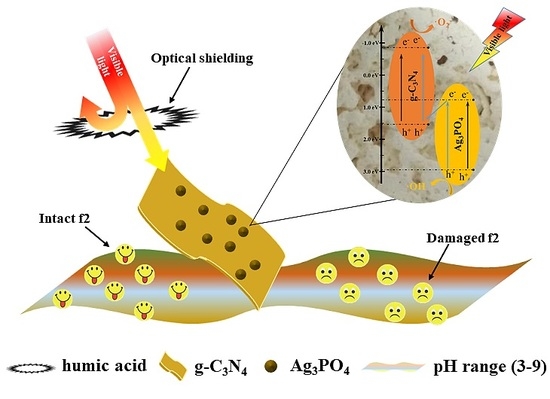
Photocatalytic Inactivation of Bacteriophage f2 with Ag3PO4/g-C3N4 Composite under Visible Light Irradiation: Performance and Mechanism
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of As-Prepared Photocatalysts
2.1.1. X-ray Diffraction (XRD) Analysis
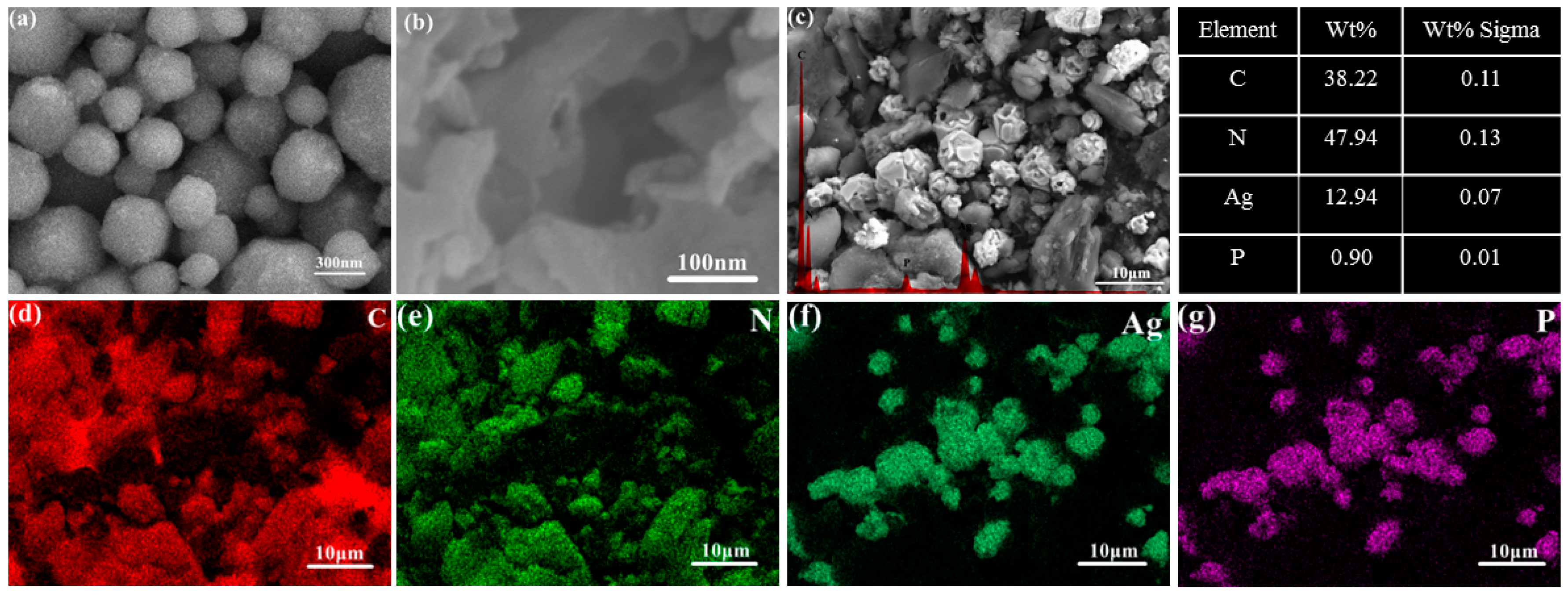
2.1.2. Morphology Analysis
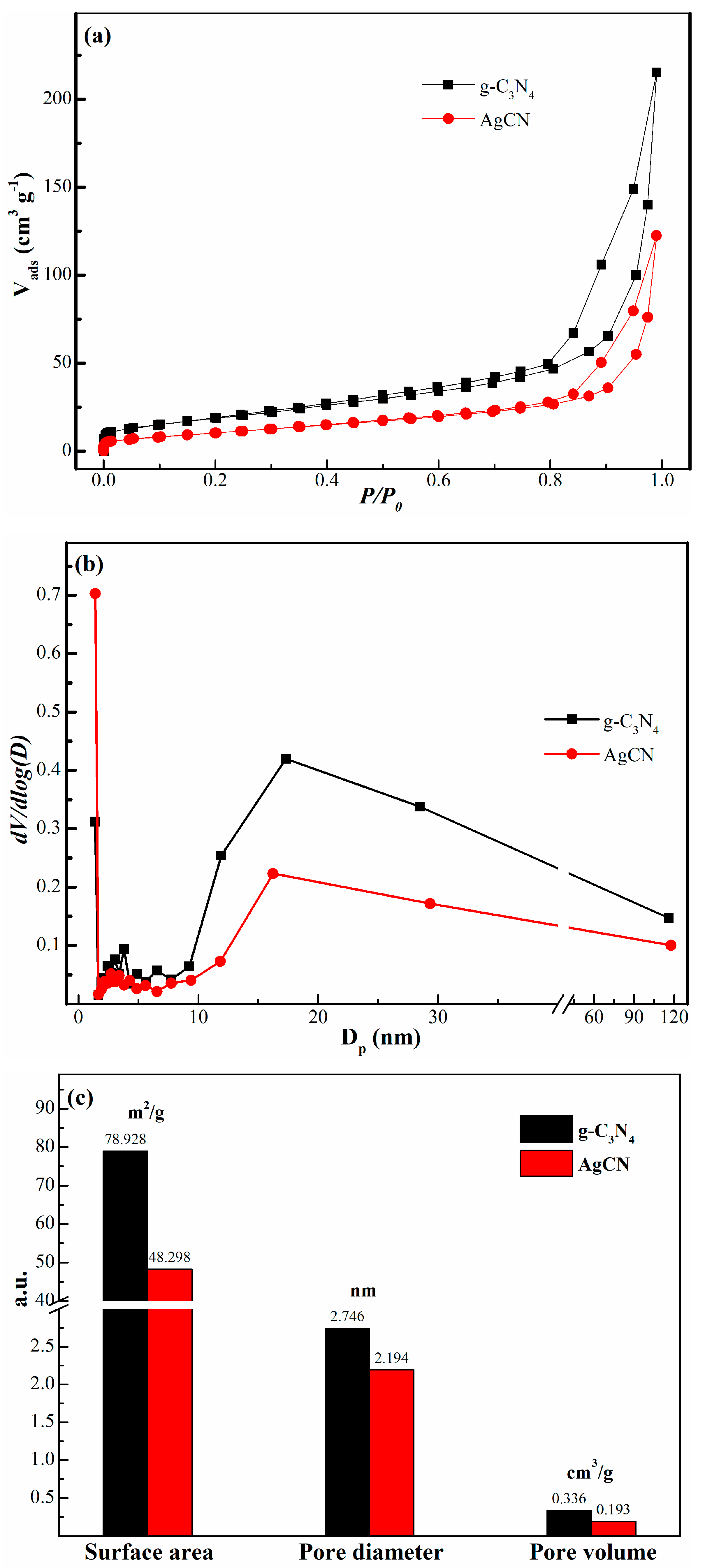
2.1.3. Pore Structure Analysis
2.1.4. DRS Analysis
2.1.5. PLS Analysis
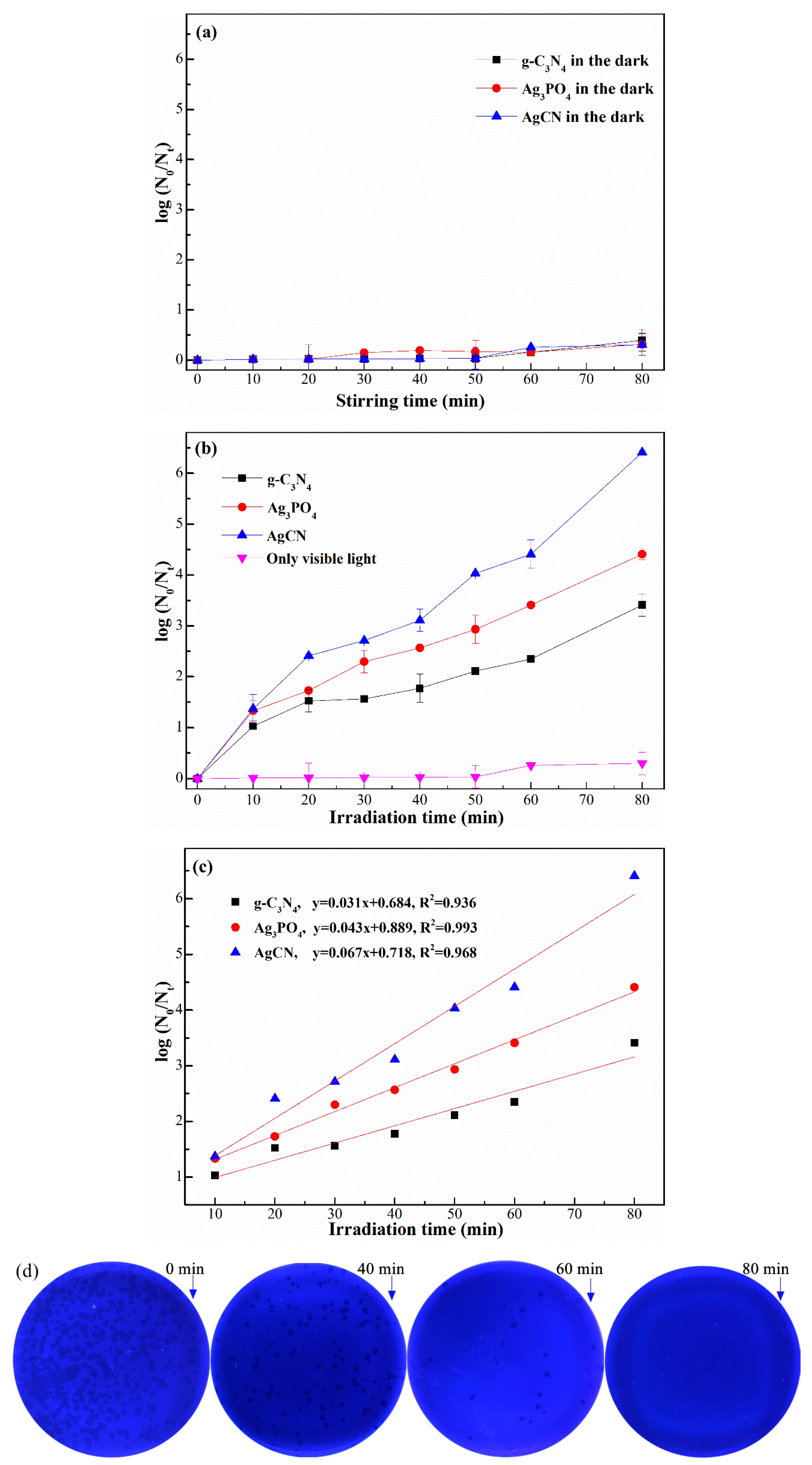
2.2. Photocatalytic Disinfection Performance to Bacteriophage f2
2.2.1. Kinetics Analysis of Photocatalytic Inactivation Process
2.2.2. Effect of pH Value
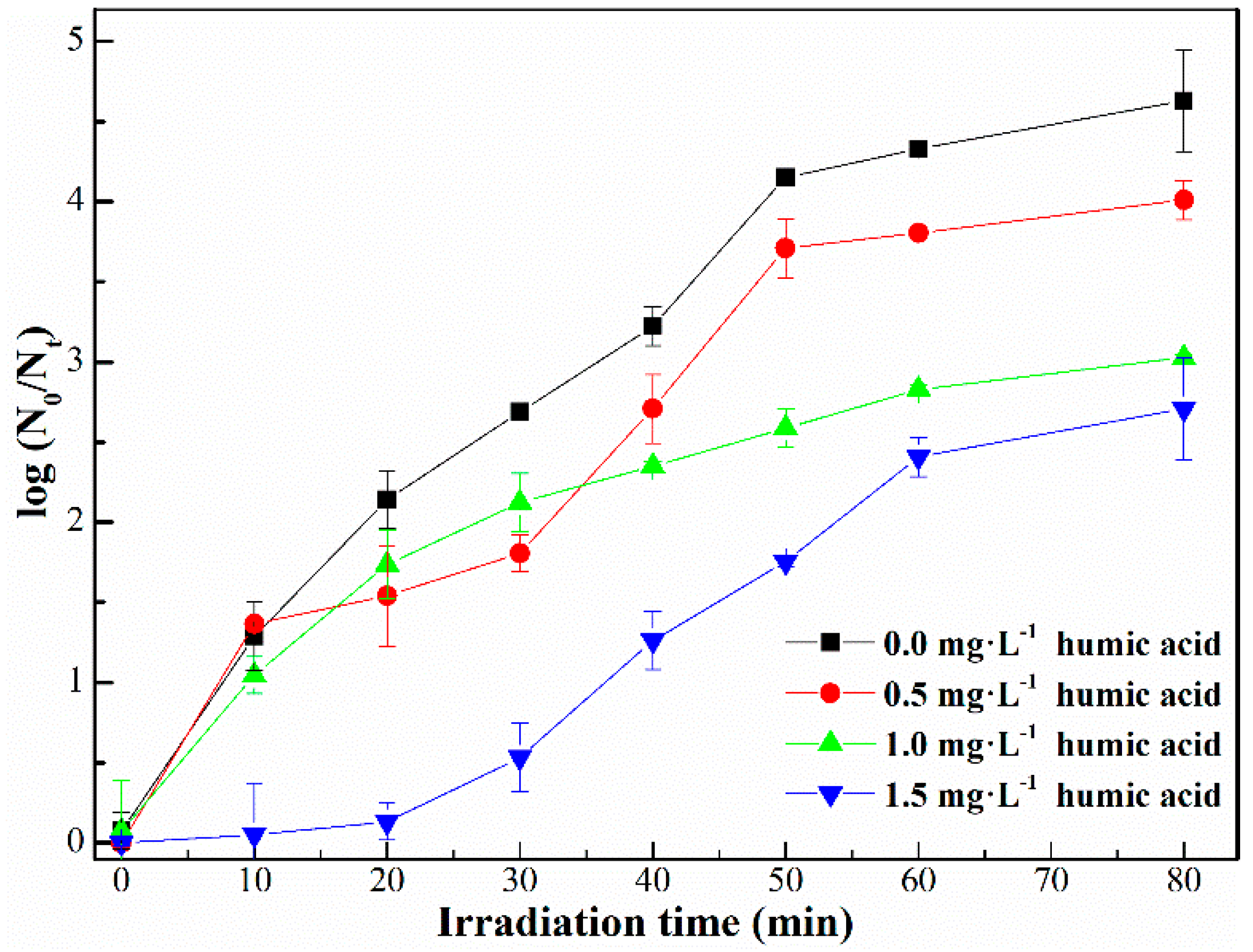
2.2.3. Effect of Humic Acid
2.2.4. Reusability of AgCN Composite Photocatalysts
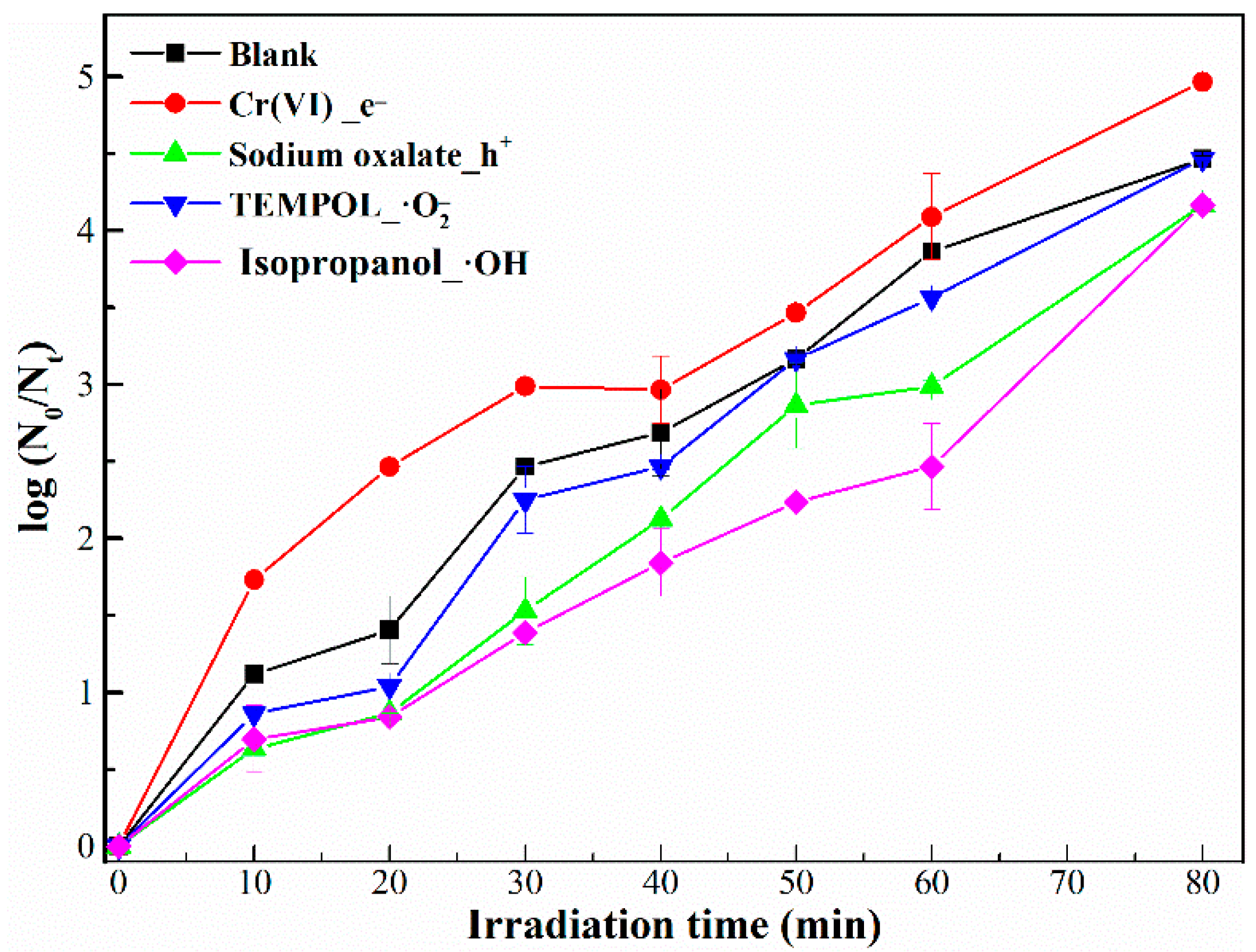
2.3. Inactivation Mechanisms of Bacteriophage f2
3. Materials and Methods
3.1. Materials and Chemicals
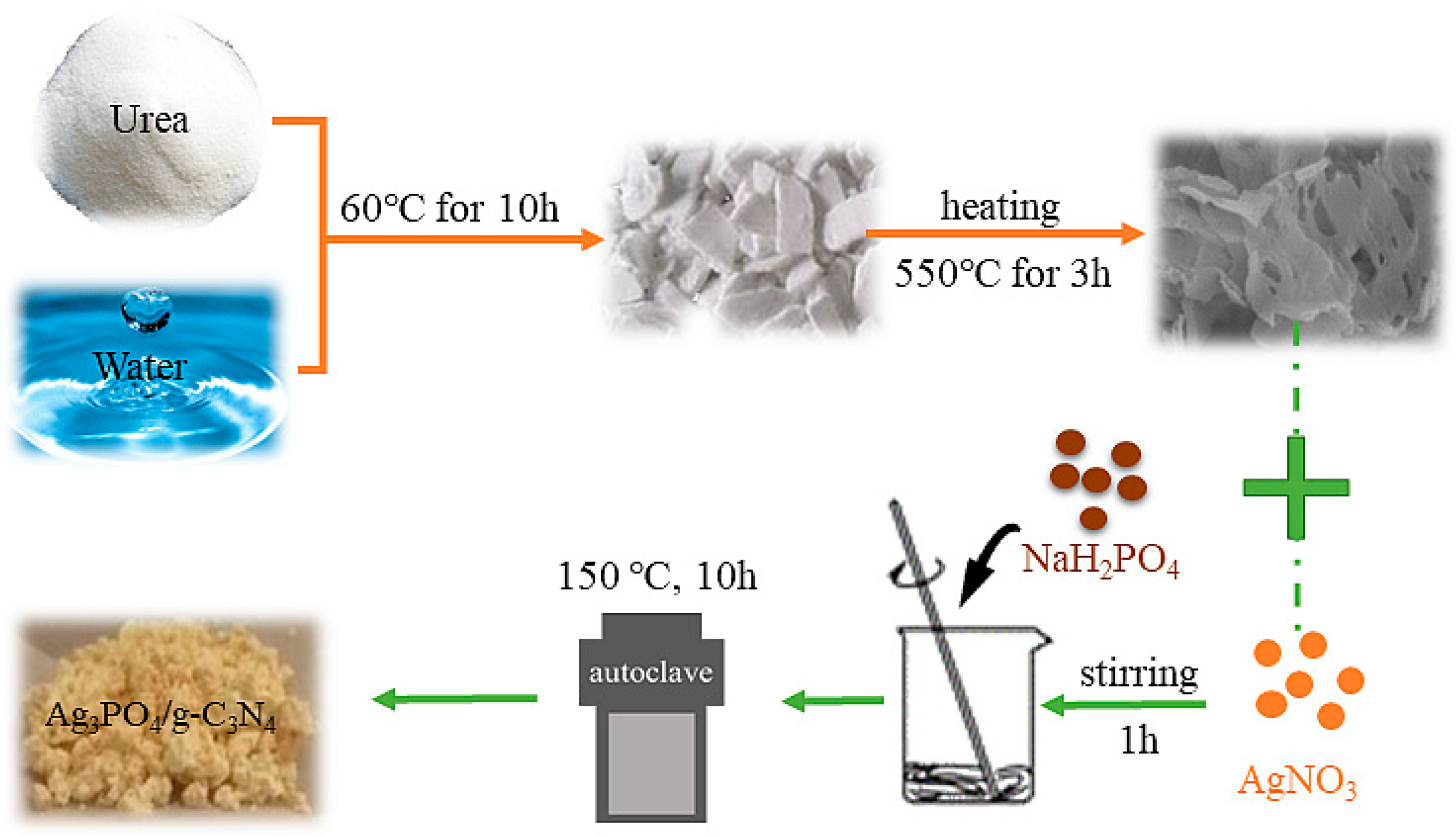
3.2. Preparation of g-C3N4
3.3. Preparation of the Ag3PO4/g-C3N4 Composite
3.4. Characterization
3.5. Photocatalytic Disinfection Experiment
3.5.1. Experimental Installation
3.5.2. Culture and Counting of Bacteriophage f2
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prevost, B.; Goulet, M.; Lucas, F.S.; Joyeux, M.; Moulin, L.; Wurtzer, S. Viral persistence in surface and drinking water: Suitability of pcr pre-treatment with intercalating dyes. Water Res. 2016, 91, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.E. Viral pathogens in water: Occurrence, public health impact, and available control strategies. Curr. Opion Virol. 2014, 4, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Gall, A.M.; Mariñas, B.J.; Lu, Y.; Shisler, J.L. Waterborne viruses: A barrier to safe drinking water. PLoS Pathog. 2015, 11, e1004867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, B.; Liu, Y.; Cai, X.; Liu, X.; Dai, R. Removal and inactivation of virus by drinking water treatment in the presence of bromide or iodide. J. Water Chem. Technol. 2015, 37, 96–101. [Google Scholar] [CrossRef]
- Zyara, A.; Torvinen, E.; Veijalainen, A.-M.; Heinonen-Tanski, H. The effect of uv and combined chlorine/uv treatment on coliphages in drinking water disinfection. Water 2016, 8, 130. [Google Scholar] [CrossRef]
- Rodriguez, R.A.; Bounty, S.; Beck, S.; Chan, C.; McGuire, C.; Linden, K.G. Photoreactivation of bacteriophages after uv disinfection: Role of genome structure and impacts of uv source. Water Res. 2014, 55, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Liga, M.V.; Bryant, E.L.; Colvin, V.L.; Li, Q. Virus inactivation by silver doped titanium dioxide nanoparticles for drinking water treatment. Water Res. 2011, 45, 535–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Martha, S.; Nashim, A.; Parida, K.M. Facile synthesis of highly active g-C3N4 for efficient hydrogen production under visible light. J. Mater. Chem. A 2013, 1, 7816–7824. [Google Scholar] [CrossRef]
- Sun, S.; Liang, S. Recent advances in functional mesoporous graphitic carbon nitride (mpg-C3N4) polymers. Nanoscale 2017, 9, 1544–1578. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Antonietti, M. Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: From photochemistry to multipurpose catalysis to sustainable chemistry. Angew. Chem. Int. Ed. 2011, 51, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shi, R.; Zhao, Y.; Bian, T.; Zhao, Y.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Alkali-assisted synthesis of nitrogen deficient graphitic carbon nitride with tunable band structures for efficient visible-light-driven hydrogen evolution. Adv. Mater. 2017, 1605148. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Xu, Q.; Zhang, J.; Yang, Z.; Si, M.; Yan, Z.; Xue, D. Defect-related ferromagnetism in ultrathin metal-free g-C3N4 nanosheets. Nanoscale 2014, 6, 2577. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhan, S.; Jia, Y.; Shi, Q.; Zhou, Q. Enhanced disinfection application of ag-modified g-C3N4 composite under visible light. Appl. Catal. B Environ. 2016, 186, 77–87. [Google Scholar] [CrossRef]
- Hong, J.; Xia, X.; Wang, Y.; Xu, R. Mesoporous carbon nitride with in situ sulfur doping for enhanced photocatalytic hydrogen evolution from water under visible light. J. Mater. Chem. 2012, 22, 15006. [Google Scholar] [CrossRef]
- Deng, J.; Liang, J.; Li, M.; Tong, M. Enhanced visible-light-driven photocatalytic bacteria disinfection by g-C3N4-AgBr. Colloid Surf. B 2017, 152, 49–57. [Google Scholar] [CrossRef]
- Hong, Y.; Jiang, Y.; Li, C.; Fan, W.; Yan, X.; Yan, M.; Shi, W. In-situ synthesis of direct solid-state z-scheme V2O5/g-C3N4 heterojunctions with enhanced visible light efficiency in photocatalytic degradation of pollutants. Appl. Catal. B Environ. 2016, 180, 663–673. [Google Scholar] [CrossRef]
- Sulaeman, U.; Febiyanto, F.; Yin, S.; Sato, T. The highly active saddle-like Ag3PO4 photocatalyst under visible light irradiation. Catal. Commun. 2016, 85, 22–25. [Google Scholar] [CrossRef]
- Yeo, B.-E.; Seo, Y.; Park, H.; Huh, Y.-D. Facet effect of Ag3PO4 crystals on antibacterial activities. Bull. Korean Chem. Soc. 2015, 36, 1904–1907. [Google Scholar] [CrossRef]
- Kumar, S.; Surendar, T.; Baruah, A.; Shanker, V. Synthesis of a novel and stable g-C3N4–Ag3PO4 hybrid nanocomposite photocatalyst and study of the photocatalytic activity under visible light irradiation. J. Mater. Chem. Coruña 2013, 1, 5333. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893. [Google Scholar] [CrossRef]
- Sun, J.-X.; Yuan, Y.-P.; Qiu, L.-G.; Jiang, X.; Xie, A.-J.; Shen, Y.-H.; Zhu, J.-F. Fabrication of composite photocatalyst g-C3N4–ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans. 2012, 41, 6756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Wu, G.; Chen, W. Porous graphitic carbon nitride synthesized via direct polymerization of urea for efficient sunlight-driven photocatalytic hydrogen production. Nanoscale 2012, 4, 5300. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, P.; Feng, Y.; Xie, Z.; Liu, Y.; Su, Y.; Zhang, Q.; Wang, Y.; Yao, K.; Lv, W.; et al. Facile synthesis of n-doped carbon dots/g-c 3 n 4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B Environ. 2017, 207, 103–113. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Zhang, W.; Wang, P.; Wang, C. Metal-free Virucidal effects induced by g-C3N4 under visible light irradiation: Statistical analysis and parameter optimization. Chemosphere 2018, 195, 551–558. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Xie, T.; Wang, D. Low-temperature synthesis and high visible-light-induced photocatalytic activity of bioi/tio2heterostructures. J. Phys. Chem. C 2009, 113, 7371–7378. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.; Cheng, C.; Shi, L.; Cheng, R.; Dong, J. Electrospinning Cu-TiO2 nanofibers used for photocatalytic disinfection of bacteriophage f2: Preparation, optimization and characterization. RSC Adv. 2017, 7, 52172–52179. [Google Scholar] [CrossRef]
- Muela, A.; Garcia-Bringas, J.M.; Arana, I.; Barcina, I. Humic Materials Offer Photoprotective Effect to Escherichia coli Exposed to Damaging Luminous Radiation. Microb. Ecol. 2000, 40, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Li, G.; Shi, L.; Xue, X.; Kang, M.; Zheng, X. The mechanism for bacteriophage f2 removal by nanoscale zero-valent iron. Water Res. 2016, 105, 429–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, R.; Shen, L.-j.; Yu, J.-h.; Xiang, S.-y.; Zheng, X. Photocatalytic Inactivation of Bacteriophage f2 with Ag3PO4/g-C3N4 Composite under Visible Light Irradiation: Performance and Mechanism. Catalysts 2018, 8, 406. https://doi.org/10.3390/catal8100406
Cheng R, Shen L-j, Yu J-h, Xiang S-y, Zheng X. Photocatalytic Inactivation of Bacteriophage f2 with Ag3PO4/g-C3N4 Composite under Visible Light Irradiation: Performance and Mechanism. Catalysts. 2018; 8(10):406. https://doi.org/10.3390/catal8100406
Chicago/Turabian StyleCheng, Rong, Liang-jie Shen, Jin-hui Yu, Shao-yu Xiang, and Xiang Zheng. 2018. "Photocatalytic Inactivation of Bacteriophage f2 with Ag3PO4/g-C3N4 Composite under Visible Light Irradiation: Performance and Mechanism" Catalysts 8, no. 10: 406. https://doi.org/10.3390/catal8100406