Novel Bifunctional Mesoporous Catalysts Based on Preyssler Heteropolyacids for Green Pyrrole Derivative Synthesis
Abstract
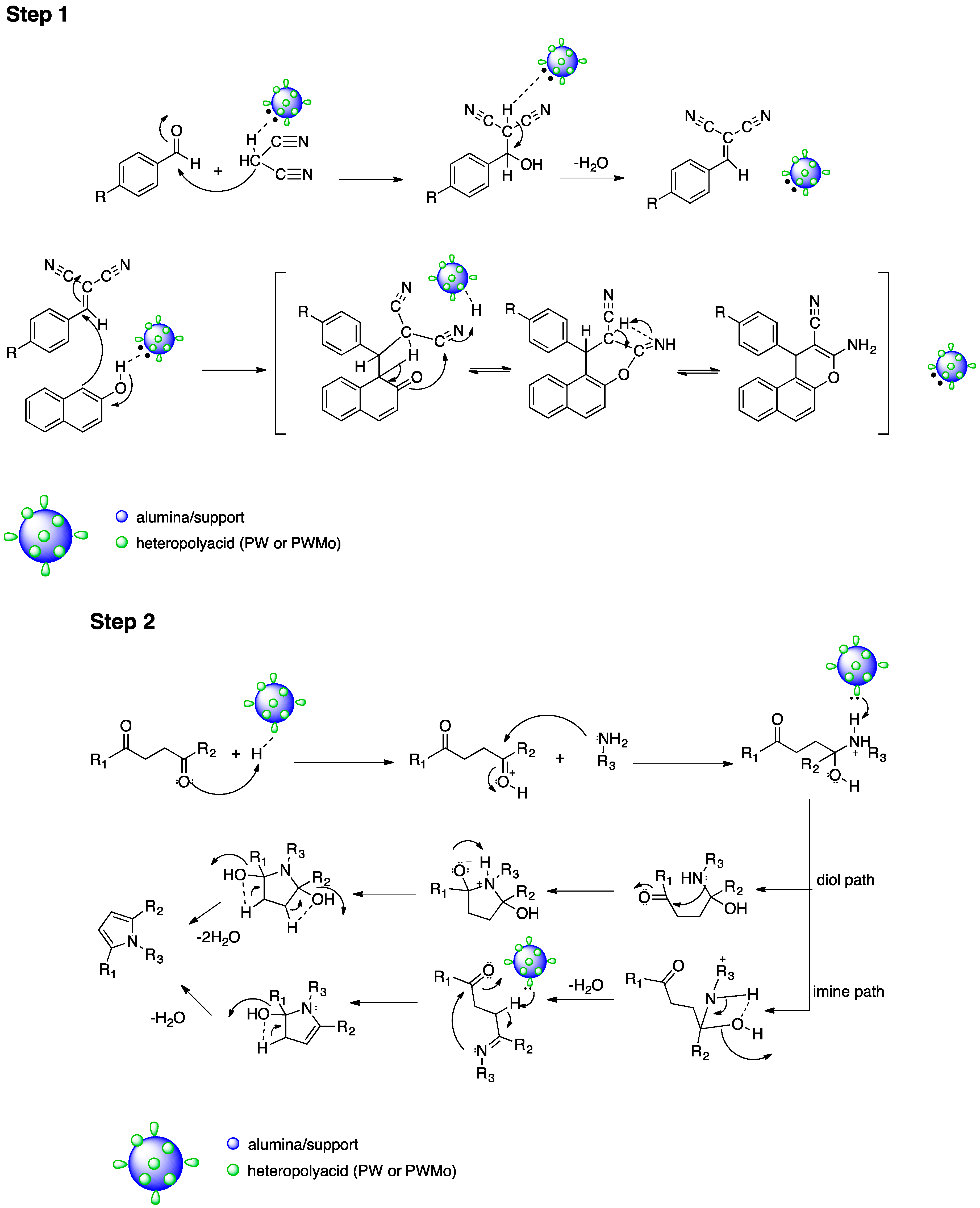
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.1.1. Textural Properties
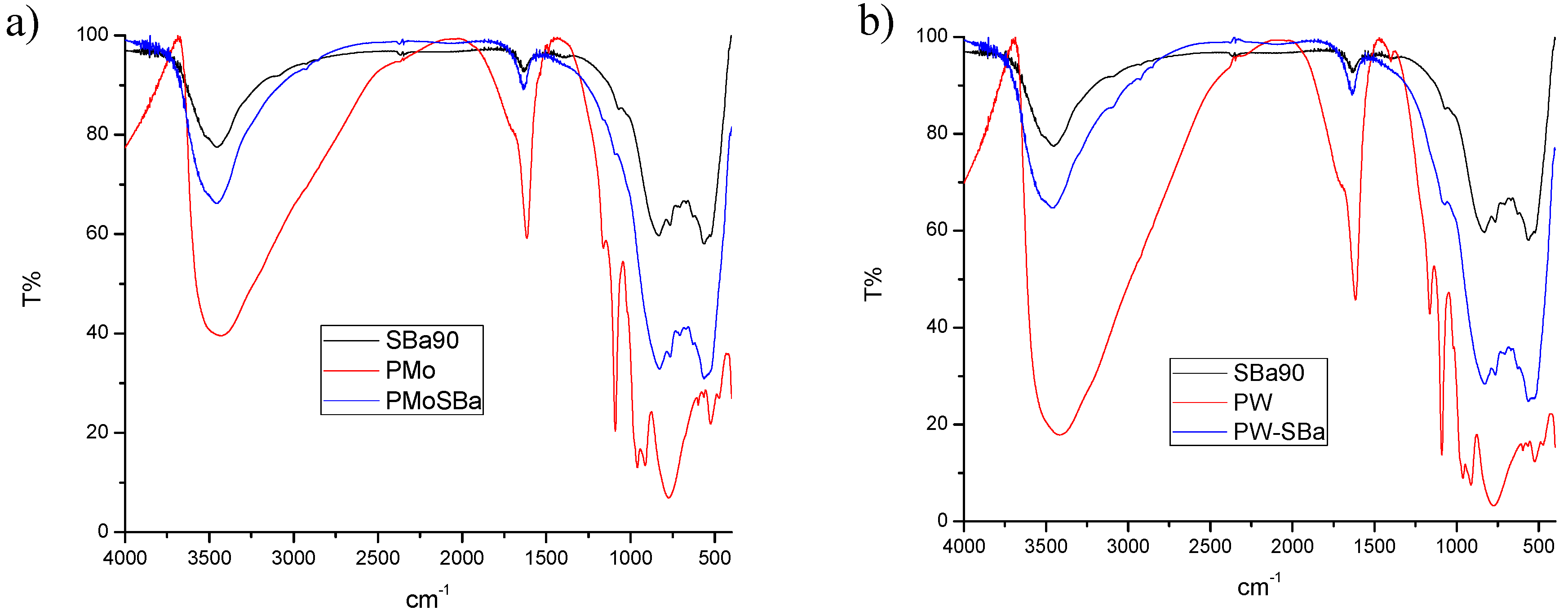
2.1.2. Fourier Transform-Infrared Spectroscopy
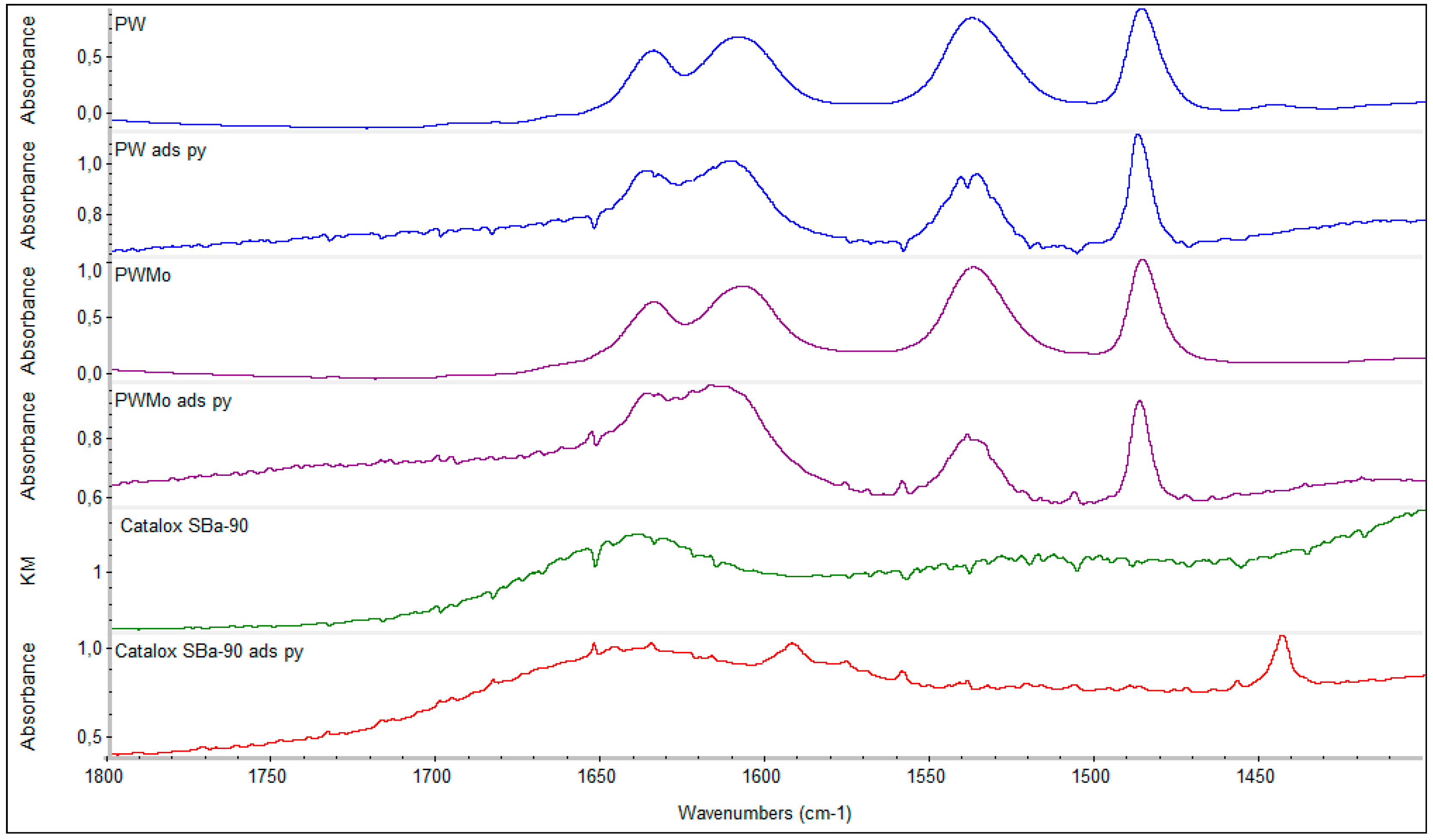
2.1.3. In Situ FT-IR Analysis of Pyridine (py) Adsorption and Desorption
2.1.4. Potentiometric Titration
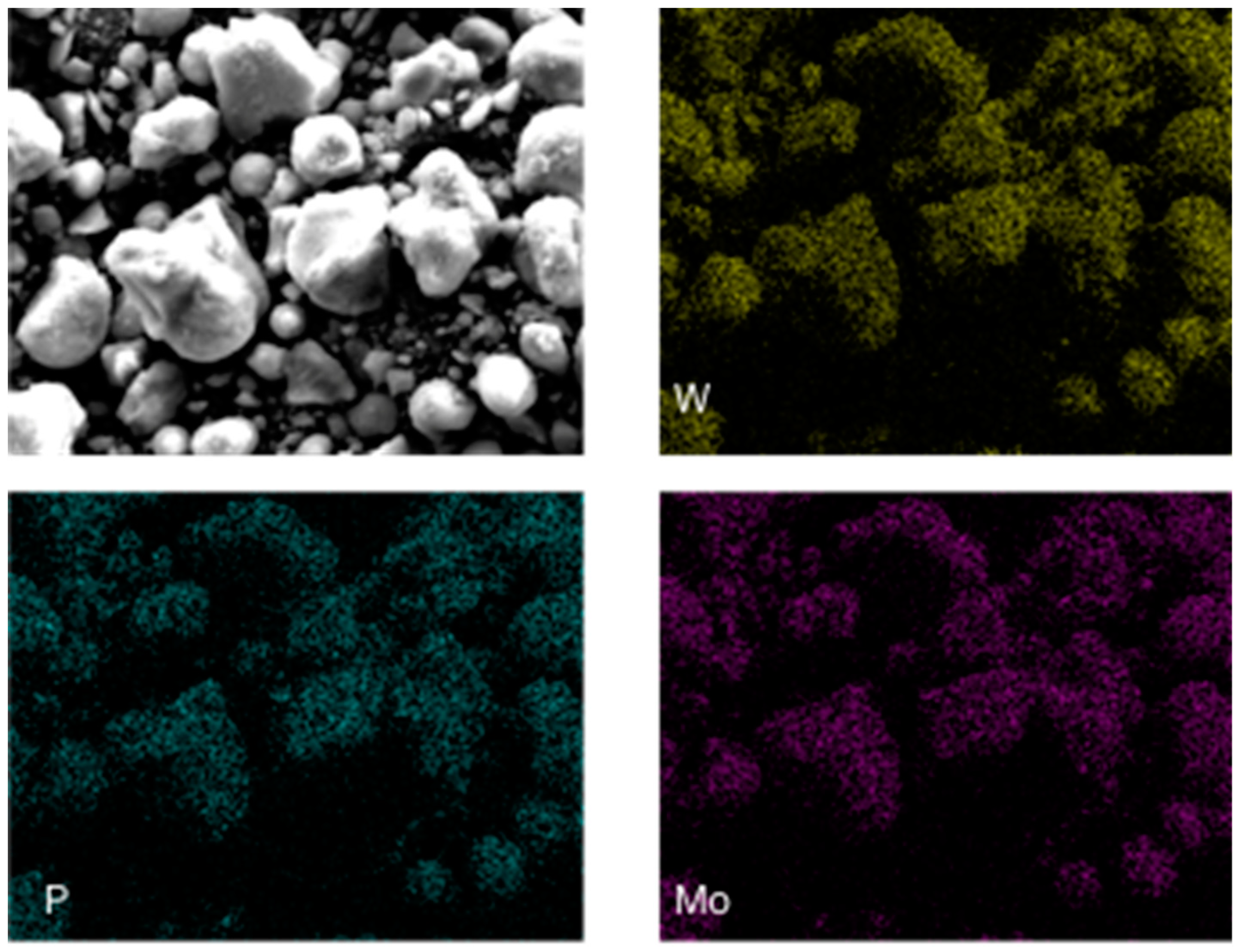
2.1.5. SEM and EDX Spectra
2.2. Catalytic Tests
3. Materials and Methods
3.1. General
3.2. Catalyst Synthesis
3.2.1. Bulk Preyssler Heteropolyacid Synthesis
3.2.2. Alumina-Supported Preyssler Catalyst Synthesis
3.3. Catalyst Characterization
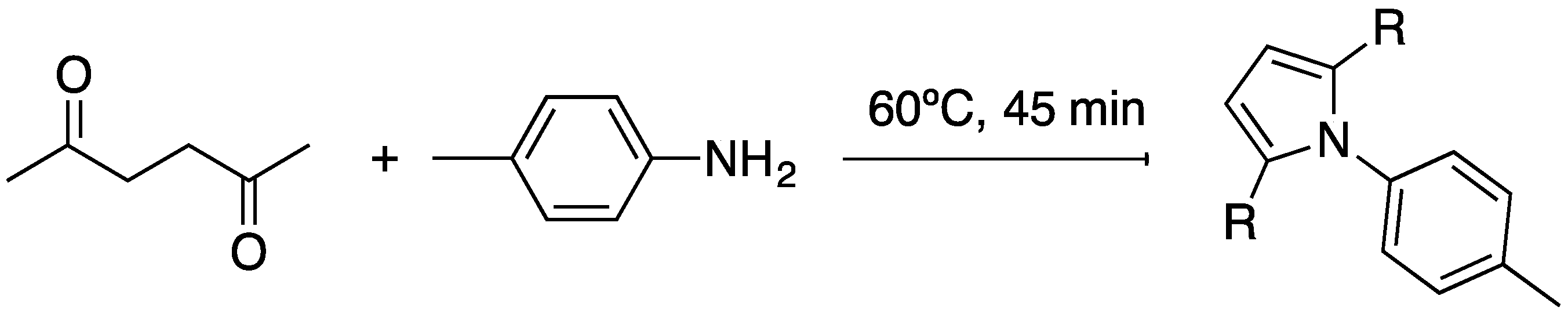
3.4. Catalytic Synthesis of Pyrrole Derivatives
3.4.1. General Procedure for the Synthesis of Pyrrole Derivatives
3.4.2. Melting Point, 1H-NMR, and 13C-NMR of Synthesized Compounds
3.4.3. Catalyst Reuse
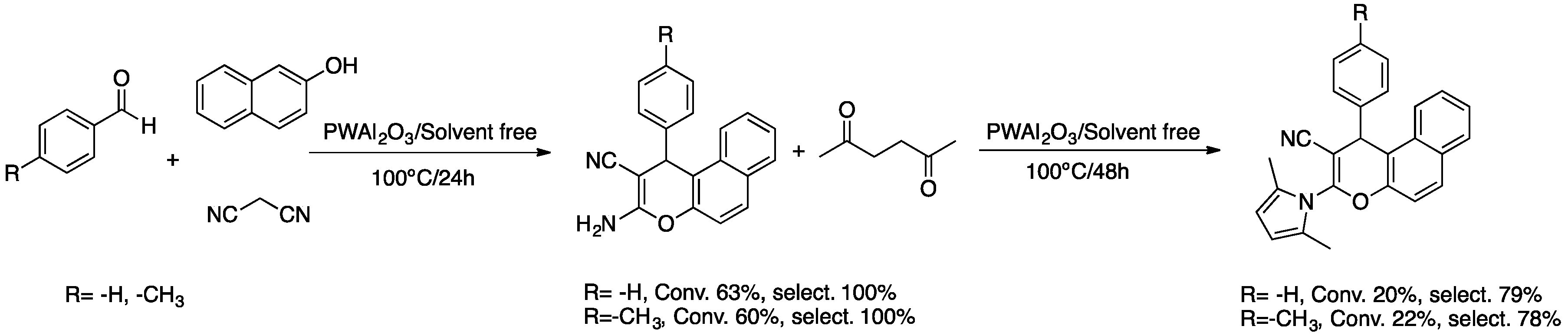
3.4.4. Specific Process for the Multicomponent Synthesis of Chromene–Pyrrole Derivative
3.4.5. Mass Spectrum of Synthetized Chromene–Pyrrole Derivatives
3.4.6. Specific Procedure for the Multicomponent Synthesis of Chromene–Pyrrole Carboxylic Acid Derivative
3.4.7. 1H-NMR, 13C-NMR, and IR Spectra of Synthesized Chromene–Pyrrole Carboxylic Acid Derivative
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Ji, S.-J.; Wang, S.-Y.; Zhang, Y.; Loh, T.-P. Facile synthesis of bis(indolyl)methanes using catalytic amount of iodine at room temperature under solvent-free conditions. Tetrahedron 2004, 60, 2051–2055. [Google Scholar] [CrossRef]
- Khurana, J.M.; Kumar, S. Tetrabutylammonium bromide (TBAB): A neutral and efficient catalyst for the synthesis of biscoumarin and 3,4-dihydropyrano[c]chromene derivatives in water and solvent-free conditions. Tetrahedron Lett. 2009, 50, 4125–4127. [Google Scholar] [CrossRef]
- Ko, S.; Yao, C.F. Heterogeneous catalyst: Amberlyst-15 catalyzes the synthesis of 14-substituted-14H-dibenzo[a,j]xanthenes under solvent-free conditions. Tetrahedron Lett. 2006, 47, 8827–8829. [Google Scholar] [CrossRef]
- Bose, D.S.; Fatima, L.; Mereyala, H.B. Green Chemistry Approaches to the Synthesis of 5-Alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones by a Three-Component Coupling of One-Pot Condensation Reaction: Comparison of Ethanol, Water, and Solvent-free Conditions. J. Org. Chem. 2003, 68, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Wei, C. Highly efficient Grignard-type imine additions via C–H activation in water and under solvent-free conditions. Chem. Commun. 2002, 3, 268–269. [Google Scholar] [CrossRef]
- Enache, D.I.; Edwards, J.K.; Landon, P.; Solsona-Espriu, B.; Carley, A.F.; Herzing, A.A.; Watanabe, M.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Solvent-Free Oxidation of Primary Alcohols to Aldehydes Using Au-Pd/TiO2 Catalysts. Science 2006, 311, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, H.; Zheng, Z.; Jin, C.; Zhang, X.; Su, W. An approach to the Paal–Knorr pyrroles synthesis catalyzed by Sc(OTf)3 under solvent-free conditions. Tetrahedron Lett. 2006, 47, 5383–5387. [Google Scholar] [CrossRef]
- Harreus, A.L. Pyrrole. In Ullman´s Encyclopedia of Industrial Chemistry, 7th ed.; Elvers, B., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; Volume 30, pp. 615–617. [Google Scholar]
- Mal, D.; Shome, B.; Dinda, B.K. Heterocycles in Natural Product Synthesis; Majumdar, K.C., Chattopadhyay, S.K., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 187–215. [Google Scholar]
- Teixeira, C.; Barbault, F.; Rebehmed, J.; Liu, K.; Xie, L.; Lu, H.; Jiang, S.; Fan, B.T.; Maurel, F. Molecular modeling studies of N-substituted pyrrole derivatives-Potential HIV-1 gp41 inhibitors. Bioorg. Med. Chem. 2008, 16, 3039–3048. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, V.; Gumber, D.; Abbot, V.; Dhiman, S.; Sharma, P. Pyrrole: A resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. 2015, 5, 15233–15266. [Google Scholar] [CrossRef]
- Wurz, R.P.; Charette, A.B. Doubly activated cyclopropanes as synthetic precursors for the preparation of 4-nitro- and 4-cyano-dihydropyrroles and pyrroles. Org. Lett. 2005, 7, 2313–2316. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J. Heterocyclic Chemistry in Drug Discovery; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 18–51. [Google Scholar]
- Wang, Z. Comprehensive Organic Name Reactions and Reagents; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 1–4. [Google Scholar]
- Brummond, K.M.; Curran, D.P.; Mitasev, B.; Fischer, S. Heterocyclic α-alkylidene cyclopentenones obtained via a Pauson-Khand reaction of amino acid derived allenynes. A scope and limitation study directed toward the preparation of a tricyclic pyrrole library. J. Org. Chem. 2005, 70, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Vekariya, R.H.; Patel, H.D. Sulfonated polyethylene glycol (PEG-OSO3H) as a polymer supported biodegradable and recyclable catalyst in green organic synthesis: Recent advances. RSC Adv. 2015, 5, 49006–49030. [Google Scholar] [CrossRef]
- Wang, B.; Gu, Y.; Luo, C.; Yang, T.; Yang, L.; Suo, J. Pyrrole synthesis in ionic liquids by Paal-Knorr condensation under mild conditions. Tetrahedron Lett. 2004, 45, 3417–3419. [Google Scholar] [CrossRef]
- Sharma, A.; Piplani, P. Microwave-activated Synthesis of Pyrroles: A Short Review. J. Heterocycl. Chem. 2017, 54, 27–34. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Li, J.J.; Li, T.S. Ultrasound-assisted synthesis of pyrroles catalyzed by zirconium chloride under solvent-free conditions. Ultrason. Sonochem. 2008, 15, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, G.P.; Autino, J.C. Recent Applications of Heteropolyacids and Related Compounds in Heterocycles Synthesis. Mini. Rev. Org. Chem. 2009, 6, 359–366. [Google Scholar] [CrossRef]
- Hekmatshoar, R.; Heravi, M.M.; Sadjadi, S.; Oskooie, H.A.; Bamoharram, F.F. Catalytic performance of Preyssler heteropolyacid, [NaP5W30O110]14-in liquid phase alkylation of phenol with 1-octene. Catal. Commun. 2008, 9, 837–841. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Jahangir, M.; Gharib, A. Preyssler catalyst, [NaP5W30O110]14-: A green, efficient and reusable catalyst for esterification of salicylic acid with aliphatic and benzylic alcohols. Appl. Catal. A Gen. 2006, 302, 42–47. [Google Scholar] [CrossRef]
- Ruiz, D.M.; Romanelli, G.P.; Vázquez, P.G.; Autino, J.C. Preyssler catalyst: An efficient catalyst for esterification of cinnamic acids with phenols and imidoalcohols. Appl. Catal. A Gen. 2010, 374, 110–119. [Google Scholar] [CrossRef]
- Heravi, M.M.; Bamoharram, F.F.; Rajabzadeh, G.; Seifi, N.; Khatami, M. Preyssler heteropolyacid [NaP5W30O110]14-, as a new, green and recyclable catalyst for the synthesis of [1,2,4]triazino[4,3-b][1,2,4,5]tetrazines. J. Mol. Catal. A Chem. 2006, 259, 213–217. [Google Scholar] [CrossRef]
- Páez, A.; Rojas, H.A.; Portilla, O.; Sathicq, G.; Afonso, C.A.M.; Romanelli, G.P.; Martínez, J.J. Preyssler Heteropolyacids in the Self-Etherification of 5-Hydroxymethylfurfural to 5,5′-[Oxybis(methylene)]bis-2-furfural Under Mild Reaction Conditions. ChemCatChem 2017, 9, 3322–3329. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Ayati, A.; Baharara, J.; Jafari, A.M.; Ebrahimi, M. Acidic cesium salt of Preyssler nanoparticles: A new, green and recyclable nanocatalyst for the synthesis of 6-aryl-1H-pyrazolo[3,4-d]pyrimidin-4[5H]-ones. J. Nanostructure Chem. 2014, 4, 93. [Google Scholar] [CrossRef]
- Javid, A.; Khojastehnezhad, A.; Eshghi, H.; Moeinpour, F.; Bamoharram, F.F.; Ebrahimi, J. Synthesis of Pyranopyrazoles using a Magnetically Separable Modified Preyssler Heteropoly Acid. Org. Prep. Proced. Int. 2016, 48, 377–384. [Google Scholar] [CrossRef]
- Tanhaei, B.; Ayati, A.; Bamoharram, F.F.; Lahtinen, M.; Sillanpää, M. A novel magnetic Preyssler acid grafted chitosan nano adsorbent: Synthesis, characterization and adsorption activity. J. Chem. Technol. Biotechnol. 2016, 91, 1452–1460. [Google Scholar] [CrossRef]
- Rohani, M.; Bamoharram, F.F.; Khosravi, M.; Baharara, J.; Heravi, M.M. Preparation and characterisation of Preyssler heteropolyacid-cellulose acetate hybrid nanofibers: A new, green and recyclable nanocatalyst for photodegradation of methyl orange as the model dye. J. Exp. Nanosci. 2017, 12, 1–13. [Google Scholar] [CrossRef]
- Tanhaei, B.; Ayati, A.; Bamoharram, F.F.; Sillanpää, M. Magnetic EDTA Functionalized Preyssler Cross Linked Chitosan Nanocomposite for Adsorptive Removal of Pb(II) Ions. Clean Soil Air Water 2017, 45, 1–19. [Google Scholar] [CrossRef]
- Yadav, J.S.; Raghavendra, S.; Satyanarayana, M.; Balanarsaiah, E. Phosphomolybdic acid supported on silica gel: An efficient, mild and reusable catalyst for the chemoselective hydrolysis of acetonides. Synlett 2005, 16, 2461–2464. [Google Scholar] [CrossRef]
- Tarlani, A.; Abedini, M.; Nemati, A.; Khabaz, M.; Amini, M.M. Immobilization of Keggin and Preyssler tungsten heteropolyacids on various functionalized silica. J. Colloid Interface Sci. 2006, 303, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Urabe, K. Catalysis of Heteropoly Acids Entrapped in Activated Carbon. Chem. Lett. 1981, 10, 663–666. [Google Scholar] [CrossRef]
- Bhorodwaj, S.K.; Dutta, D.K. Activated clay supported heteropoly acid catalysts for esterification of acetic acid with butanol. Appl. Clay Sci. 2011, 53, 347–352. [Google Scholar] [CrossRef]
- Yadav, J.S.; Subba Reddy, B.V.; Srinivasa Rao, T.; Narender, R.; Gupta, M.K. PMA/SiO2 as efficient, cost-effective and recyclable catalytic system for the synthesis of highly substituted pyrroles. J. Mol. Catal. A Chem. 2007, 278, 42–46. [Google Scholar] [CrossRef]
- Jafari, A.A.; Mahmoudi, H.; Mirjalili, B.F. Tungstophosphoric Acid Supported on Silica Gel (H2PW12O40/SiO2), Reusable and Heterogeneous Catalyst for the Synthesis of Pyrroles in Solution or under Solvent-Free Microwave Irradiation. J. Iran. Chem. Soc. 2011, 8, 851–856. [Google Scholar] [CrossRef]
- Kumar, M.A.; Krishna, A.B.; Babu, B.H.; Reddy, C.B.; Reddy, C.S. Phosphomolybdic Acid/SiO2 as Heterogeneous Solid Acid Catalyst for the Rapid Synthesis of N -Substituted Pyrroles. Synth. Commun. 2008, 38, 3456–3464. [Google Scholar] [CrossRef]
- Villabrille, P.; Romanelli, G.; Quaranta, N.; Vázquez, P. An efficient catalytic route for the preparation of silyl ethers using alumina-supported heteropolyoxometalates. Appl. Catal. B Environ. 2010, 96, 379–386. [Google Scholar] [CrossRef]
- Rafiee, E.; Eavani, S. Heterogenization of heteropoly compounds: A review of their structure and synthesis. RSC Adv. 2016, 6, 46433–46466. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, X.; Yang, X.; Wang, X.; Chu, W.; Hu, Y. Heterogenization of heteropolyacids: A general discussion on the preparation of supported acid catalysts. Ind. Eng. Chem. Res. 1996, 35, 2546–2560. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, M.; Chen, H.; Chen, J.; Chen, H. Convenient Method for Synthesis of Substituted 2-Amino-2-chromenes. Synth. Commun. 2007, 37, 231–235. [Google Scholar] [CrossRef]
- Şen, B.; Lolak, N.; Paralı, Ö.; Koca, M.; Şavk, A.; Akocak, S.; Şen, F. Bimetallic PdRu/graphene oxide based Catalysts for one-pot three-component synthesis of 2-amino-4H-chromene derivatives. Nano-Struct. Nano-Objects 2017, 12, 33–40. [Google Scholar] [CrossRef]
- Abbat, S.; Dhaked, D.; Arfeen, M.; Bharatam, P.V. Mechanism of the Paal-Knorr reaction: The importance of water mediated hemialcohol pathway. RSC Adv. 2015, 5, 88353–88366. [Google Scholar] [CrossRef]
- Kharat, A.N.; Abedini, M.; Amini, M.M.; Pendleton, P.; Badalyan, A. Investigation of the Preyssler phosphotungstate heteropolyanion, [NaP5W30O110]14-, properties with different counter ions. Transit. Met. Chem. 2003, 28, 339–344. [Google Scholar] [CrossRef]
- Jeannin, Y.; Martin-Frere, J. Inorganic Syntheses; Ginsberg, A.P., Ed.; Jhon Wiley & Sons: Mississauga, ON, Canada, 1990; Volume 27, pp. 115–118. [Google Scholar]
- Andraos, J. Global green chemistry metrics analysis algorithm and spreadsheets: Evaluation of the material efficiency performances of synthesis plans for oseltamivir phosphate (Tamiflu) as a test case. Org. Process Res. Dev. 2009, 13, 161–185. [Google Scholar] [CrossRef]
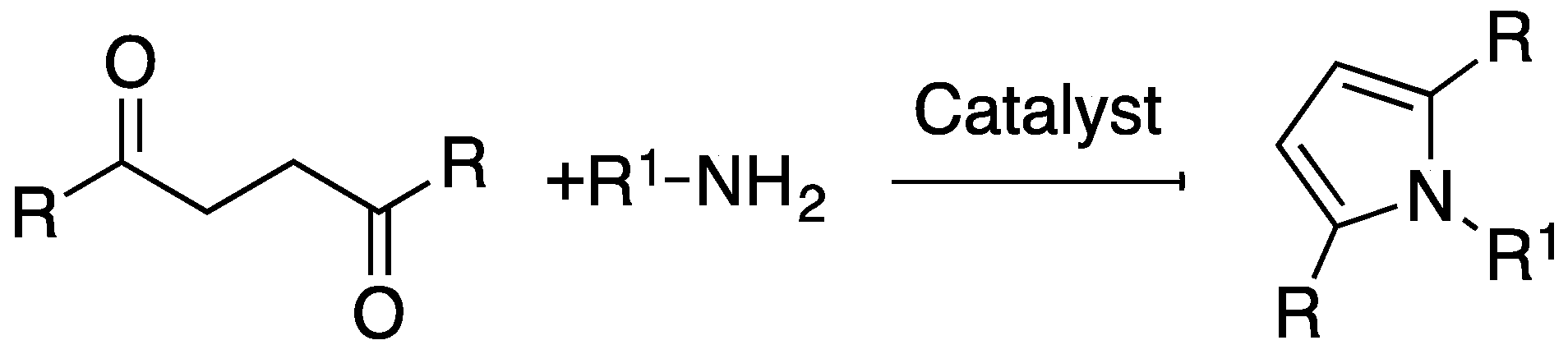



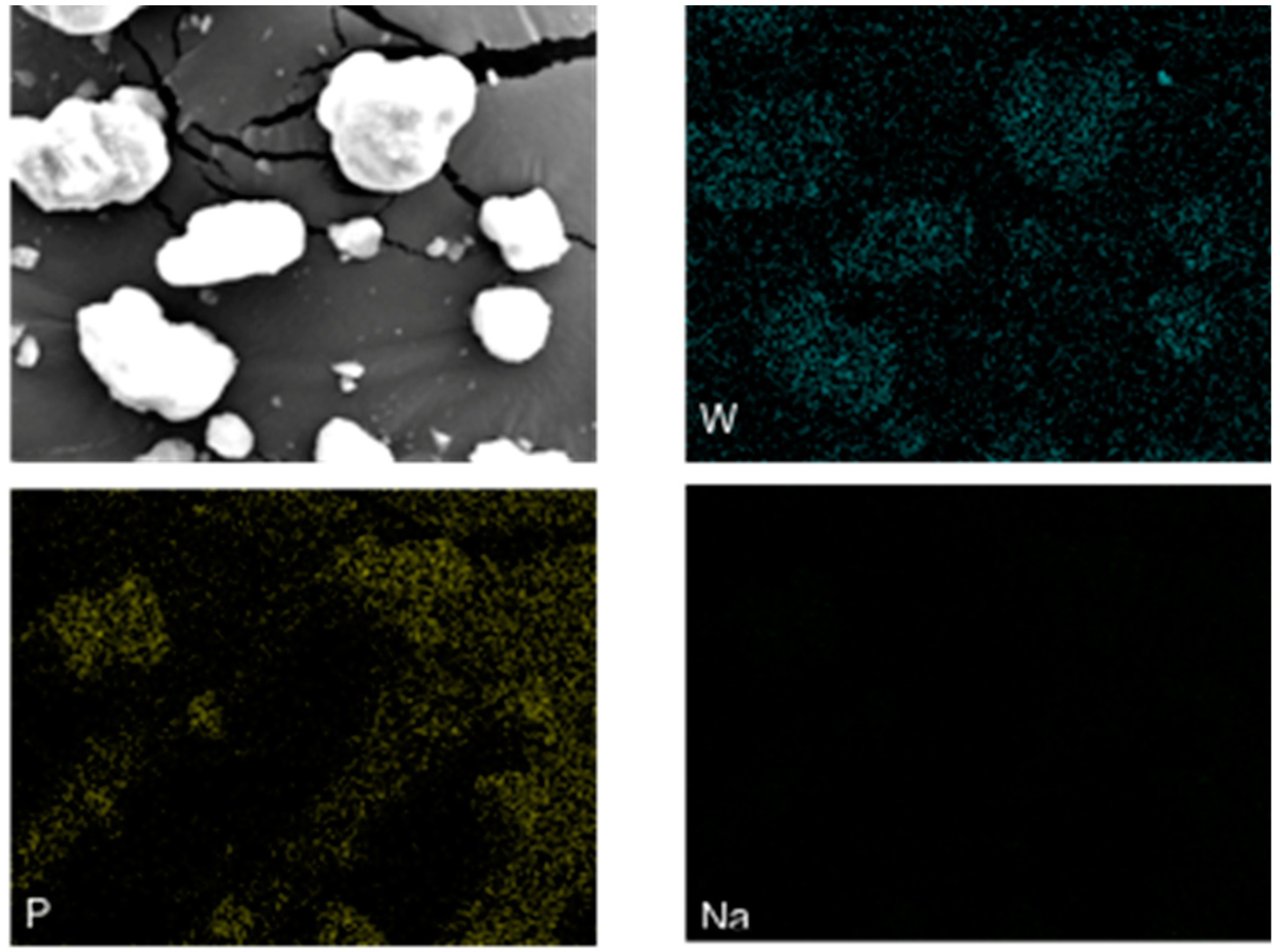
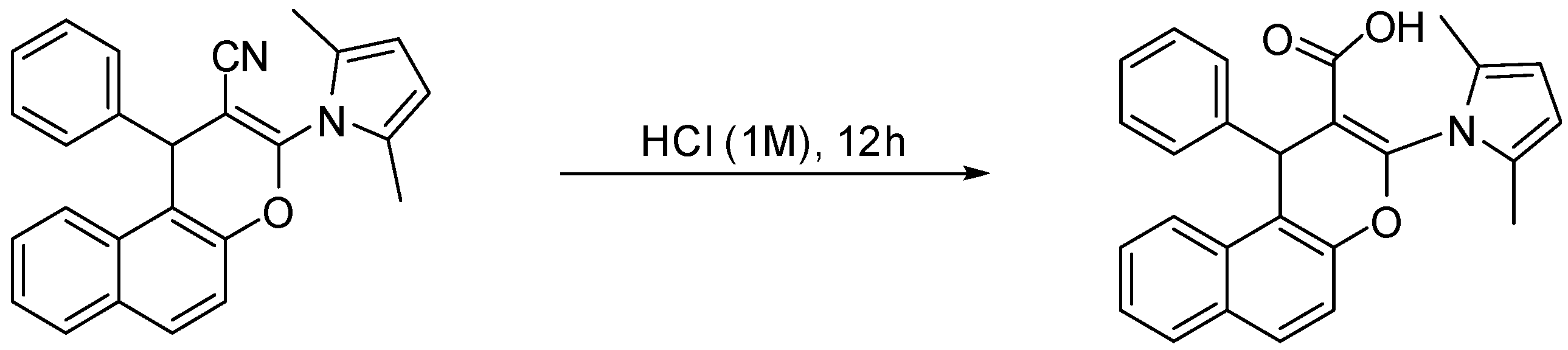
| Catalyst | SBET (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| PW | <10 | - | - |
| PWMo | <10 | - | - |
| Al2O3 | 106 | 0.3 | 14.6 |
| PWAl2O3 | 101 | 0.3 | 13.2 |
| PWMoAl2O3 | 110 | 0.3 | 12.1 |
| Catalyst | Ei (U/mV) |
|---|---|
| PW | 779 |
| PWMo | 657 |
| Al2O3 | 74 |
| PWAl2O3 | 497 |
| PWMoAl2O3 | 452 |
| Entry | Amount of Catalyst (mg) | Yields (%) a |
|---|---|---|
| 1 | 0 | 47 |
| 2 | 4 | 55 |
| 3 | 10 | 58 |
| 4 | 20 | 96 |
| 5 | 40 | 98 |
| 6 | 80 | 85 |
| Entry | Temperature (°C) | Yields (%) a |
|---|---|---|
| 1 | 20 | 10 |
| 2 | 40 | 29 |
| 3 | 60 | 98 |
| 4 | 80 | 85 b |
| 5 | 100 | 77 b |
| Entry | Time (min) | Yields (%) a |
|---|---|---|
| 1 | 30 | 72 |
| 2 | 45 | 98 |
| 3 | 60 | 96 |
| 4 | 120 | 85 b |
| Entry | Catalyst Cycle | Yield (%) a |
|---|---|---|
| 1 | 1 | 98 |
| 2 | 2 | 95 |
| 3 | 3 | 85 b |
| 4 | 4 | 80 b |
| 5 | 5 | 77 b |
| Entry | Catalyst | Yields (%) a |
|---|---|---|
| 1 | None | 47 |
| 2 | PW | 55 |
| 3 | PWMo | 50 |
| 4 | Al2O3 | 50 |
| 5 | PWAl2O3 | 83 b (81, 80, 80) c |
| 6 | PWMoAl2O3 | 78 b |
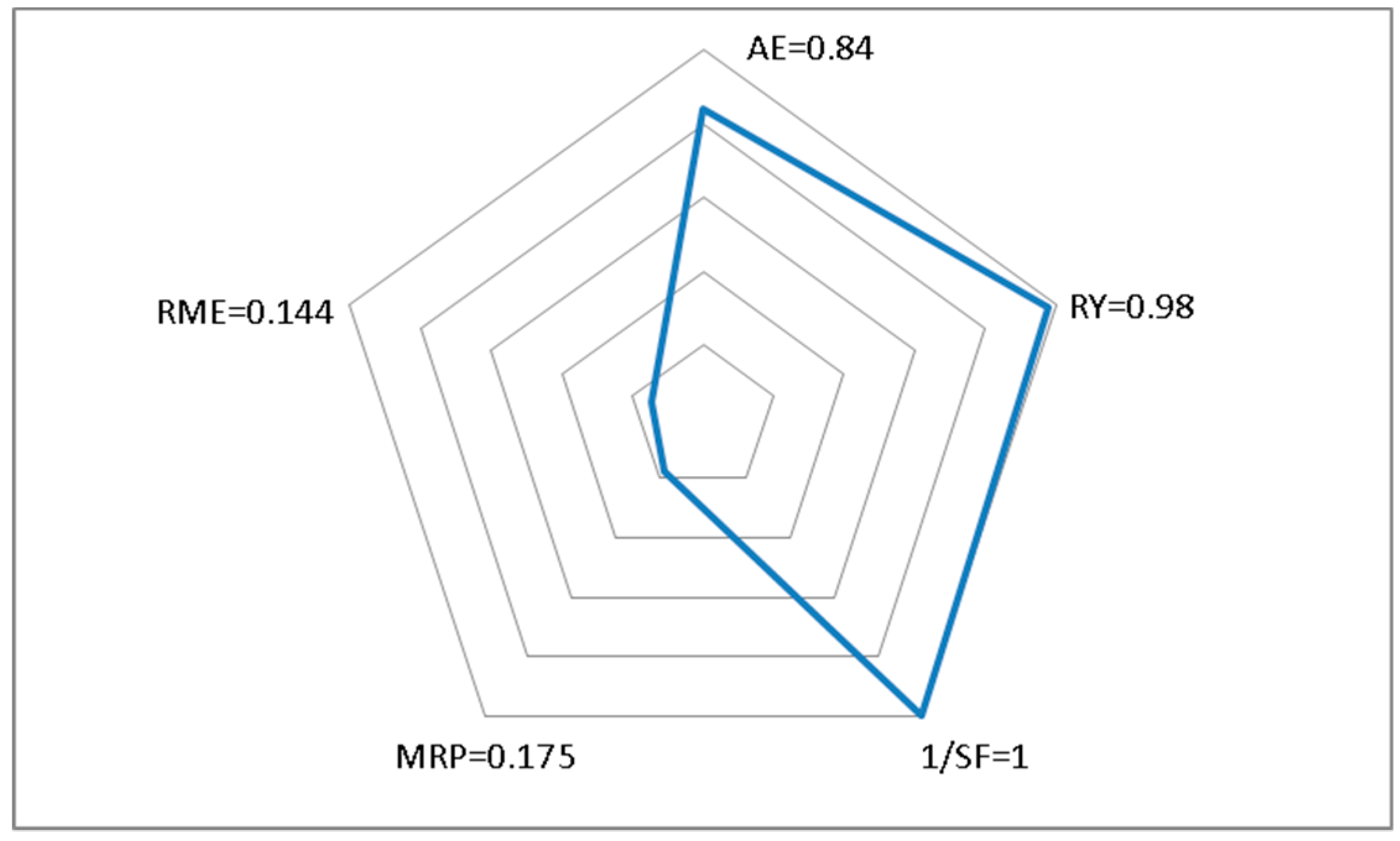
| Entry | Product a | t (h) | Y (%) b PW Al2O3PW | AE b (%) | EF b | PMI b | TON b | TOF b |
|---|---|---|---|---|---|---|---|---|
| 1 2 |  | 2 | 85 79 | 83 | 0.699 0.828 | 1.699 1.828 | 3.63 33.8 | 1.800 16.86 |
| 3 4 |  | 0.5 | 98 85 | 84 | 0.440 0.660 | 1.440 1.660 | 4.53 39.3 | 9.060 78.60 |
| 5 6 |  | 4 | 86 83 | 85 | 0.593 0.650 | 1.593 1.650 | 4.42 42.6 | 1.080 10.68 |
| 7 8 | 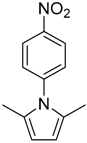 | 18 | 73 70 | 86 | 0.852 0.931 | 1.852 1.931 | 3.94 37.8 | 0.240 2.100 |
| 9 10 | 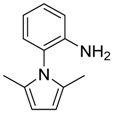 | 2 | 90 82 | 84 | 0.565 0.718 | 1.565 1.718 | 4.18 38.1 | 2.100 19.08 |
| 11 12 | 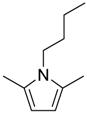 | 3.5 | 92 81 | 81 | 0.634 0.856 | 1.634 1.856 | 3.47 30.6 | 1.020 8.760 |
| 13 14 | 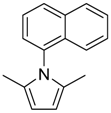 | 3 | 85 75 | 86 | 0.581 0.792 | 1.581 1.792 | 4.70 41.4 | 1.560 13.80 |
| 15 16 |  | 1.5 | 98 87 | 86 | 0.374 0.547 | 1.374 1.547 | 5.38 47.7 | 3.600 31.80 |
| 17 18 | 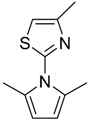 | 3 | 84 76 | 84 | 0.662 0.837 | 1.662 1.837 | 4.03 36.5 | 1.320 12.18 |
| 19 20 | 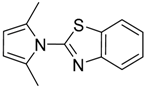 | 3 | 98 90 | 86 | 0.361 0.481 | 1.361 1.481 | 5.59 51.3 | 1.860 17.10 |
| 21 22 |  | 3 | 78 69 | 88 | 0.653 0.869 | 1.653 1.869 | 5.12 45.3 | 1.680 15.12 |
| 23 24 |  | 3 | 50 53 | 88 | 1.557 1.412 | 2.557 2.412 | 3.41 36.2 | 1.140 12.060 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portilla-Zúñiga, O.; Sathicq, Á.; Martínez, J.; Rojas, H.; De Geronimo, E.; Luque, R.; Romanelli, G.P. Novel Bifunctional Mesoporous Catalysts Based on Preyssler Heteropolyacids for Green Pyrrole Derivative Synthesis. Catalysts 2018, 8, 419. https://doi.org/10.3390/catal8100419
Portilla-Zúñiga O, Sathicq Á, Martínez J, Rojas H, De Geronimo E, Luque R, Romanelli GP. Novel Bifunctional Mesoporous Catalysts Based on Preyssler Heteropolyacids for Green Pyrrole Derivative Synthesis. Catalysts. 2018; 8(10):419. https://doi.org/10.3390/catal8100419
Chicago/Turabian StylePortilla-Zúñiga, Omar, Ángel Sathicq, José Martínez, Hugo Rojas, Eduardo De Geronimo, Rafael Luque, and Gustavo P. Romanelli. 2018. "Novel Bifunctional Mesoporous Catalysts Based on Preyssler Heteropolyacids for Green Pyrrole Derivative Synthesis" Catalysts 8, no. 10: 419. https://doi.org/10.3390/catal8100419
APA StylePortilla-Zúñiga, O., Sathicq, Á., Martínez, J., Rojas, H., De Geronimo, E., Luque, R., & Romanelli, G. P. (2018). Novel Bifunctional Mesoporous Catalysts Based on Preyssler Heteropolyacids for Green Pyrrole Derivative Synthesis. Catalysts, 8(10), 419. https://doi.org/10.3390/catal8100419






