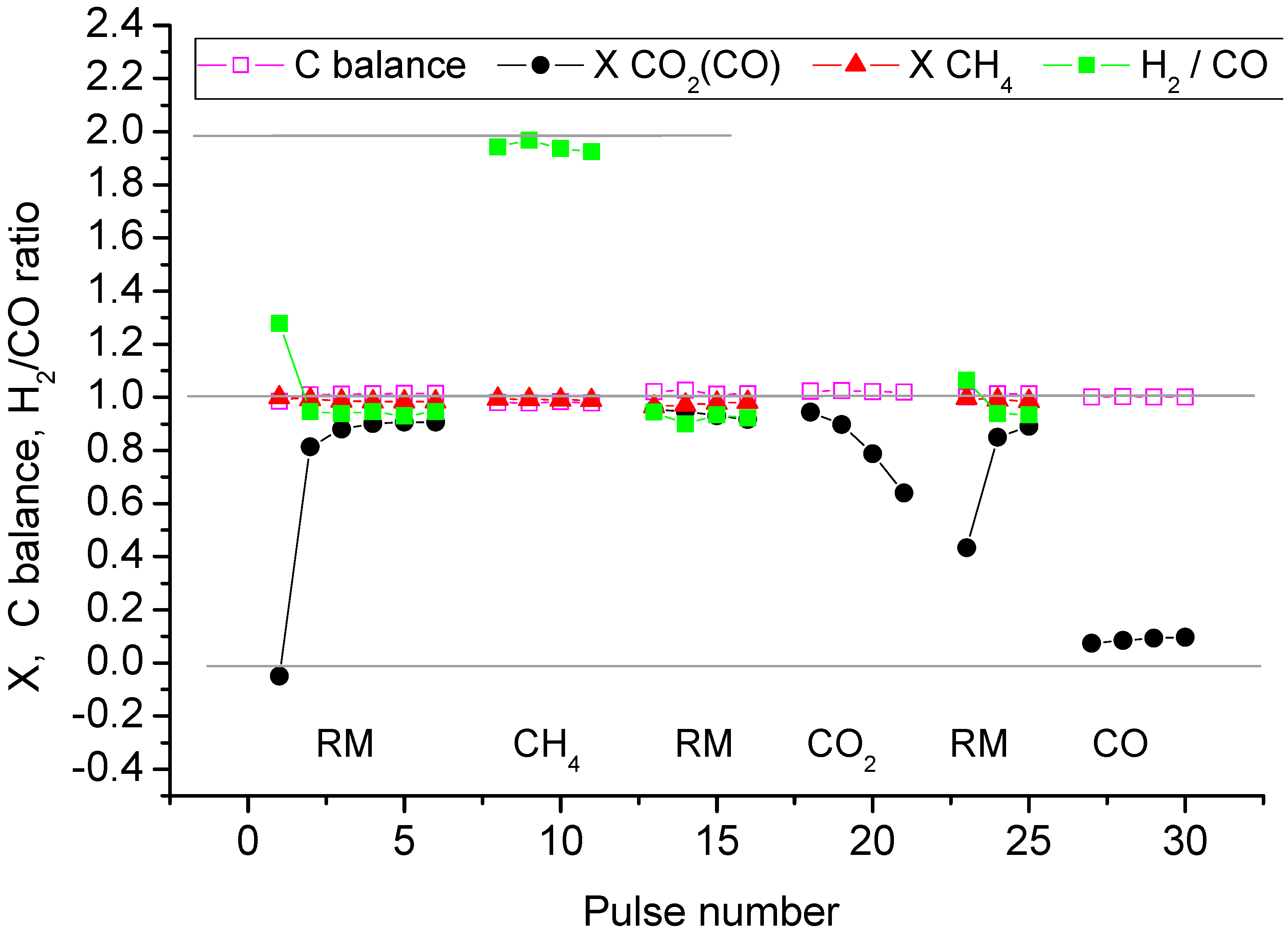
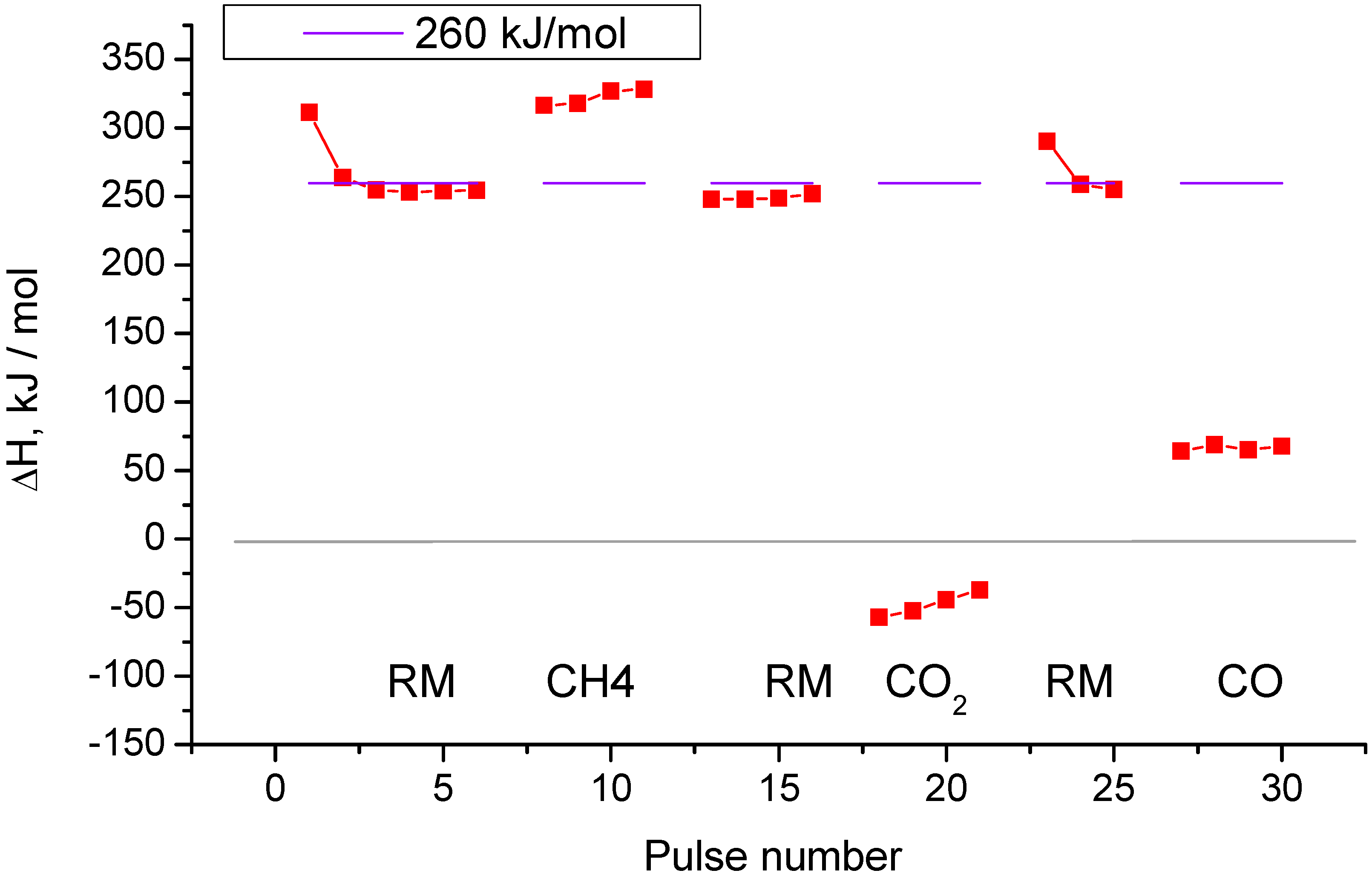
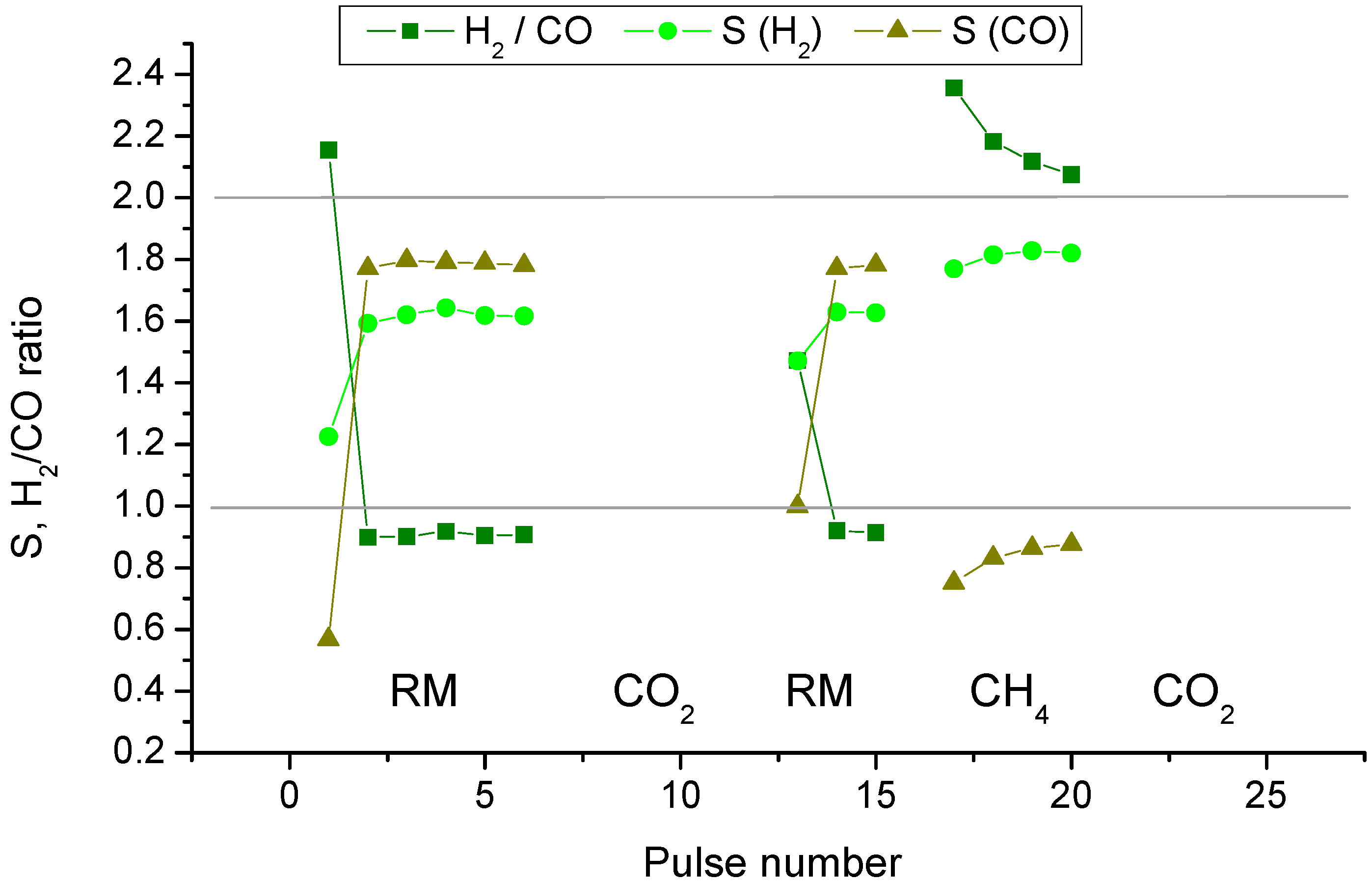
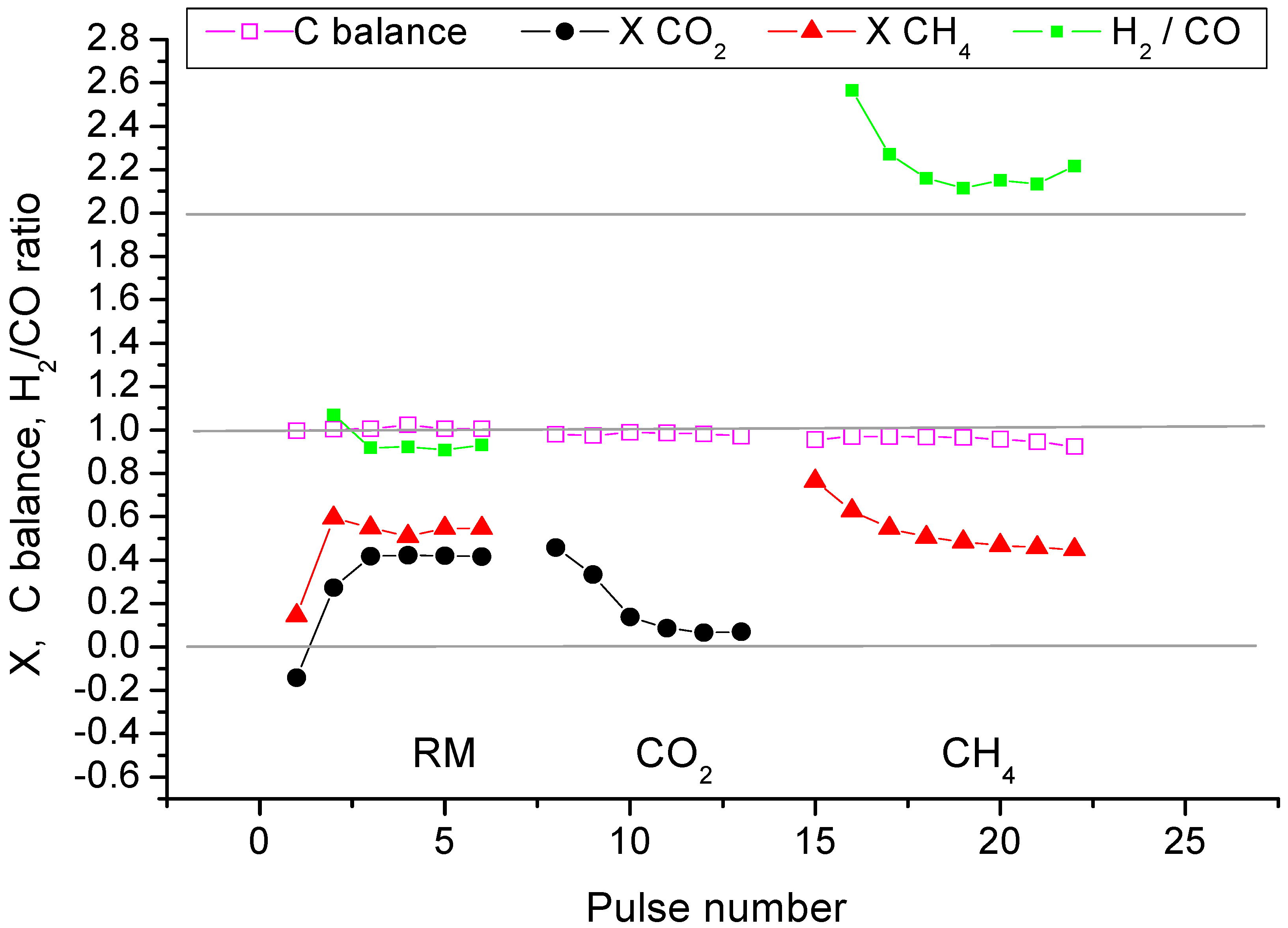
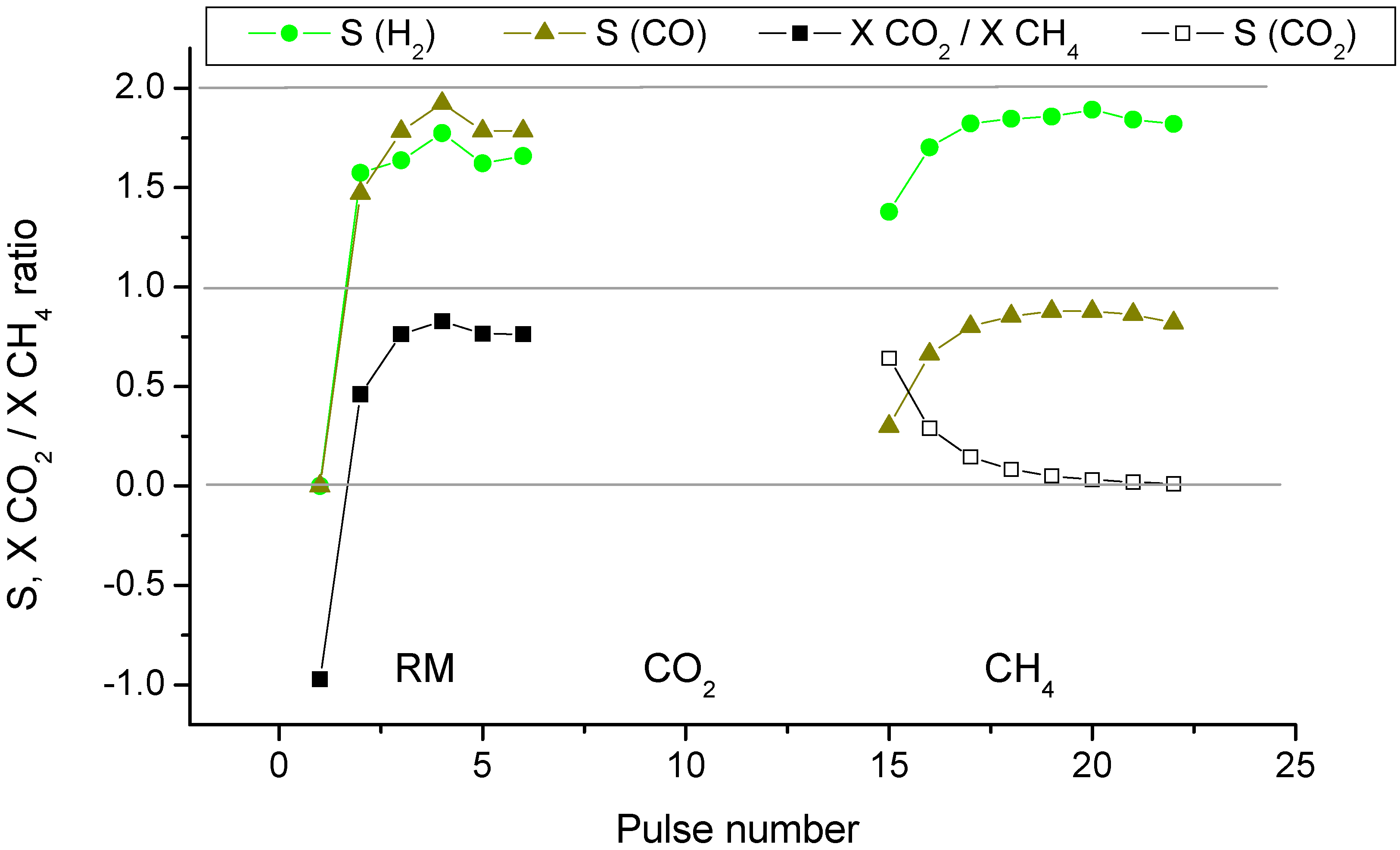
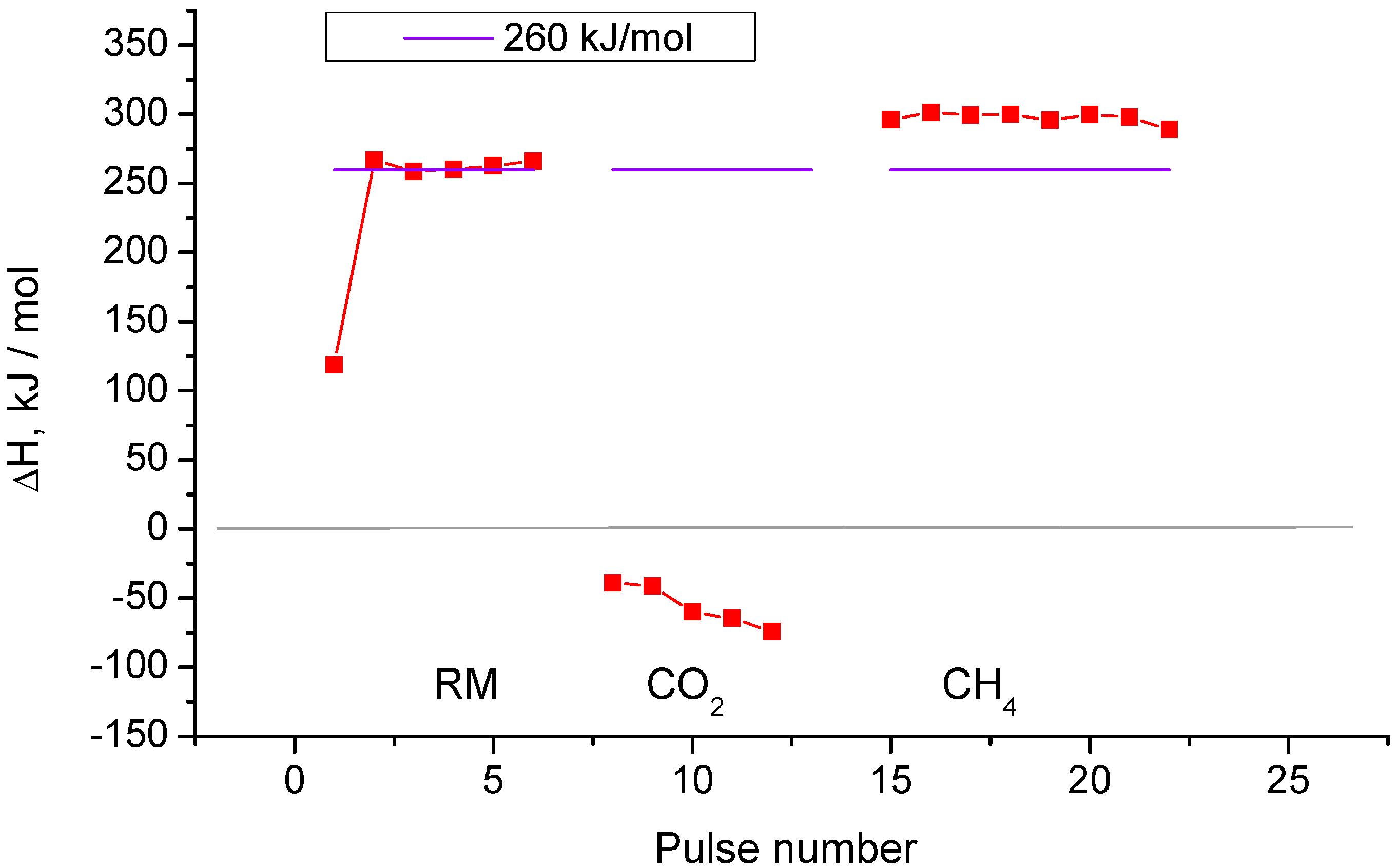
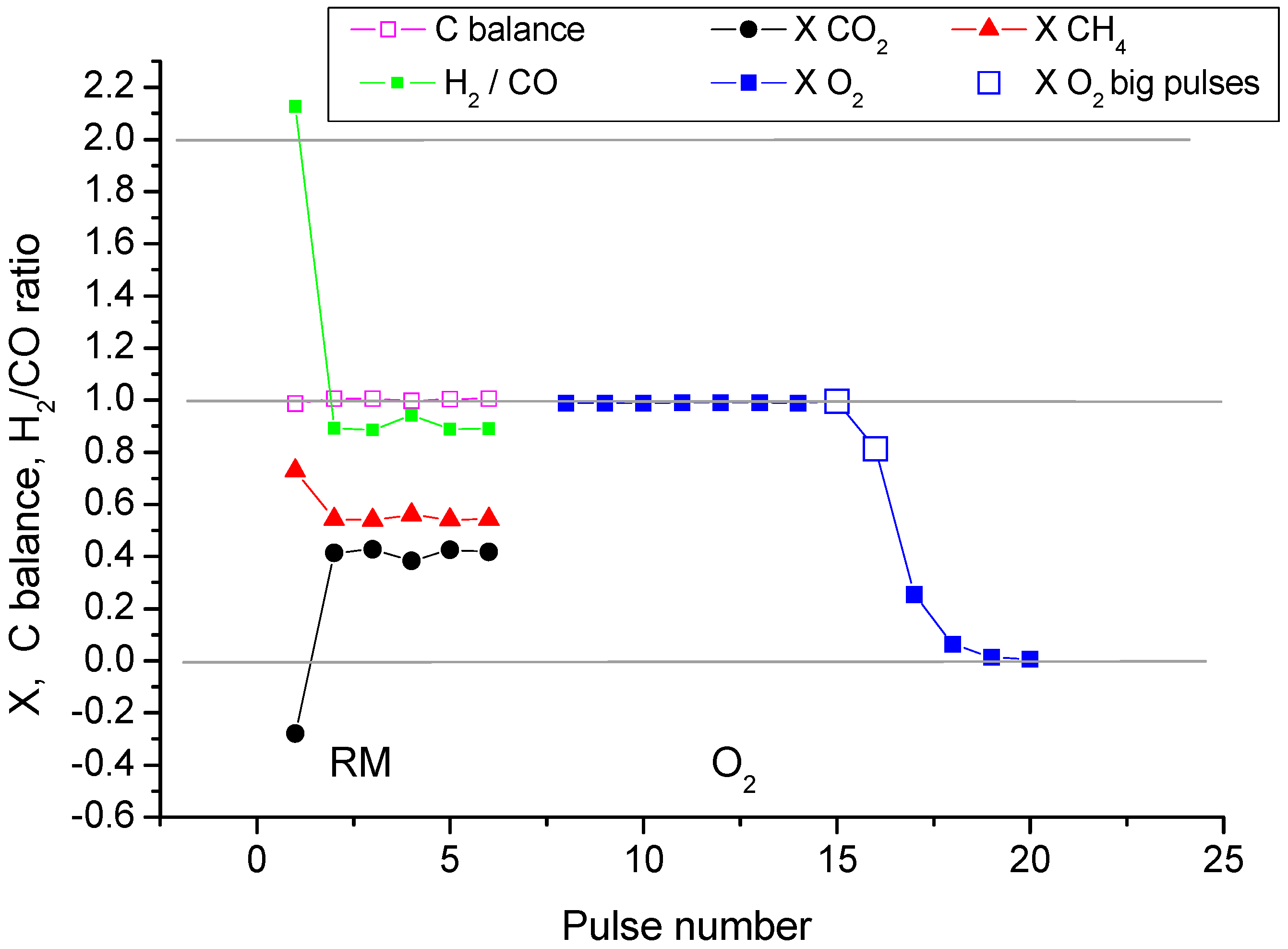
2.2. Pulse Microcalorimetry at 973 K
- Continuous flow of reaction mixture (5% CH4 and CO2) in He at 973 K for 30 min. After that small (pulse volume 0.85 mL) probing pulses and big (pulse volume 3.6 mL) pulses of reaction mixture were fed into the reactor by turns. Mixtures of products from small pulses were analysed by GC (gas chromatography), whereas products from big pulses were not analysed due to GC column overloading. Big pulses were used to achieve steady-state of the catalyst more quickly. It is important to note that the use of alternating small and big pulses was equivalent to the use of only small pulses, and the achieved steady state of the catalyst depended only on the temperature of the reactor and composition of the pulse.
- Pulses of 5% CH4 in He (pulse volume 0.85 mL).
- Small (pulse volume 0.85 mL) probing pulses and big (pulse volume 3.6 mL) pulses of reaction mixture.
- Pulses of 5% CO2 in He.
- Small (pulse volume 0.85 mL) probing pulses and big (pulse volume 3.6 mL) pulses of reaction mixture.
- Pulses of CO in He.
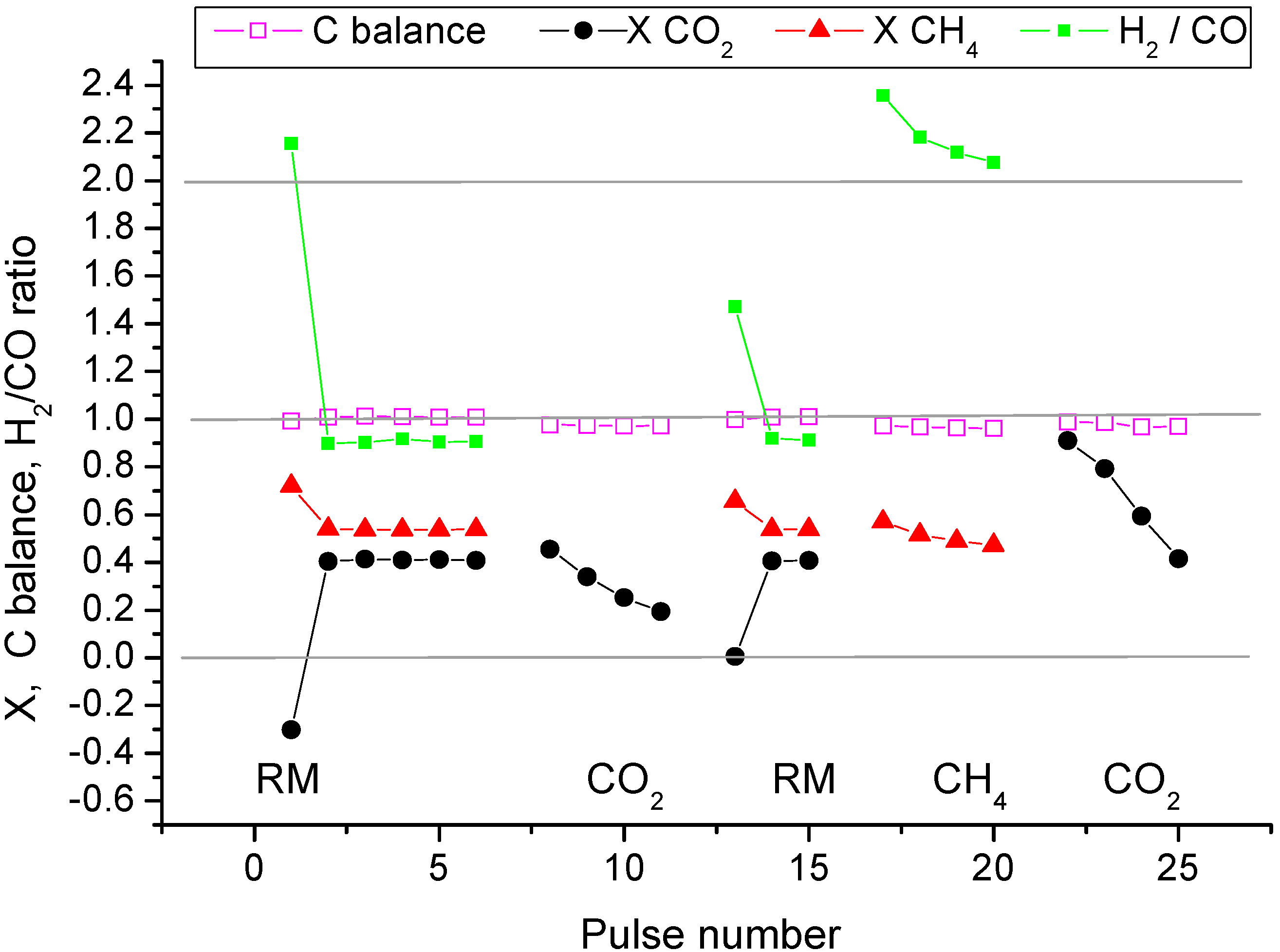
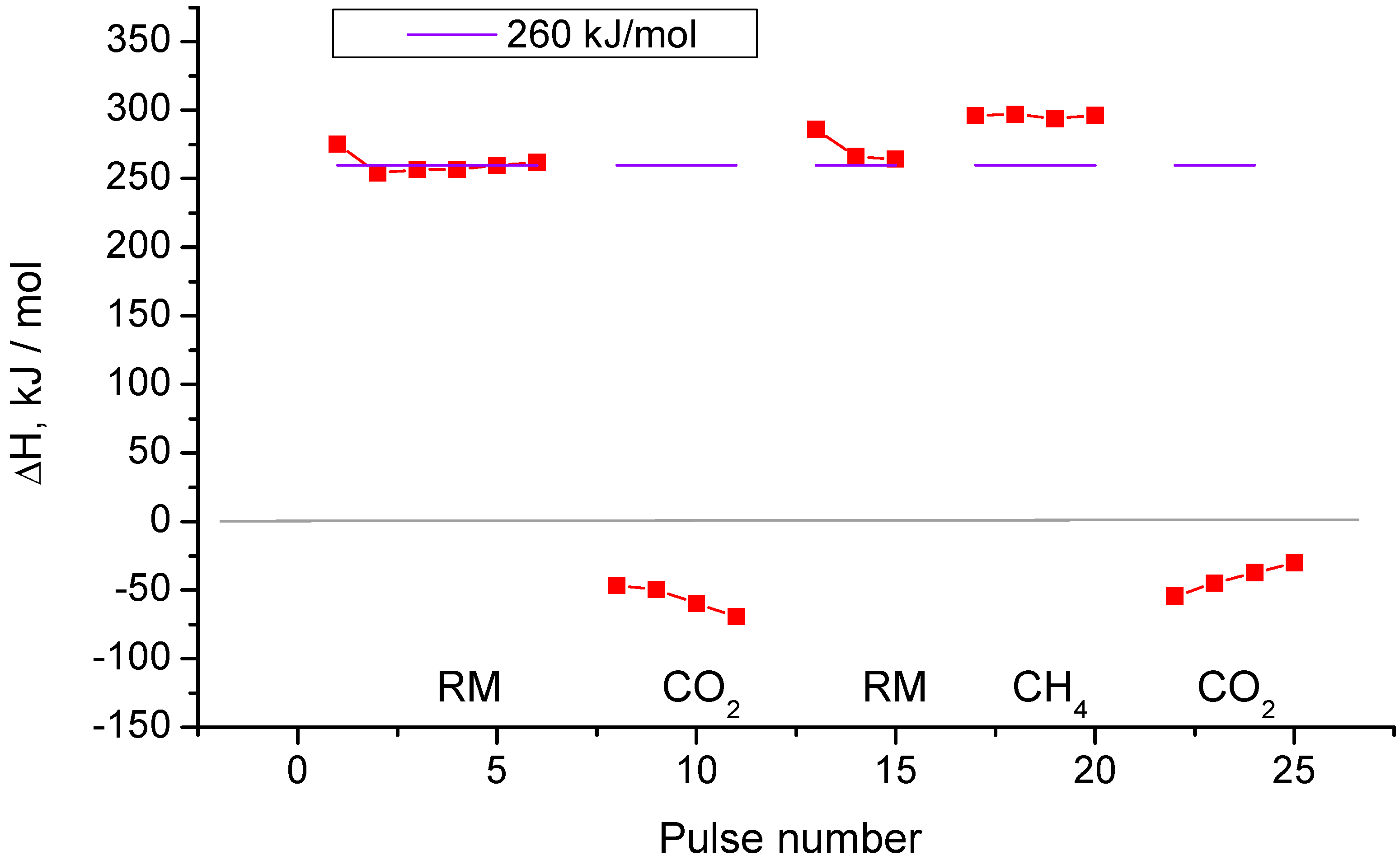
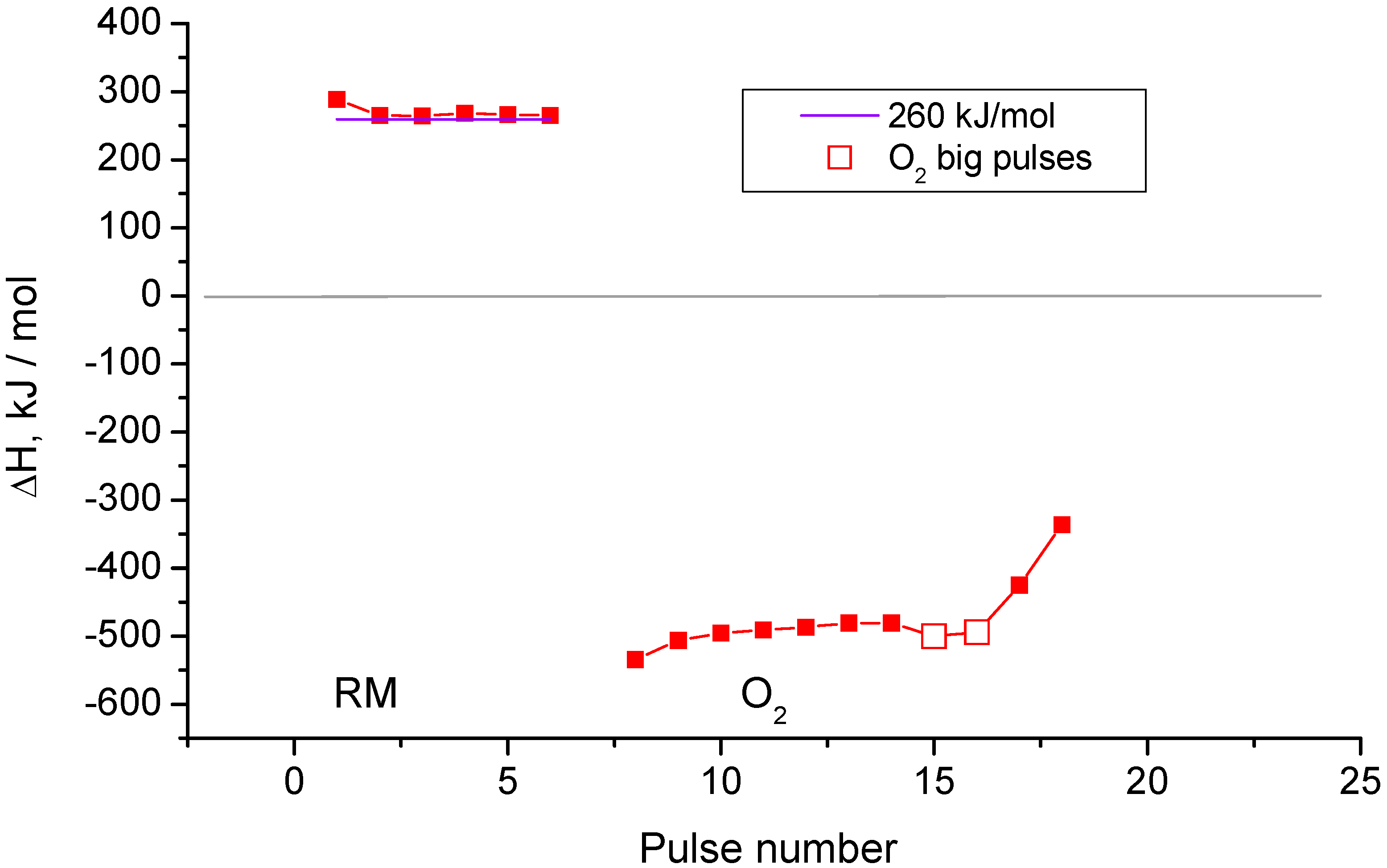
2.3. Pulse Microcalorimetry at 773 K
- (1)
- Continuous flow of reaction mixture (5% СН4 and СО2) in He at 773 K for 30 min. After that small (pulse volume 0.85 mL) probing pulses and big (pulse volume 3.6 mL) pulses of reaction mixture were fed into the reactor by turns. Analysis method was the same as at 973 K described above.
- (2)
- Pulses of 5% CO2 in He.
- (3)
- Small (pulse volume 0.85 mL) probing pulses and big (pulse volume 3.6 mL) pulses of the reaction mixture.
- (4)
- Pulses of 5% CH4 in He (pulse volume 0.85 mL).
- (5)
- Pulses of 5% CO2 in He.
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koubaissy, B.; Pietraszek, A.; Roger, A.C.; Kiennemann, A. CO2 reforming of methane over Ce-Zr-Ni-Me mixed catalysts. Catal. Today 2010, 157, 436–439. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, Ch. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Huerta, M.V.M.; Fierro, J.L.G. The effect of CeO2 on the surface and catalytic properties of Pt/CeO2-ZrO2 catalysts for methane dry reforming. Appl. Catal. B 2009, 89, 149–159. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Gubanova, E.L.; Sazonova, N.N.; Pokrovskaya, S.A.; Chumakova, N.A.; Mezentseva, N.V.; Bobin, A.S.; Gulyaev, R.V.; Ishchenko, A.V.; Krieger, T.A.; et al. Dry reforming of methane over Pt/PrCeZrO catalyst: Kinetic and mechanistic features by transient studies and their modeling. Catal. Today 2011, 171, 140–149. [Google Scholar] [CrossRef]
- Pavlova, S.N.; Sazonova, N.N.; Sadykov, V.A.; Alikina, G.M.; Lukashevich, A.I.; Gubanova, E.L.; Bunina, R.V. Study of synthesis gas production over structured catalysts based on LaNi(Pt)Ox- and Pt(LaPt)-CeO2-ZrO2 supported on corundum. Stud. Surf. Sci. Catal. 2007, 167, 343–348. [Google Scholar]
- Bitter, J.H.; Seshan, K.; Lercher, J.A. Mono and bifunctional pathways of CO2/CH4 reforming over Pt and Rh based catalysts. J. Catal. 1998, 176, 93–101. [Google Scholar] [CrossRef]
- Bychkov, V.; Tyulenin, Yu.; Krylov, O.; Korchak, V. Methane reforming with carbon dioxide on the Co/-Al2O3 catalyst: The formation, state, and transformations of surface carbon. Kinet. Catal. 2002, 43, 724–730. [Google Scholar] [CrossRef]
- Bychkov, V.; Tyulenin, Yu.; Krylov, O.; Korchak, V. The mechanism of methane reforming with carbon dioxide: Comparison of supported Pt and Ni (Co) catalysts. Kinet. Catal. 2003, 44, 353–359. [Google Scholar] [CrossRef]
- Schuurman, Y.; Mirodatos, C. Uses of transient kinetics for methane activation studies. Appl. Catal. A 1997, 151, 305–331. [Google Scholar] [CrossRef]
- Slagtern, Y.; Schuurman, C.; Leclercq, X.; Verykios, C. Mirodatos, Specific features concerning the mechanism of methane reforming by carbon dioxide over Ni/La2O3 catalyst. J. Catal. 1997, 172, 118–126. [Google Scholar] [CrossRef]
- Pavlova, S.N.; Sadykov, V.A.; Bulgakov, N.N.; Bredikhin, M.N. The influence of support on the low-temperature activity of Pd in the reaction of CO oxidation: 3. Kinetics and mechanism of the reaction. J. Catal. 1996, 161, 517–523. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Tikhov, S.F.; Bulgakov, N.N.; Gerasev, A.P. Catalytic oxidation of CO on CuOx revisited: Impact of the surface state on the apparent kinetic parameters. Catal. Today 2009, 144, 324–333. [Google Scholar] [CrossRef]
- Sadykov, V.; Rogov, V.; Ermakova, E.; Arendarsky, D.; Mezentseva, N.; Alikina, G.; Sazonova, N.; Bobin, A.; Pavlova, S.; Schuurman, Y.; et al. Mechanism of CH4 dry reforming by pulse microcalorimetry: Metal nanoparticles on perovskite/fluorite supports with high oxygen mobility. Thermochim. Acta 2013, 567, 27–34. [Google Scholar] [CrossRef]
- Smirnova, M.Y.; Pavlova, S.N.; Krieger, T.A.; Bespalko, Y.N.; Anikeev, V.I.; Chesalov, Y.A.; Kaichev, V.V.; Mezentseva, N.V.; Sadykov, V.A. Synthesis of Ce1−xZrxO2 oxides in supercritical alcohols and catalysts of methane dry reforming on their bases. Supercritical Fluids Theory Pract. 2017, in press. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonov, M.N.; Rogov, V.A.; Smirnova, M.Y.; Sadykov, V.A. Pulse Microcalorimetry Study of Methane Dry Reforming Reaction on Ni/Ceria-Zirconia Catalyst. Catalysts 2017, 7, 268. https://doi.org/10.3390/catal7090268
Simonov MN, Rogov VA, Smirnova MY, Sadykov VA. Pulse Microcalorimetry Study of Methane Dry Reforming Reaction on Ni/Ceria-Zirconia Catalyst. Catalysts. 2017; 7(9):268. https://doi.org/10.3390/catal7090268
Chicago/Turabian StyleSimonov, Mikhail N., Vladimir A. Rogov, Marina Yu. Smirnova, and Vladislav A. Sadykov. 2017. "Pulse Microcalorimetry Study of Methane Dry Reforming Reaction on Ni/Ceria-Zirconia Catalyst" Catalysts 7, no. 9: 268. https://doi.org/10.3390/catal7090268