SnO2 Composite Films for Enhanced Photocatalytic Activities
Abstract
:1. Introduction
2. Results and Discussion
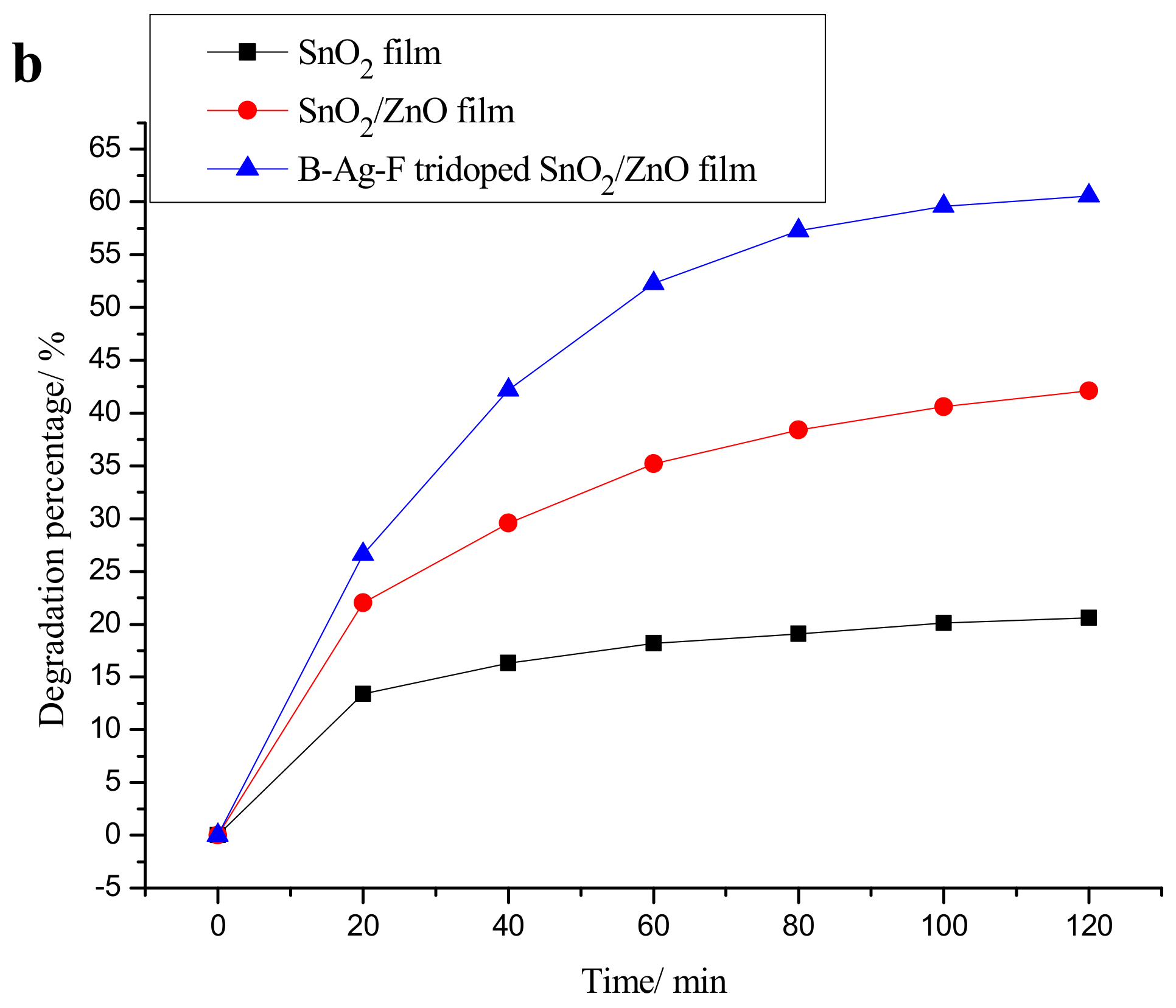
2.1. Photocatalytic Activity
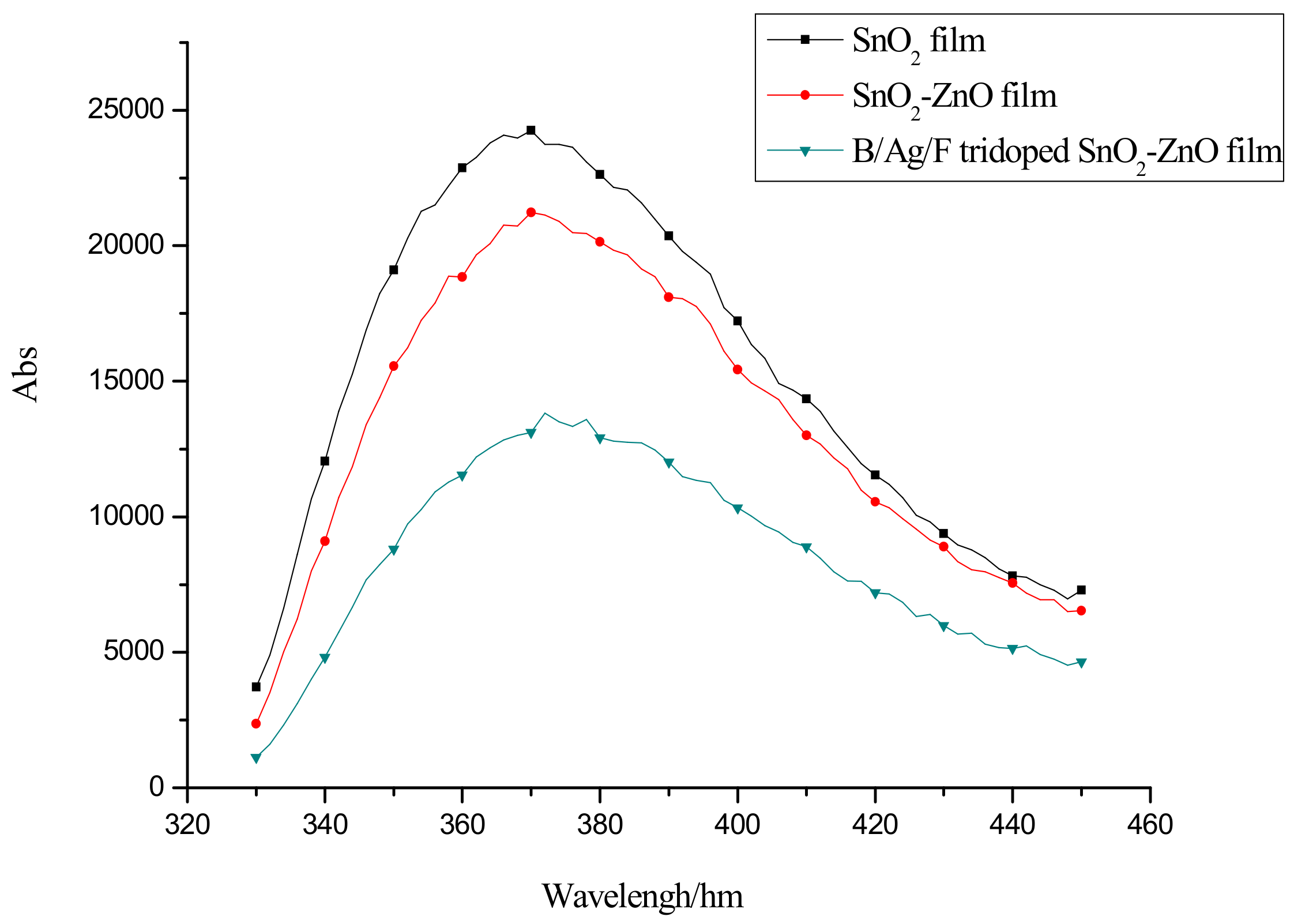
2.2. Optical Absorption
2.3. Photoluminescence (PL) Analysis
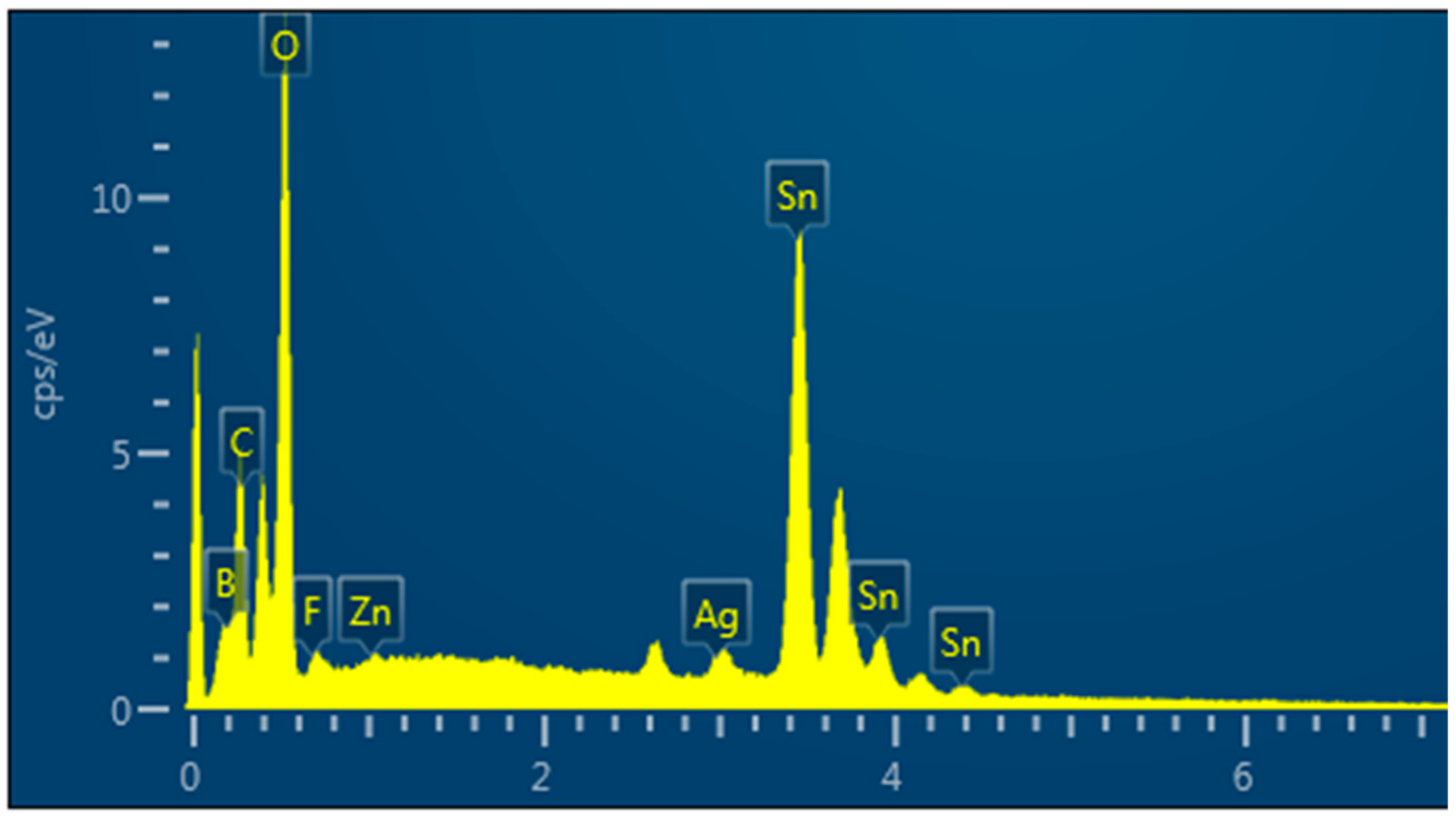
2.4. Crystal Structure
2.5. Thermal Analysis
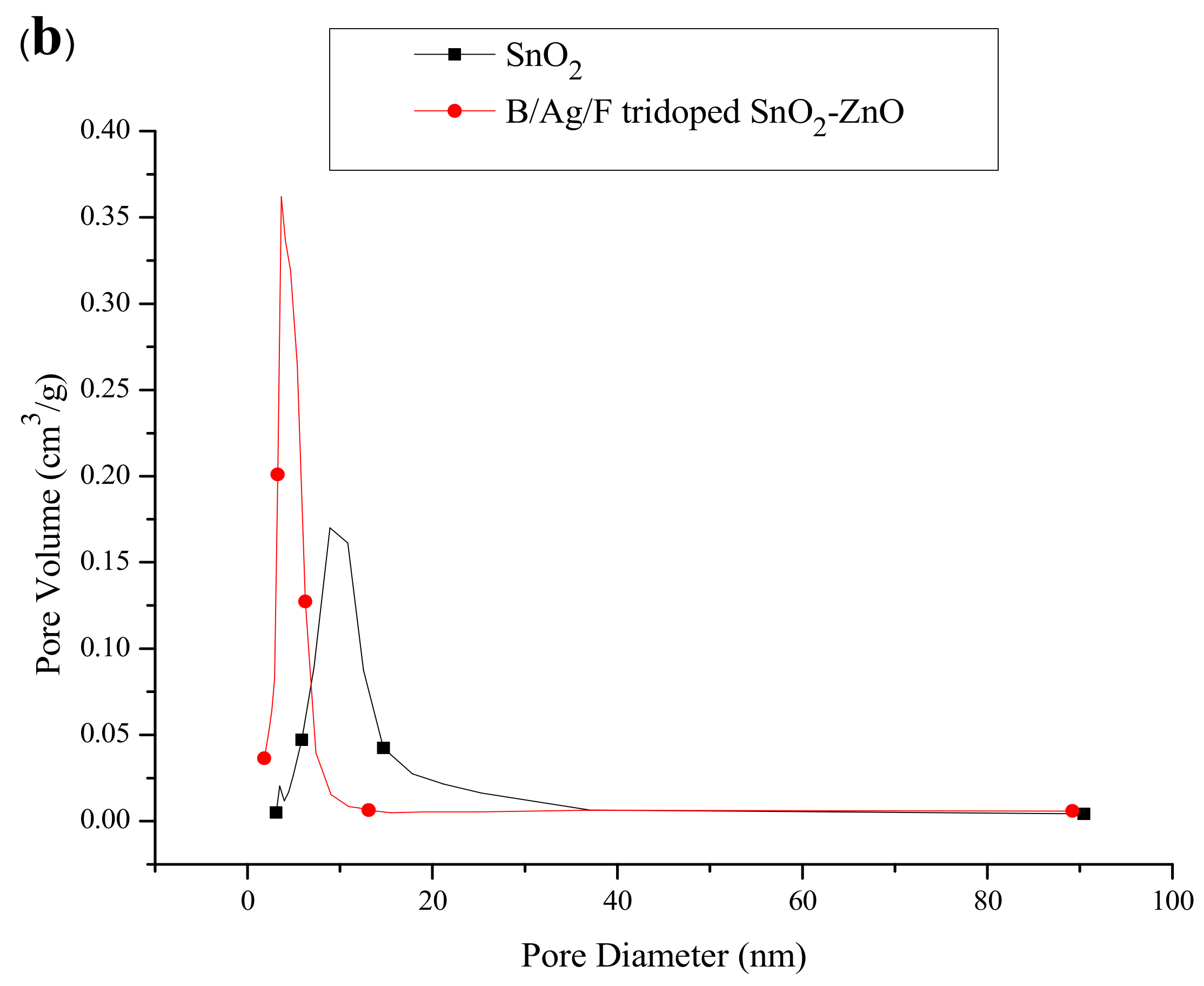
2.6. Surface Areas
2.7. Surface Morphology
3. Experimental
3.1. Film Preparation
3.2. Catalyst Characterization
3.3. Catalyst Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Mehta, A.; Mishra, A.; Singh, J.; Rawat, M.; Basu, S. Biosynthesis of tin oxide nanoparticles using Psidium Guajava leave extract for photocatalytic dye degradation under sunlight. Mater. Lett. 2018, 215, 121–124. [Google Scholar] [CrossRef]
- Patzsch, J.; Bloh, J.Z. Improved photocatalytic ozone abatement over transition metal-grafted titanium dioxide. Catal. Today 2018, 300, 2–11. [Google Scholar] [CrossRef]
- Tsai, M.-T.; Chang, Y.-S.; Liu, Y.-C. Photocatalysis and luminescence properties of zinc stannate oxides. Ceram. Int. 2017, 43, S428–S434. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, C.; Zheng, Y.; Zhan, Y.; Cao, Y.; Lin, X.; Zheng, Q.; Wei, K.; Zhu, J. Photocatalytic activity of ZnO/Sn1−xZnxO2−x nanocatalysts: A synergistic effect of doping and heterojunction. Appl. Catal. B Environ. 2014, 148–149, 44–50. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Jana, S.; Mitra, B.C.; Bera, P.; Sikdar, M.; Mondal, A. Photocatalytic activity of galvanically synthesized nanostructure SnO2 thin films. J. Alloy Compd. 2014, 602, 42–48. [Google Scholar] [CrossRef]
- Al-Hamdi, A.M.; Sillanpää, M.; Dutta, J. Photocatalytic degradation of phenol by iodine doped tin oxide nanoparticles under UV and sunlight irradiation. J. Alloy Compd. 2015, 618, 366–371. [Google Scholar] [CrossRef]
- Masjedi-Arani, M.; Salavati-Niasari, M. Metal (Mn, Co, Ni and Cu) doped ZnO-Zn2SnO4-SnO2 nanocomposites: Green sol-gel synthesis, characterization and photocatalytic activity. J. Mol. Liq. 2017, 248, 197–204. [Google Scholar] [CrossRef]
- Ivetić, T.B.; Finčur, N.L.; Abramović, B.F.; Dimitrievska, M.; Štrbac, G.R.; Čajko, K.O.; Miljević, B.B.; Đačanin, L.R.; Lukić-Petrović, S.R. Environmentally friendly photoactive heterojunction zinc tin oxide nanoparticles. Ceram. Int. 2016, 42, 3575–3583. [Google Scholar] [CrossRef]
- Kong, X.-B.; Li, F.; Qi, Z.-N.; Qi, L.; Yao, M.-M. Boron-doped tin dioxide films for environmental applications. Surf. Rev. Lett. 2017, 24, 1750059. [Google Scholar] [CrossRef]
- Hamrouni, A.; Moussa, N.; Parrino, F.; Di Paola, A.; Houas, A.; Palmisano, L. Sol–gel synthesis and photocatalytic activity of ZnO–SnO2 nanocomposites. J. Mol. Catal. A Chem. 2014, 390, 133–141. [Google Scholar] [CrossRef]
- Raj, N.P.M.J.; Rajesh, P.; Ramasamy, P.; Vijayan, N. One step synthesis of tin oxide nanomaterials and their sintering effect in dye degrdation. Optik 2017, 135, 434–445. [Google Scholar]
- Chiang, Y.-J.; Lin, C.-C. Photocatalytic decolorization of methylene blue in aqueous solutions using coupled ZnO/SnO2 photocatalysts. Powder Technol. 2013, 246, 137–143. [Google Scholar] [CrossRef]
- Ilkhechi, N.N.; Ghorbani, M.; Mozammel, M.; Khajeh, M. The optical, photo catalytic behavior and hydrophilic properties of silver and tin co doped TiO2 thin films using sol–gel method. J. Mater. Sci. Mater. Electron. 2016, 28, 3571–3580. [Google Scholar] [CrossRef]
- de Luna, M.D.G.; Laciste, M.T.; Tolosa, N.C.; Lu, M.C. Effect of catalyst calcination temperature in the visible light photocatalytic oxidation of gaseous formaldehyde by multi-element doped titanium dioxide. Environ. Sci. Pollut. Res. Int. 2018, 25, 15216–15225. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, A.S.; Renukadevi, K.B. Influence of fluorine incorporation on the photocatalytic activity of tin oxide thin films. Mater. Res. Bull. 2017, 94, 85–91. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Guan, L.; Li, F.; Yao, M. Visible light activeTiO2–ZnO composite films by cerium and fluorine codoping for photocatalytic decontamination. Mater. Sci. Semicond. Process. 2015, 40, 310–318. [Google Scholar] [CrossRef]
- Kong, X.; Li, F.; Qi, Z.; Qi, L.; Yao, M. SnO2-based thin films with excellent photocatalytic performance. J. Mater. Sci. Mater. Electron. 2017, 28, 7660–7667. [Google Scholar] [CrossRef]
- Li, F.; Yin, X.; Yao, M.; Li, J. Investigation on F–B–S tri-doped nano-TiO2 films for the photocatalytic degradation of organic dyes. J. Nanopart. Res. 2011, 13, 4839–4846. [Google Scholar] [CrossRef]
- Yamashita, H.; Harada, M.; Misaka, J.; Takeuchi, M.; Neppolian, B.; Anpo, M. Photocatalytic degradation of organic compounds diluted in water using visible light-responsive metal ion-implanted TiO2 catalysts: Fe ion-implanted TiO2. Catal. Today 2003, 84, 191–196. [Google Scholar] [CrossRef]
- Al-Hamdi, A.M.; Sillanpää, M.; Dutta, J. Gadolinium doped tin dioxide nanoparticles: An efficient visible light active photocatalyst. J. Rare Earths 2015, 33, 1275–1283. [Google Scholar] [CrossRef]
- Tang, W.; Wang, J.; Yao, P.-J.; Du, H.-Y.; Sun, Y.-H. Preparation, characterization and gas sensing mechanism of ZnO-doped SnO2 nanofibers. Acta Phys. Chim. Sin. 2014, 30, 781–788. [Google Scholar]
- Zhang, W.; Song, N.; Guan, L.; Li, F.; Yao, M. Photocatalytic degradation of formaldehyde by nanostructured TiO2 composite films. J. Exp. Nanosci. 2015, 11, 185–196. [Google Scholar] [CrossRef]










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, K.; Peng, X.-L.; Li, F.; Yao, M.-M. SnO2 Composite Films for Enhanced Photocatalytic Activities. Catalysts 2018, 8, 453. https://doi.org/10.3390/catal8100453
Han K, Peng X-L, Li F, Yao M-M. SnO2 Composite Films for Enhanced Photocatalytic Activities. Catalysts. 2018; 8(10):453. https://doi.org/10.3390/catal8100453
Chicago/Turabian StyleHan, Ke, Xue-Lei Peng, Fang Li, and Ming-Ming Yao. 2018. "SnO2 Composite Films for Enhanced Photocatalytic Activities" Catalysts 8, no. 10: 453. https://doi.org/10.3390/catal8100453
APA StyleHan, K., Peng, X.-L., Li, F., & Yao, M.-M. (2018). SnO2 Composite Films for Enhanced Photocatalytic Activities. Catalysts, 8(10), 453. https://doi.org/10.3390/catal8100453



