Evolution of Copper Supported on Fe3O4 Nanorods for WGS Reaction
Abstract
:1. Introduction
2. Results and Discussion
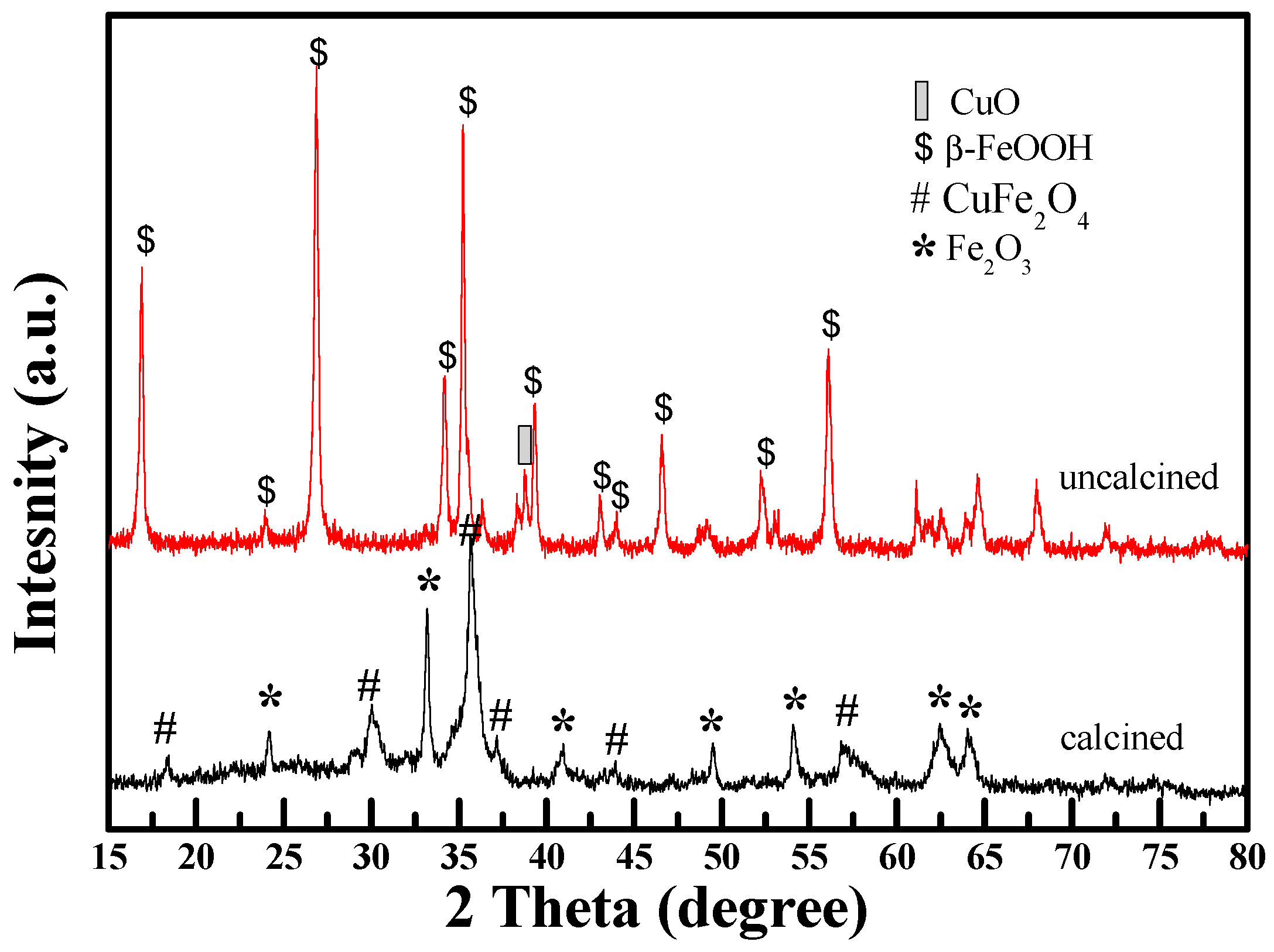
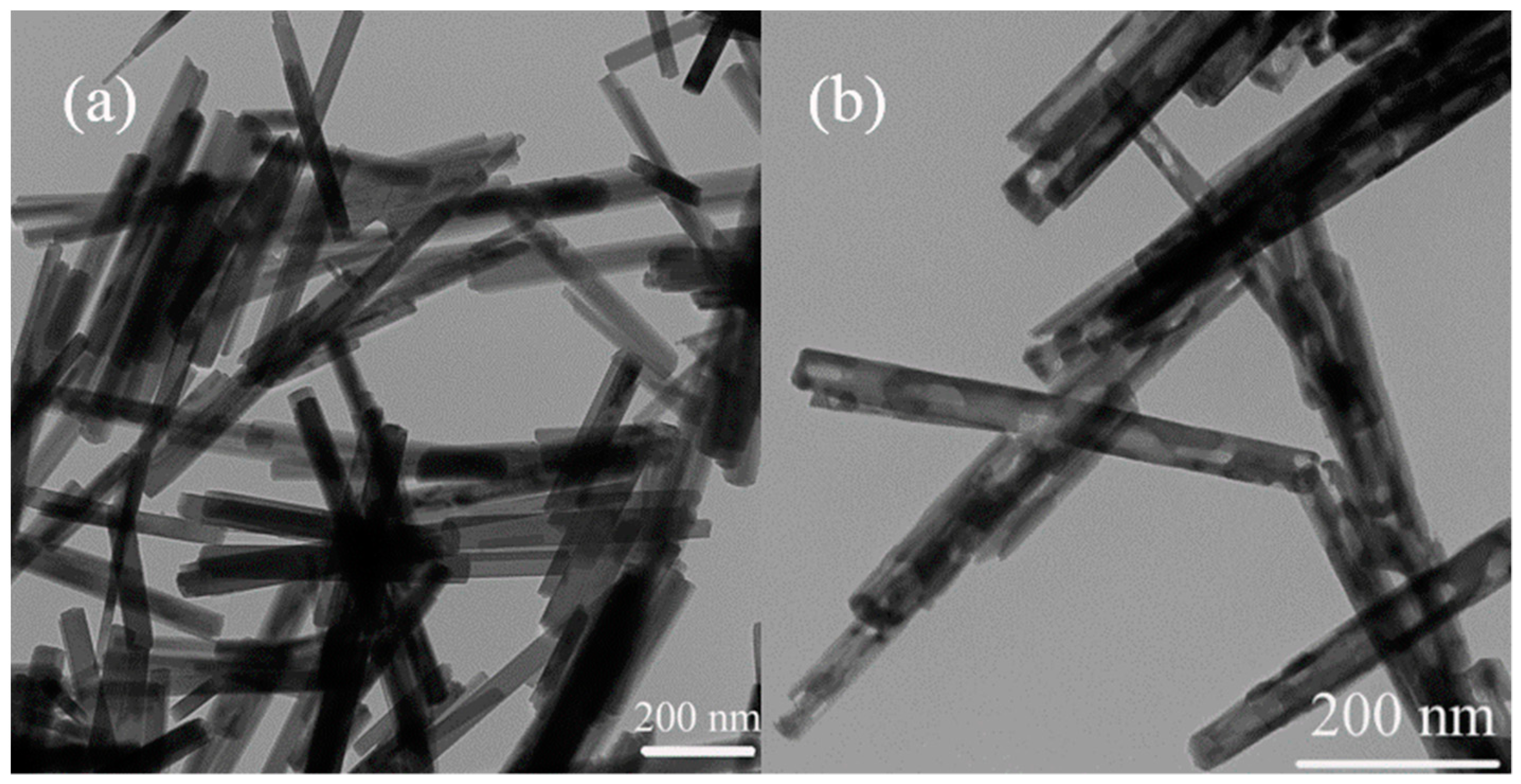
2.1. Structure and Morphology of Cu1Fe9Ox Nanorods Catalyst
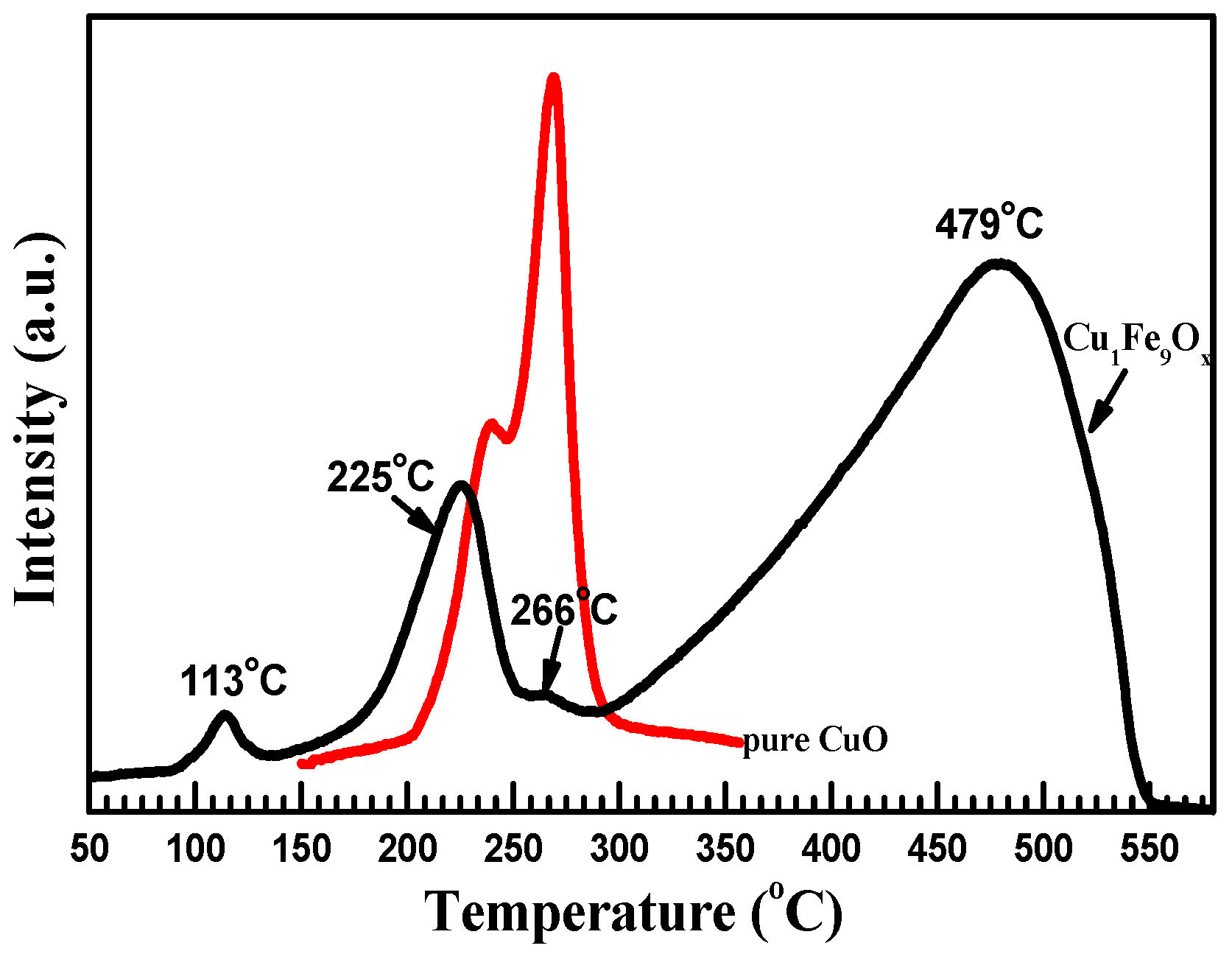
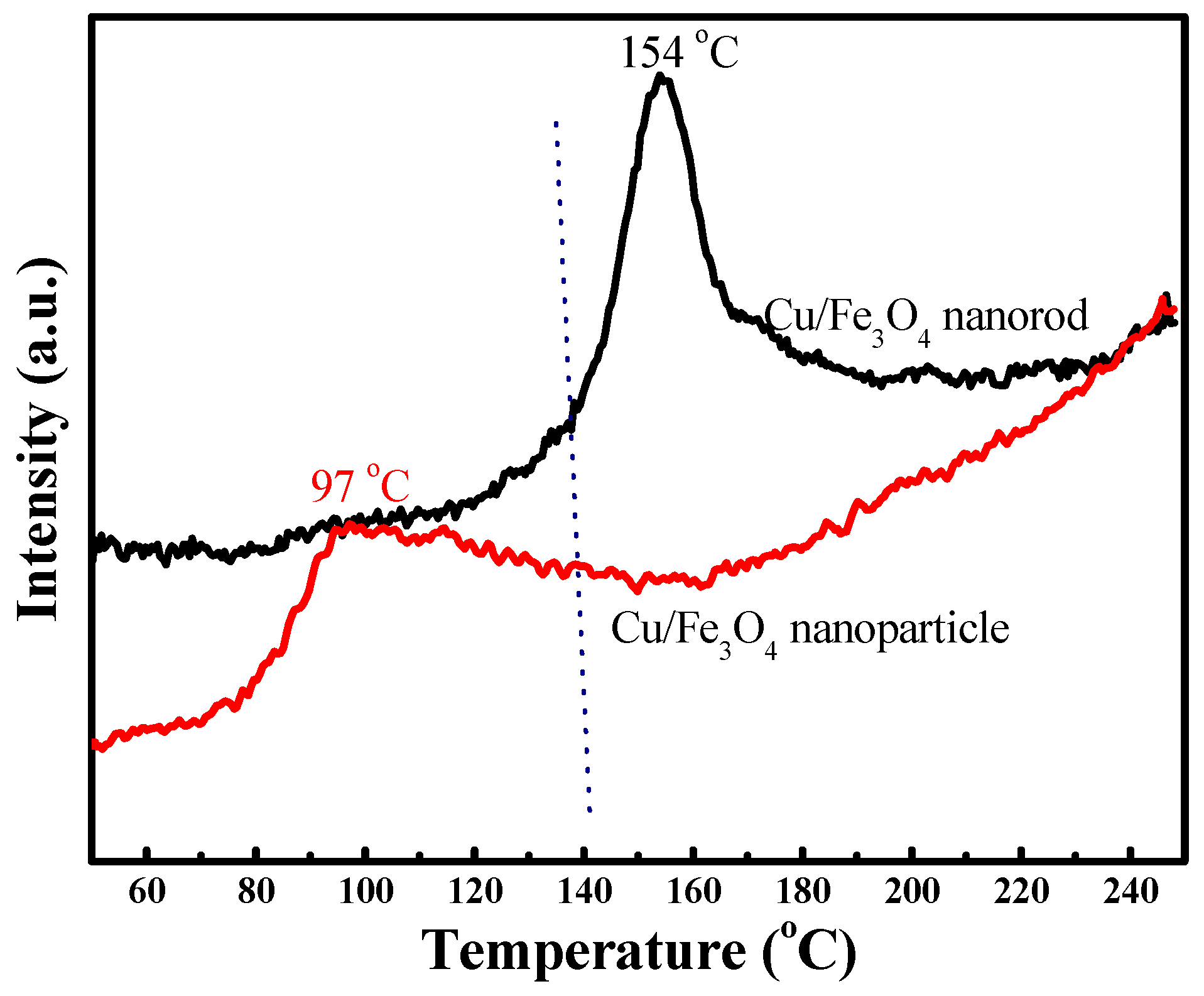
2.2. Reducibility of Cu1Fe9Ox Nanorod
2.3. Morphology Structure of As-Prepared Catalyst after Reduction
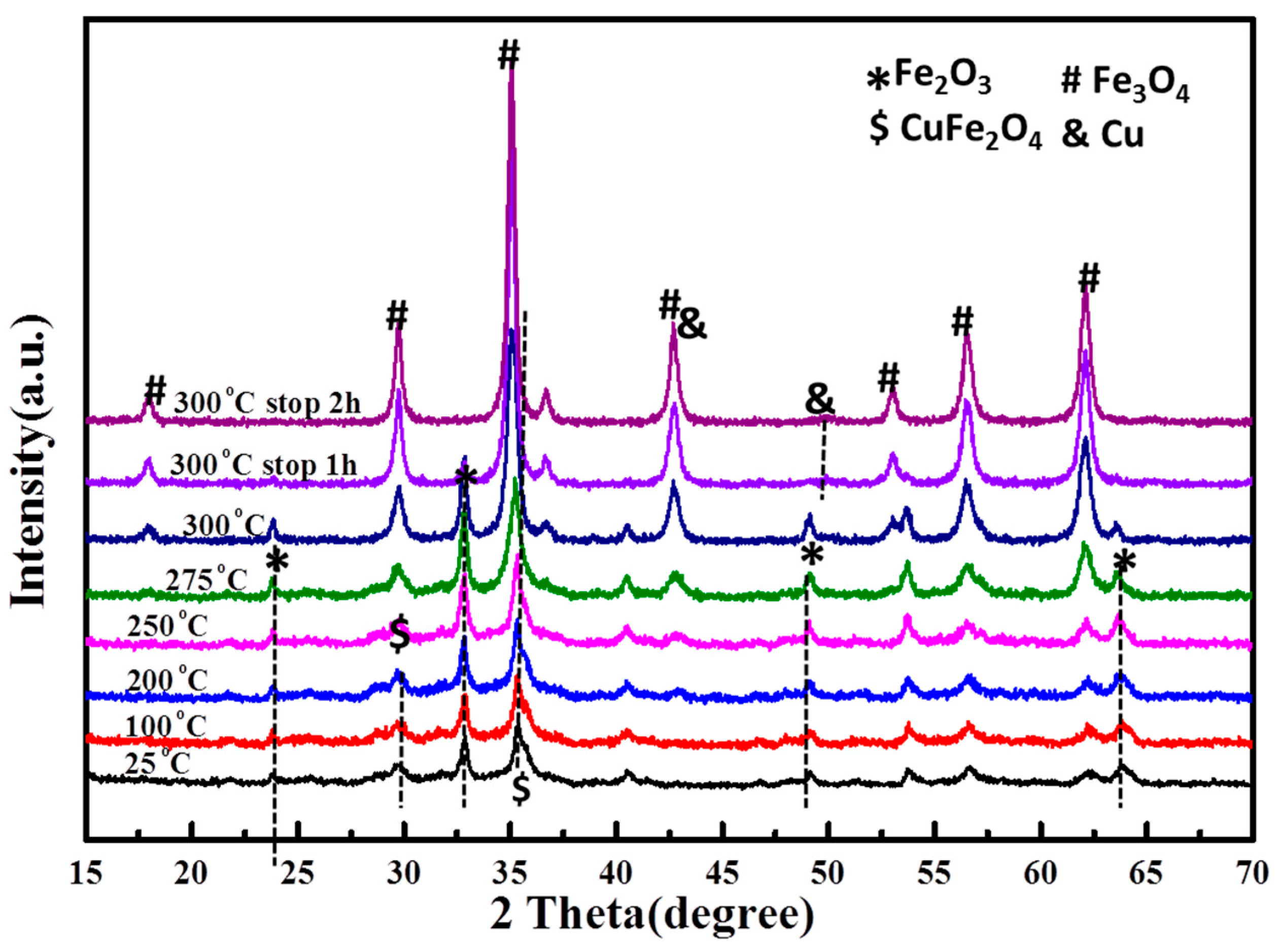
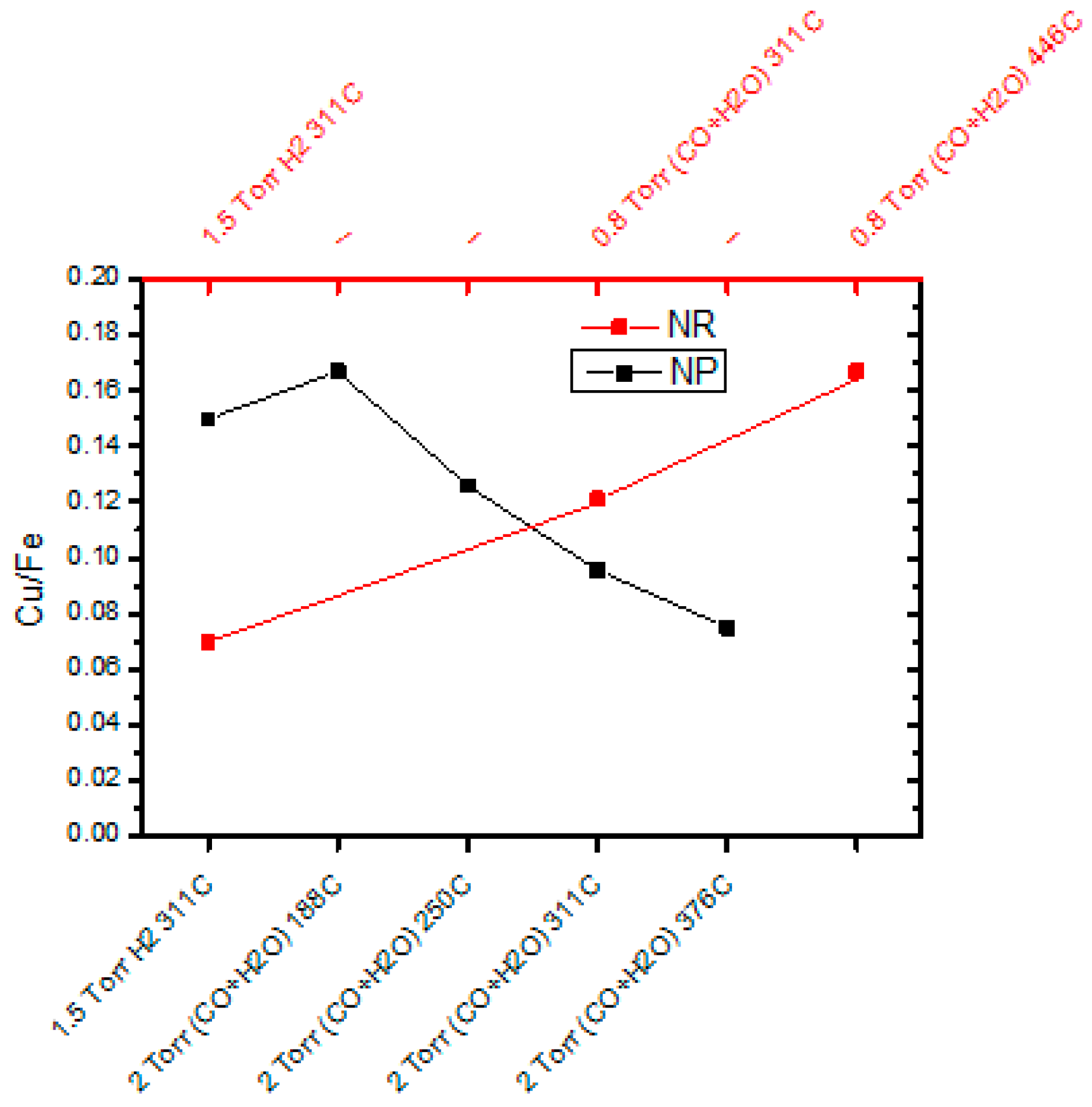
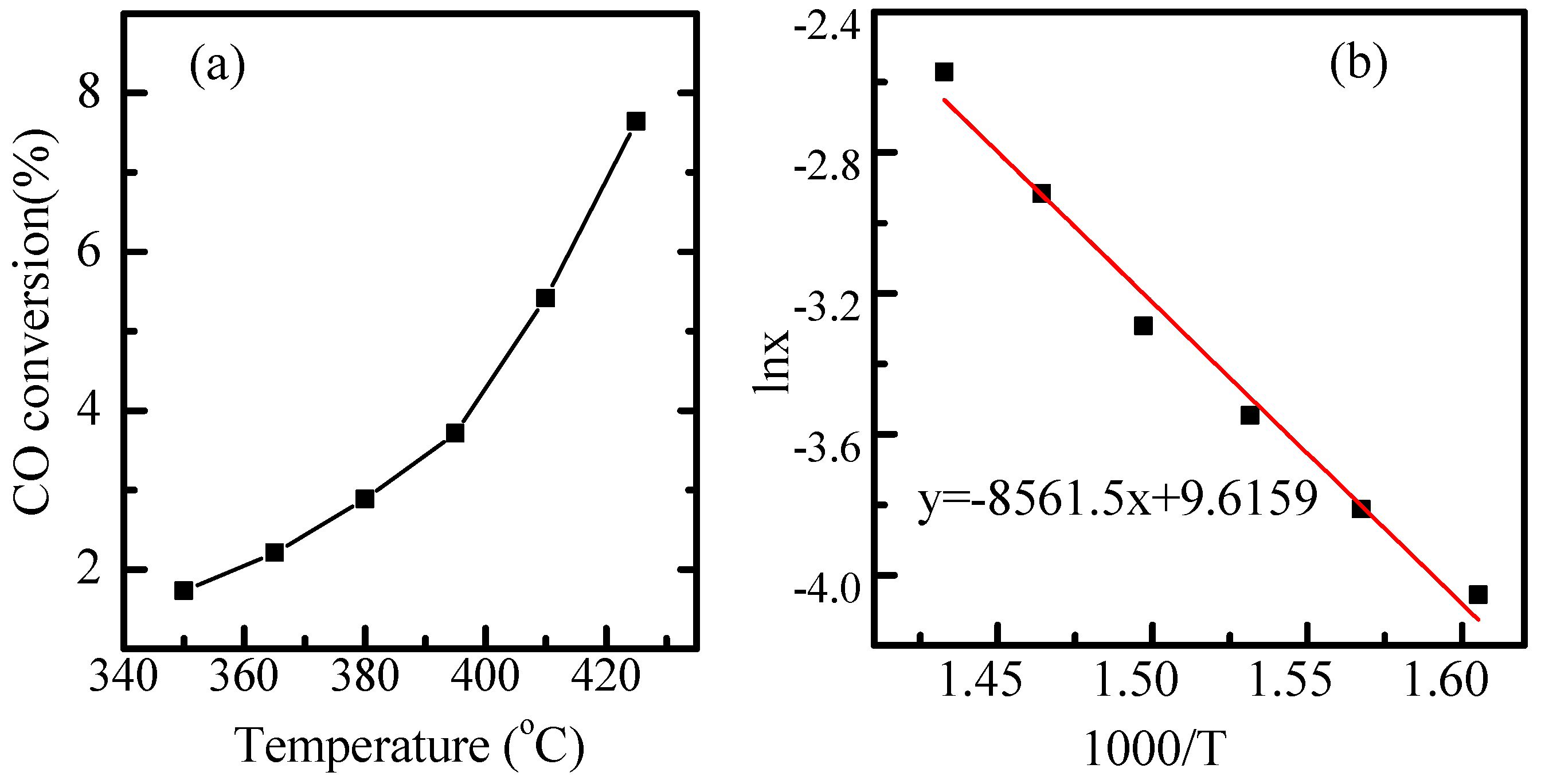
2.4. Water Gas Shift (WGS) Reaction Kinetics and In Situ Studies
3. Materials and Methods
3.1. Materials and Methods
3.2. Characterization of Catalyst Structure
3.3. Ambient Pressure X-Ray Photoelectron SpectroFscopy (AP-XPS) Studies of Evolution of Surface Species
3.4. Measurement of Catalytic Performance
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jacobson, M.Z.; Colella, W.G.; Golden, D.M. Cleaning the air and improving health with hydrogen fuel-cell vehicles. Science 2005, 308, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- Tonkovich, A.Y.; Zilka, J.L.; Lamont, M.J.; Wang, Y. Microchannel reactors for fuel processing applications I. Water gas shift reactor. Chem. Eng. Sci. 1999, 54, 2947–2951. [Google Scholar] [CrossRef]
- Spivey, J.J. Catalysis in the development of clean energy technologies. Catal. Today 2005, 100, 171–180. [Google Scholar] [CrossRef]
- Ertl, G.; Knözinger, H.; Weitkamp, J. Handbook of Heterogeneous Catalysis; VCH: Berlin, Germany, 1997. [Google Scholar]
- Newsome, D.S. The water-gas shift reaction. Catal. Rev. Sci. Eng. 1980, 21, 275–318. [Google Scholar] [CrossRef]
- Zhu, M.; Wachs, I.E. Iron-based catalysts for the high-temperature water-gas shift (HT-WGS) reaction: A review. ACS Catal. 2016, 6, 722–732. [Google Scholar] [CrossRef]
- Ratnasamy, C.; Wagner, J.P. Water gas shift catalysis. Catal. Rev. Sci. Eng. 2009, 51, 325–440. [Google Scholar] [CrossRef]
- Andreeva, A.; Idakieva, V.; Mihajlovaa, D.; Shopova, D. Iron-based catalysts for the water-gas shift reaction promoted by first-row transition metal oxides. Appl. Catal. 1986, 22, 385–387. [Google Scholar] [CrossRef]
- Kappen, P.; Grunwaldt, J.D.; Hammershoi, B.S.; Troger, L.; Clausen, B.S. The state of Cu promoter atoms in high-temperature shift catalysts—An in situ fluorescence XAFS study. J. Catal. 2001, 198, 56–65. [Google Scholar] [CrossRef]
- Gunawardana, P.; Lee, H.C.; Kim, D.H. Performance of copper-ceria catalysts for water gas shift reaction in medium temperature range. Int. J. Hydrogen Energy 2009, 34, 1336–1341. [Google Scholar] [CrossRef]
- Tanaka, Y.; Utaka, T.; Kikuchi, R.; Sasaki, K.; Eguchi, K. CO removal from reformed fuel over Cu/ZnO/Al2O3 catalysts prepared by impregnation and coprecipitation methods. Appl. Catal. A Gen. 2003, 238, 11–18. [Google Scholar] [CrossRef]
- Estrella, M.; Barrio, L.; Zhou, G.; Wang, X.; Wang, Q.; Wen, W.; Hanson, J.C.; Frenkel, A.I.; Rodriguez, J.A. In situ characterization of CuFe2O4 and Cu/Fe3O4 water-gas shift catalysts. J. Phys. Chem. C 2009, 113, 14411–14417. [Google Scholar] [CrossRef]
- Kameoka, S.; Tanabe, T.; Tsai, A.P. Spinel CuFe2O4: A precursor for copper catalyst with high thermal stability and activity. Catal. Lett. 2005, 100, 89–93. [Google Scholar] [CrossRef]
- Mou, X.; Zhang, B.; Li, Y.; Yao, L.; Wei, X.; Su, D.; Shen, W. Rod-shaped Fe2O3 as an efficient catalyst for the selective reduction of nitrogen oxide by ammonia. Angew. Chem. Int. Ed. 2012, 51, 2989–2993. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Ma, L.; Ma, H.; Yue, M. In situ characterization of Cu-Fe-Ox catalyst for water-gas shift reaction. J. Mater. Sci. 2017, 53, 1065–1075. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, L.; Wang, J.; Ding, W.; Chen, Y. Activities of supported copper oxide catalysts in the NO + CO reaction at low temperatures. J. Mol. Catal. A Chem. 2000, 162, 307–316. [Google Scholar] [CrossRef]
- Li, X.; Shen, M.; Hong, X.; Zhu, H.; Gao, F.; Kong, Y.; Dong, L.; Chen, Y. Dispersion and reduction of copper oxide supported on WO3-modified Ce0.5Zr0.5O2 solid solution. J. Phys. Chem. B 2005, 109, 3949–3955. [Google Scholar]
- Hu, Y.; Dong, L.; Shen, M.; Liu, D.; Wang, J.; Ding, W.; Chen, Y. Influence of supports on the activities of copper oxide species in the low-temperature NO + CO reaction. Appl. Catal. B Environ. 2001, 31, 61–69. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Y.; Yin, L.; Chen, C.; Zhan, Y.; Li, D. Characterization and catalytic performance of copper-based WGS catalysts derived from copper ferrite. Int. J. Hydrogen Energy 2014, 39, 6424–6432. [Google Scholar] [CrossRef]
- Gervasini, A.; Bennici, S. Dispersion and surface states of copper catalysts by temperature-programmed-reduction of oxidized surfaces (s-TPR). Appl. Catal. A Gen. 2005, 281, 199–205. [Google Scholar] [CrossRef]
- Keiski, R.L.; Salmi, T.; Niemistö, P.; Ainassaari, J.; Pohjola, V.J. Stationary and transient kinetics of the high temperature water-gas shift reaction. Appl. Catal. A Gen. 1996, 137, 349–370. [Google Scholar] [CrossRef]
- Bohlbro, H.; Jørgensen, M.H. Catalysts for conversion of carbon monoxide. Chem. Eng. World 1970, 5, 46–49. [Google Scholar]
- Rhodes, C.; Hutchings, G.J. Studies of the role of the copper promoter in the iron oxide/chromia high temperature water gas shift catalyst. Phys. Chem. Chem. Phys. 2003, 5, 2719–2723. [Google Scholar] [CrossRef]
- Hla, S.S.; Park, D.; Duffy, G.J.; Edwards, J.H.; Roberts, D.G.; Ilyushechkin, A.; Morpeth, L.D.; Nguyen, T. Kinetics of high-temperature water-gas shift reaction over two iron-based commercial catalysts using simulated coal-derived syngases. Chem. Eng. J. 2009, 146, 148–154. [Google Scholar] [CrossRef]
- Ma, H.; Ma, L.; Hou, M.; Yue, M. Rod-like CuFe4Ox composite: Controllable synthesis and catalytic performance in isoamylic alcohol dehydrogenation. Chin. J. Inorg. Chem. 2017, 33, 2193–2200. [Google Scholar]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Wen, C.; Zhu, Y.; Ye, Y.; Zhang, S.; Cheng, F.; Liu, Y.; Wang, P.; Tao, F. Water-gas shift reaction on metal nanoclusters encapsulated in mesoporous ceria studied with ambient-pressure X-ray photoelectron spectroscopy. ACS Nano 2012, 6, 9305–9313. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Ma, H.; Han, D.; Qiu, M.; Guan, Y.; Hu, Y. Evolution of Copper Supported on Fe3O4 Nanorods for WGS Reaction. Catalysts 2018, 8, 415. https://doi.org/10.3390/catal8100415
Ma L, Ma H, Han D, Qiu M, Guan Y, Hu Y. Evolution of Copper Supported on Fe3O4 Nanorods for WGS Reaction. Catalysts. 2018; 8(10):415. https://doi.org/10.3390/catal8100415
Chicago/Turabian StyleMa, Lingjuan, Hongbin Ma, Dawei Han, Mingyue Qiu, Yafei Guan, and Yanlei Hu. 2018. "Evolution of Copper Supported on Fe3O4 Nanorods for WGS Reaction" Catalysts 8, no. 10: 415. https://doi.org/10.3390/catal8100415




