Ultrasonic-Assisted Synthesis of 2D α-Fe2O3@g-C3N4 Composite with Excellent Visible Light Photocatalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
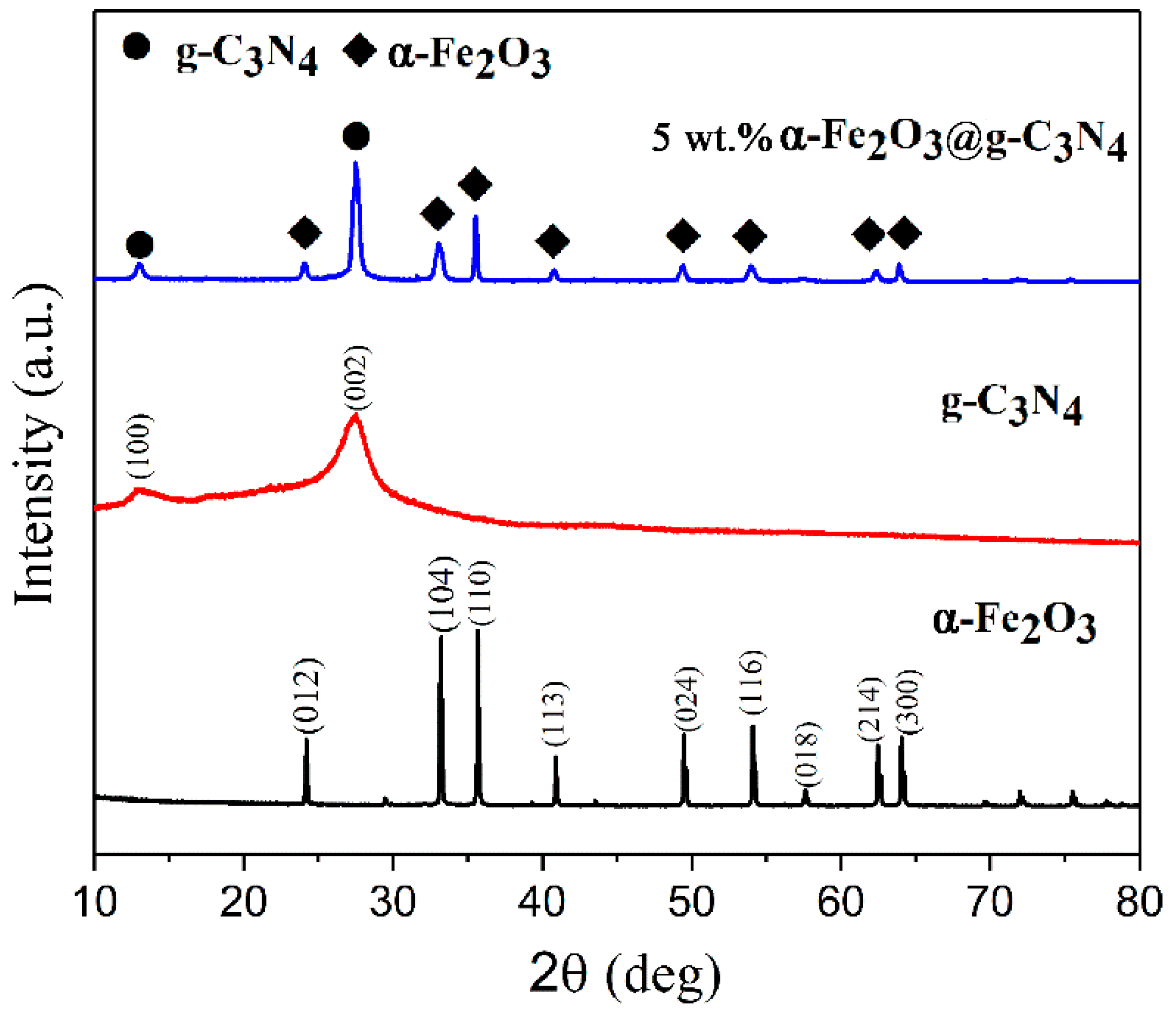
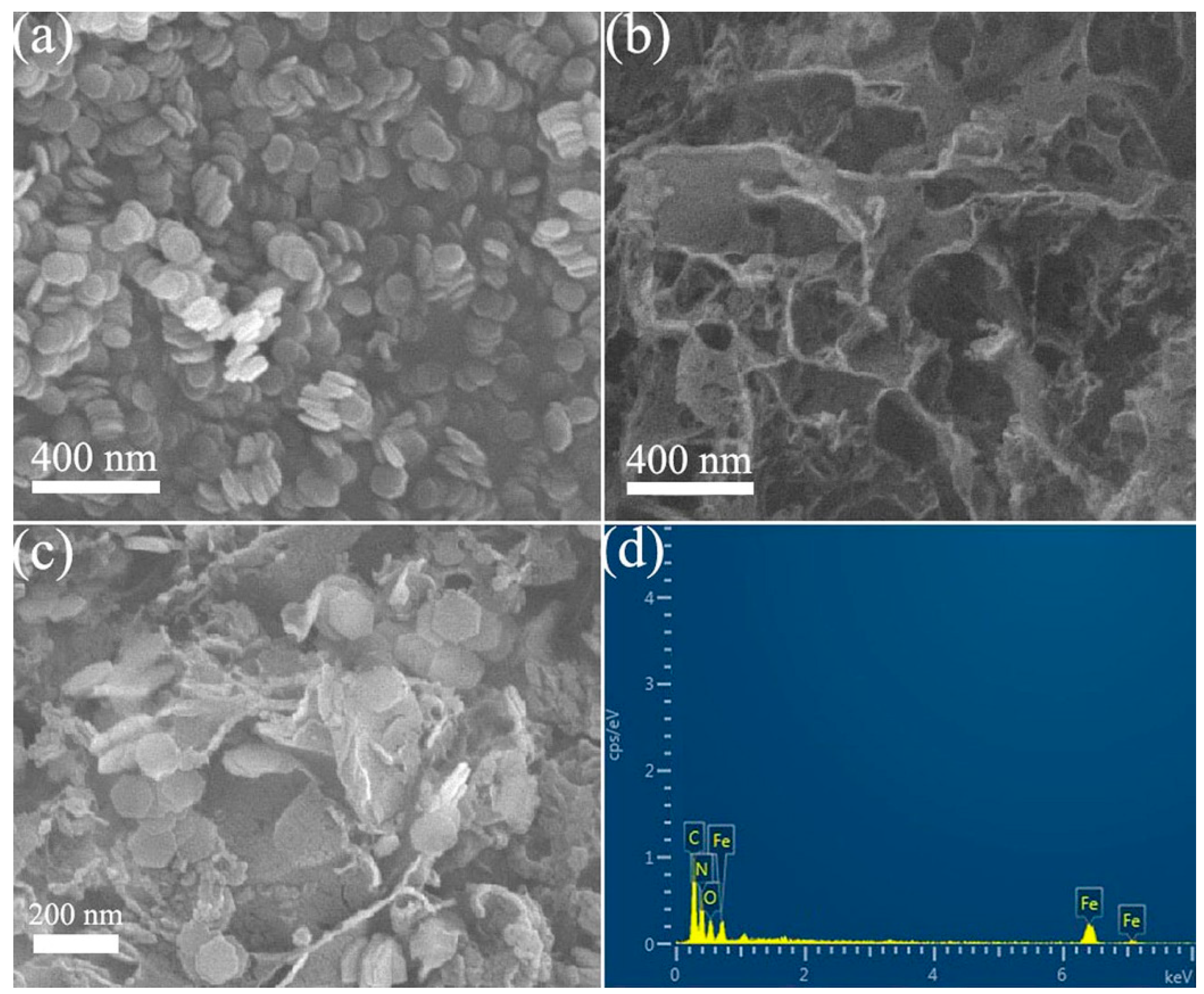
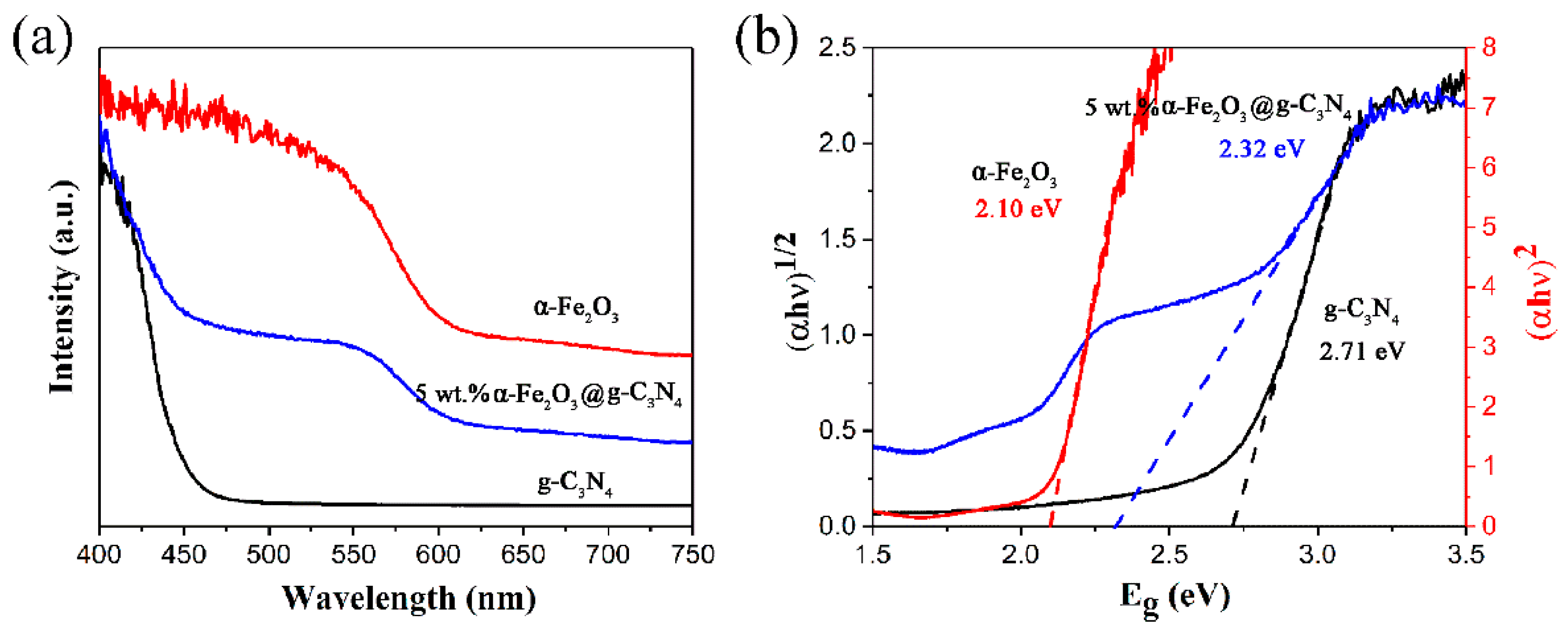
2.1. Catalyst Characterization
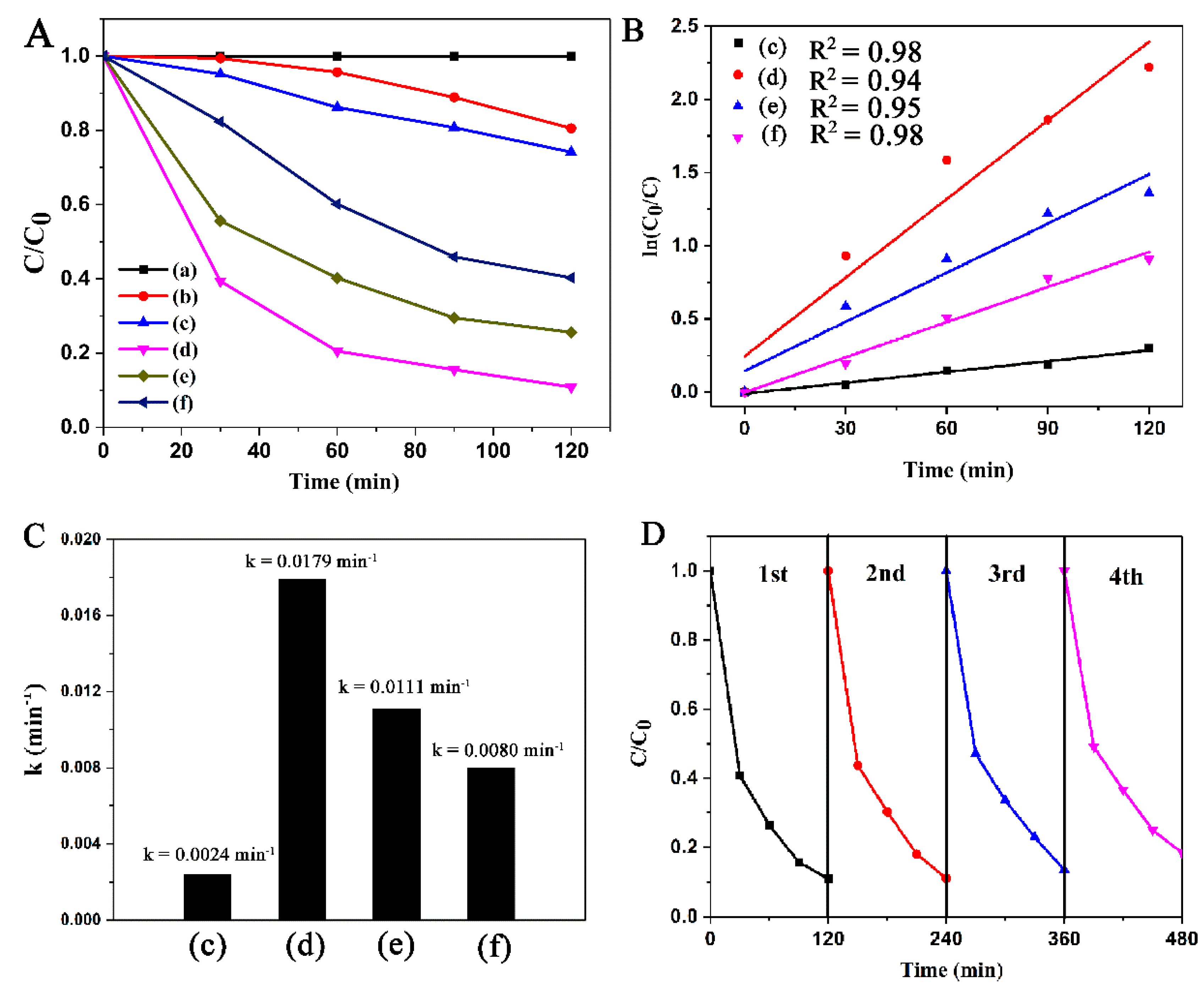
2.2. Catalytic Performance
2.3. Photocatalytic Mechanism
3. Materials and Methods
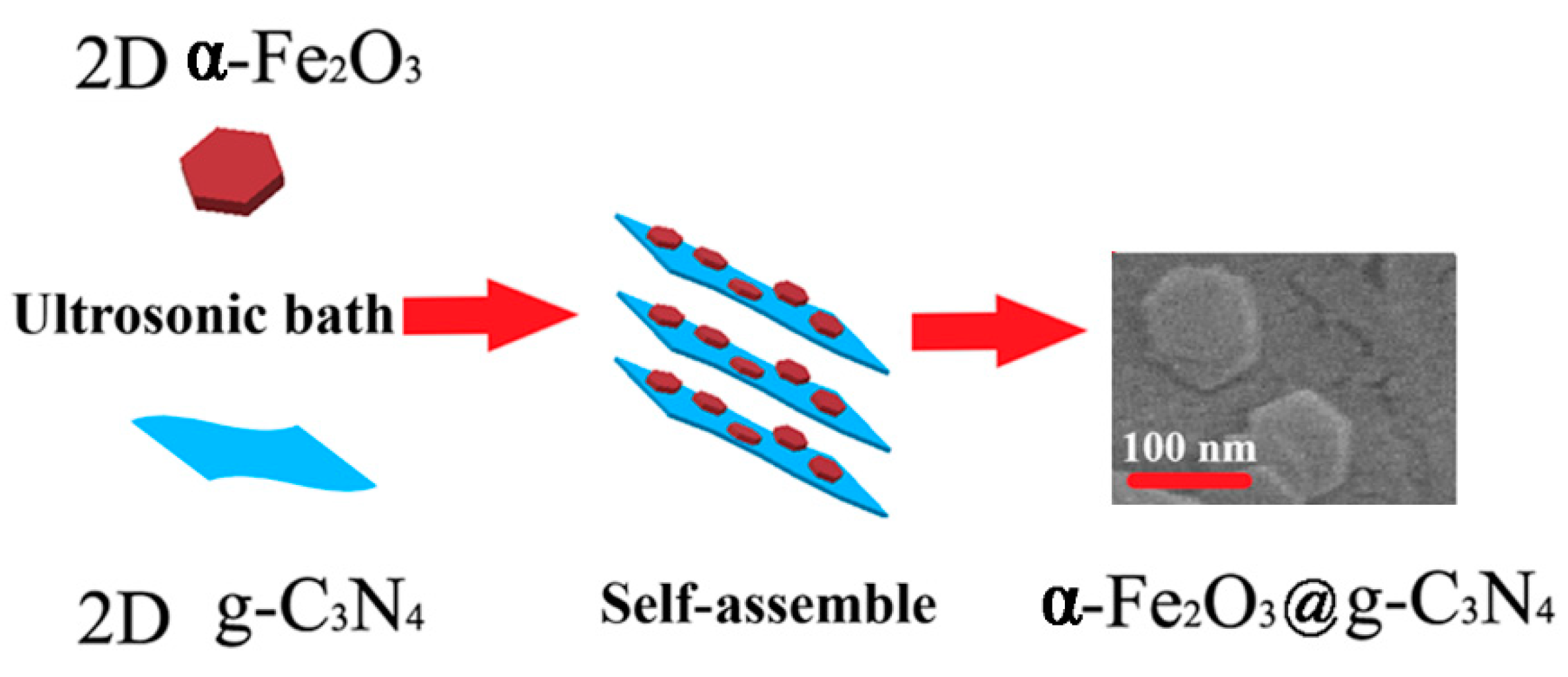
3.1. Synthesis
3.2. Characterizations
3.3. Photocatalytic Tests
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Low, J.; Jiang, C.; Cheng, B.; Wageh, S.; AI-Ghamdi, A.A.; Yu, J. A review of direct Z-Scheme photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; AI-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yan, X.; Mo, Q.; Liu, B.; Wang, J.; Yang, X.; Li, L. Facile synthesis of g-C3N4/BiVO4 heterojunctions with enhanced visible light photocatalytic performance. Ceram. Int. 2017, 43, 301–307. [Google Scholar] [CrossRef]
- Tian, Y.; Chang, B.; Fu, J.; Zhou, B.; Liu, J.; Xi, F.; Dong, X. Graphitic carbon nitride/Cu2O heterojunctions: Preparation, characterization, and enhanced photocatalytic activity under visible light. J. Solid State Chem. 2014, 212, 1–6. [Google Scholar] [CrossRef]
- Tang, D.; Zhang, G. Fabrication of AgFeO2/g-C3N4 nanocatalyst with enhanced and stable photocatalytic performance. Appl. Surf. Sci. 2017, 391, 415–422. [Google Scholar] [CrossRef]
- Wan, W.; Yu, S.; Dong, F.; Zhang, Q.; Zhou, Y. Efficient C3N4/graphene oxide macroscopic aerogel visible-light photocatalyst. J. Mater. Chem. A 2016, 4, 7823–7829. [Google Scholar] [CrossRef]
- Fina, F.; Ménard, H.; Irvine, J.T.S. The effect of Pt NPs crystallinity and distribution on the photocatalytic activity of Pt-g-C3N4. Phys. Chem. Chem. Phys. 2015, 17, 13929–13936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Hisatomi, T.; Jia, Q.X.; Tokudome, H.; Zhong, M.; Wang, C.Z.; Pan, Z.; Takata, T.; Nakabayashi, M.; Shibata, N.; et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 2016, 15, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Qi, Y.; Hisatomi, T.; Ding, Q.; Asai, T.; Li, Z.; Ma, S.S.K.; Zhang, F.; Domen, K.; Li, C. Efficient visible-light-driven Z-Scheme overall water splitting using a MgTa2O6-xNy /TaON heterostructure photocatalyst for H2 evolution. Angew. Chem. Int. Ed. 2015, 127, 8618–8621. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, J.; Wang, L.; Han, M.; Zhang, M.; Wang, H.; Huang, H.; Liu, Y.; Kang, Z. Carbon dots as solid-state electron mediator for BiVO4/CDs/CdS Z-scheme photocatalyst working under visible light. Appl. Catal. B Environ. 2017, 206, 501–509. [Google Scholar] [CrossRef]
- Ou, M.; Wan, S.; Zhong, Q.; Zhang, S.; Song, Y.; Guo, L.; Cai, W.; Xu, Y. Hierarchical Z-scheme photocatalyst of g-C3N4@Ag/BiVO4 (040) with enhanced visible-light-induced photocatalytic oxidation performance. Appl. Catal. B Environ. 2018, 221, 97–107. [Google Scholar] [CrossRef]
- Ng, B.J.; Putri, L.K.; Tan, L.L.; Pasbakhsh, P.; Chai, S.P. All-solid-state Z-scheme photocatalyst with carbon nanotubes as an electron mediator for hydrogen evolution under simulated solar light. Chem. Eng. J. 2017, 316, 41–49. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, J.W.; Ma, D.; Fan, Z.; Niu, C.; Wang, L. Fabrication of g-C3N4/Au/C-TiO2 hollow structures as visible-light-driven Z-scheme photocatalysts with enhanced photocatalytic H2 evolution. ChemCatChem 2017, 9, 3752–3761. [Google Scholar] [CrossRef]
- Di, T.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. A direct Z-scheme g-C3N4/SnS2 photocatalyst with superior visible-light CO2 reduction performance. J. Catal. 2017, 352, 532–541. [Google Scholar] [CrossRef]
- Qiu, B.; Zhu, Q.; Du, M.; Fan, L.; Xing, M.; Zhang, J. Efficient solar light harvesting CdS/Co9S8 hollow cubes for Z-scheme photocatalytic water splitting. Angew. Chem. Int. Ed. 2017, 129, 2728–2732. [Google Scholar] [CrossRef]
- Xia, P.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. 2D/2D g-C3N4/MnO2 nanocomposite as a direct Z-scheme photocatalyst for enhanced photocatalytic activity. ACS Sustain. Chem. Eng. 2017, 6, 965–973. [Google Scholar] [CrossRef]
- Zhou, F.Q.; Fan, J.C.; Xu, Q.J.; Min, Y.L. BiVO4 nanowires decorated with CdS nanoparticles as Z-scheme photocatalyst with enhanced H2 generation. Appl. Catal. B Environ. 2017, 201, 77–83. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Zhao, H.; Wang, G.; Xaing, L.; Xu, J.; Komarneni, S. Oxygen defects-mediated Z-scheme charge separation in g-C3N4/ZnO photocatalysts for enhanced visible-light degradation of 4-chlorophenol and hydrogen evolution. Appl. Catal. B Environ. 2017, 206, 406–416. [Google Scholar] [CrossRef]
- Meng, A.; Zhu, B.; Zhong, B.; Zhang, L.; Cheng, B. Direct Z-scheme TiO2/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Surf. Sci. 2017, 422, 518–527. [Google Scholar] [CrossRef]
- Cui, L.; Ding, X.; Wang, Y.; Shi, H.; Huang, L.; Zuo, Y.; Kang, S. Facile preparation of Z-scheme WO3/g-C3N4 composite photocatalyst with enhanced photocatalytic performance under visible light. Appl. Surf. Sci. 2017, 391, 202–210. [Google Scholar] [CrossRef]
- Yang, G.; Chen, D.; Ding, H.; Feng, J.; Zhang, J.Z.; Zhu, Y.; Hamid, S.; Bahnemann, D.W. Well-designed 3D ZnIn2S4 nanosheets/TiO2 nanobelts as direct Z-scheme photocatalysts for CO2 photoreduction into renewable hydrocarbon fuel with high efficiency. Appl. Catal. B Environ. 2017, 219, 611–618. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, G.; Wu, X.; Yin, S. Novel visible-light-driven Z-scheme Bi12GeO20/g-C3N4 photocatalyst: Oxygen-induced pathway of organic pollutants degradation and proton assisted electron transfer mechanism of Cr (VI) reduction. Appl. Catal. B Environ. 2017, 207, 17–26. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Wheeler, D.A.; Wang, G.; Ling, Y.; Li, Y.; Zhang, J.Z. Nanostructured hematite: Synthesis, characterization, charge carrier dynamics, and photoelectrochemical properties. Energy Environ. Sci. 2012, 5, 6682–6702. [Google Scholar] [CrossRef]
- She, X.; Wu, J.; Xu, H.; Zhong, J.; Wang, Y.; Song, Y.; Nie, K.; Liu, Y.; Yang, Y.; Rodrigues, M.T.F.; et al. High efficiency photocatalytic water splitting using 2D α-Fe2O3/g-C3N4 Z-Scheme Catalysts. Adv. Energy Mater. 2017, 7, 1700025. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, B.; Jiang, C.; Cheng, B.; Yu, J. Constructing 2D/2D Fe2O3/g-C3N4 direct Z-scheme photocatalysts with enhanced H2 generation performance. Sol. RRL 2018, 2, 1800006. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, W.; Li, H.; Yuan, S.; Zhao, H.; Wong, P.K. A hierarchical Z-scheme α-Fe2O3/g-C3N4 hybrid for enhanced photocatalytic CO2 reduction. Adv. Mater. 2018, 30, 1706108. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, L.; Li, M.; Hao, Y.; Lian, Y.; Li, Z.; Wei, Y. α-Fe2O3 modified ZnO flower-like microstructures with enhanced photocatalytic performance for pentachlorophenol degradation. Ceram. Int. 2015, 41, 9420–9425. [Google Scholar] [CrossRef]
- Xie, J.; Zhou, Z.; Lian, Y.; Hao, Y.; Li, P.; Wei, Y. Synthesis of α-Fe2O3/ZnO composites for photocatalytic degradation of pentachlorophenol under UV–vis light irradiation. Ceram. Int. 2015, 41, 2622–2625. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Zhang, L.; Fang, W.X.; Ren, J.; Li, Y.Y.; Yao, H.C.; Wang, J.S.; Li, Z.J. Enhanced photoreduction CO2 activity over direct Z-scheme α-Fe2O3/Cu2O heterostructures under visible light irradiation. ACS Appl. Mater. Interfaces 2015, 7, 8631–8639. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, X.; Chen, J.; Liu, J.; Wu, H.; Zhan, H.; Liang, C.; Wu, M. Continuous shape-and spectroscopy-tuning of hematite nanocrystals. Inorg. Chem. 2010, 49, 8411–8420. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wang, Y.; Sun, G.; Jia, T.; Jia, L.; Zhang, F.; Lin, L.; Zhang, B.; Cao, J.; Zhang, Z. Carbon nitride decorated ball-flower like Co₃O₄ hybrid composite: Hydrothermal synthesis and ethanol gas sensing application. Nanomaterials 2018, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Gong, Y.; Wang, Y.; Zhang, B.; Zhang, H.; Sun, G.; Bala, H.; Zhang, Z. Cocoon-like ZnO decorated graphitic carbon nitride nanocomposite: Hydrothermal synthesis and ethanol gas sensing application. Mater. Lett. 2017, 198, 76–80. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhu, C.; Cao, J.; Tang, Q.; Li, M.; Kang, P.; Shi, C.; Ma, M. Ultrasonic-Assisted Synthesis of 2D α-Fe2O3@g-C3N4 Composite with Excellent Visible Light Photocatalytic Activity. Catalysts 2018, 8, 457. https://doi.org/10.3390/catal8100457
Zhang H, Zhu C, Cao J, Tang Q, Li M, Kang P, Shi C, Ma M. Ultrasonic-Assisted Synthesis of 2D α-Fe2O3@g-C3N4 Composite with Excellent Visible Light Photocatalytic Activity. Catalysts. 2018; 8(10):457. https://doi.org/10.3390/catal8100457
Chicago/Turabian StyleZhang, Huoli, Changxin Zhu, Jianliang Cao, Qingjie Tang, Man Li, Peng Kang, Changliang Shi, and Mingjie Ma. 2018. "Ultrasonic-Assisted Synthesis of 2D α-Fe2O3@g-C3N4 Composite with Excellent Visible Light Photocatalytic Activity" Catalysts 8, no. 10: 457. https://doi.org/10.3390/catal8100457




