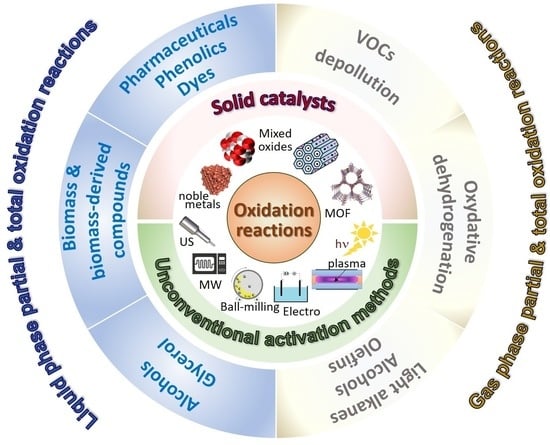
General and Prospective Views on Oxidation Reactions in Heterogeneous Catalysis
Abstract
:1. Introduction
2. Selective Oxidation Reactions in Gas Phase and Liquid Phase
2.1. Gas Phase–Solid Catalyst Selective Oxidation Reactions
- Nature of lattice oxygen anions, being either nucleophilic (partial oxidation) or electrophilic (total oxidation)
- Redox properties of the metal oxide (reduction of the cation with subsequent removal of lattice oxygen anions and their rapid reinsertion by oxygen from the gas phase) characterized by TPR and TPO methods
- Structure of the oxide should allow the redox mechanism to occur, without the collapse of the crystalline structure
- Phase cooperation, which facilitates electron transfer, thus enhances electrical conductivity, lattice oxygen anions mobility and the MvK redox mechanism
- Multifunctionality, such as α-H abstraction and O-/NH- insertion in the organic molecule
- Active site isolation, to avoid a too high lattice surface oxygen anions mobility and thus over-oxidation to CO2
- M-O bond strength, which should be neither too weak (total oxidation) nor too strong (inactivity) (Sabatier’s principle).
2.2. Liquid Phase–Solid Catalyst Selective Oxidation Reactions
2.3. Unconventional Activation Methods for Liquid Phase Selective Oxidation Reactions
3. Total Oxidation Reactions in Gas Phase and Liquid Phase
3.1. Gas Phase–Solid Catalyst Total Oxidation Reactions
3.2. Liquid Phase–Solid Catalyst Total Oxidation Reactions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Callahan, J.L.; Grasselli, R.K. A selectivity factor in vapor phase hydrocarbon oxidation catalysis. AIChE J. 1963, 9, 755–760. [Google Scholar] [CrossRef]
- Brazdil, J.F. A critical perspective in the design and development of metal oxide catalysts for selective propylene ammoxidation and oxidation. Appl. Catal. A Gen. 2017, 543, 225–233. [Google Scholar] [CrossRef]
- Brazdil, J.F. Selective oxidation in industry. In Heterogeneous Catalysis by Metal Oxides; Védrine, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 455–502. [Google Scholar]
- López Nieto, J.M.; Solsona, B. Gas phase heterogeneous partial oxidation reactions. In Heterogeneous Catalysis by Metal Oxides; Védrine, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 211–286. [Google Scholar]
- Dagnan, T.F. Industrial applications of metal oxide catalysts—An impressive past and a promising future. In Heterogeneous Catalysis by Metal Oxides; Védrine, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 503–546. [Google Scholar]
- Mars, P.; van Krevelen, D.W. Oxidation carried out by means of vanadium oxide catalysts. Chem. Eng. Sci. 1954, 3 (Suppl. 1), 41–49. [Google Scholar] [CrossRef]
- Grasselli, R.K. Fundamental principles of selective heterogeneous oxidation catalysis. Top. Catal. 2002, 21, 79–88. [Google Scholar] [CrossRef]
- Grasselli, R.K. Site isolation and phase cooperation: Two important concepts in selective oxidation catalysis: A retrospective. Catal. Today 2014, 238, 10–27. [Google Scholar] [CrossRef]
- Védrine, J.C.; Fechete, I. Heterogeneous partial oxidation catalysis on metal oxides. CR Chim. 2016, 119, 1203–1225. [Google Scholar] [CrossRef]
- Chu, W.; Luo, J.; Paul, S.; Liu, Y.; Khodakov, A.; Bordes, E. Synthesis and performance of vanadium-based catalysts for the selective oxidation of light alkanes. Catal. Today 2017, 298, 145–157. [Google Scholar] [CrossRef]
- Ivars, F.; López Nieto, J.M. Light alkanes oxidation: Targets reached and current challenges. In Advanced Methods and Processes on Oxidation Catalysis: From Laboratory to Industry; Cavani, F., Duprez, D., Eds.; Imperial College Press: London, UK, 2014; pp. 767–833. [Google Scholar]
- López Nieto, J.M. Bifunctional Mo3VOx/H4SiW12O40/Al2O3 catalysts for one-step conversion of glycerol to acrylic acid: Catalyst structural evolution and reaction pathways. Appl. Catal. B Environ. 2015, 175, 1–12. [Google Scholar]
- La Salvia, N.; Delgado, D.; Ruiz-Rodríguez, L.; Nadji, L.; Massó, A.; López Nieto, J.M. V- and Nb-containing bronzes catalysts for the aerobic transformation of ethanol and glycerol. Bulk and supported materials. Catal. Today 2017, 296, 2–9. [Google Scholar] [CrossRef]
- Chieregato, A.; Soriano, M.D.; Basile, F.; Liosi, G.; Zamora, S.; Concepción, P.; Cavania, C.; López Nieto, J.M. One-pot glycerol oxidehydration to acrylic acid on multifunctional catalysts: focus on the influence of the reaction parameters in respect to the catalytic performance. Appl. Catal. B 2014, 150–151, 37–46. [Google Scholar] [CrossRef]
- Kalevaru, V.N.; Dhachapally, N.; Martin, A. Catalytic performance of lanthanum vanadate catalysts in ammoxidation of 2-methylpyrazine. Catalysts 2016, 6, 10. [Google Scholar] [CrossRef]
- Armstrong, R.D.; Hutchings, G.J.; Taylor, S.H. An overview of recent advances of the catalytic selective oxidation of ethane to oxygenates. Catalysts 2016, 6, 71. [Google Scholar] [CrossRef]
- Liu, F.; Wang, H.; Sapi, A.; Tatsumi, H.; Zherebetskyy, D.; Han, H.-L.; Carl, L.M.; Somorjai, G.A. Molecular orientations change reaction kinetics and mechanism: A review on catalytic alcohol oxidation in gas phase and liquid phase on size-controlled Pt nanoparticles. Catalysts 2018, 8, 226. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. A review on glycerol valorization to acrolein over solid acid catalysts. J. Taiwan Inst. Chem. Eng. 2016, 67, 29–44. [Google Scholar] [CrossRef]
- Cespi, D.; Passarini, F.; Mastragostino, G.; Vassura, I.; Larocca, S.; Iaconi, A.; Chieregato, A.; Dubois, J.-L.; Cavani, F. Glycerol as feedstock in the synthesis of chemicals: A life cycle analysis for acrolein production. Green Chem. 2015, 17, 343–355. [Google Scholar] [CrossRef]
- Katryniok, B.; Kimura, H.; Skrzyńska, E.; Girardon, J.S.; Fongarland, P.; Capron, M.; Dumeignil, F. Selective catalytic oxidation of glycerol: Perspectives for high value chemicals. Green Chem. 2011, 13, 1960–1979. [Google Scholar] [CrossRef]
- Villa, A.; Dimitratos, N.; Chan-Thaw, C.E.; Hammond, C.; Prati, L.; Hutchings, G.J. Glycerol oxidation using gold-containing catalysts. Acc. Chem. Res. 2015, 48, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Gao, J.; Sun, H.; Chen, P.; Hou, Z.; Zheng, X. Selective oxidation of glycerol with oxygen in a base-free aqueous solution over MWNTs supported Pt catalysts. Appl. Catal. B 2011, 106, 423–432. [Google Scholar] [CrossRef]
- Xue, W.; Wang, Z.; Liang, Y.; Xu, H.; Liu, L.; Dong, J. Promoting role of bismuth on hydrotalcite-supported platinum catalysts in aqueous phase oxidation of glycerol to dihydroxyacetone. Catalysts 2018, 8, 20. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y. Highly efficient process for the conversion of glycerol to acrylic acid via gas phase catalytic oxidation of an allyl alcohol intermediate. ACS Catal. 2016, 6, 143–150. [Google Scholar] [CrossRef]
- Chieregato, A.; Basile, F.; Concepción, P.; Guidetti, S.; Liosi, G.; Soriano, M.D.; Trevisianut, C.; Cavani, F.; López Nieto, J.M. Glycerol oxidehydration into acrolein and acrylic acid over W–V–Nb–O bronzes with hexagonal structure. Catal. Today 2012, 197, 58–75. [Google Scholar] [CrossRef]
- Shen, L.; Yin, H.; Wang, A.; Lu, X.; Zhang, C. Gas phase oxidehydration of glycerol to acrylic acid over Mo/V and W/V oxide catalysts. Chem. Eng. J. 2014, 244, 168–177. [Google Scholar] [CrossRef]
- Pinna, F.; Olivo, A.; Trevisan, V.; Manegazzo, F.; Signoretto, M.; Manzoli, M.; Boccuzzi, F. The effects of gold nanosize for the exploitation of furfural by selective oxidation. Catal. Today 2013, 203, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Ampelli, C.; Centi, G.; Genovese, C.; Papanikolaou, G.; Pizzi, R.; Perathoner, S.; van Putten, R.-J.; Schouten, K.J.P.; Gluhoi, A.C.; van der Waal, J.C. A comparative catalyst evaluation for the selective oxidative esterification of furfural. Top. Catal. 2016, 59, 1659–1667. [Google Scholar] [CrossRef]
- Menegazzo, F.; Signoretto, M.; Pinna, F.; Manzoli, M.; Aina, V.; Cerrato, G.; Boccuzzi, F. Oxidative esterification of renewable furfural on gold-based catalysts: which is the best support? J. Catal. 2014, 309, 241–247. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US department of energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Deng, J.; Song, H.-J.; Cui, M.-S.; Du, Y.-P.; Fu, Y. Aerobic oxidation of hydroxymethylfurfural and furfural by using heterogeneous CoxOy–N@C catalysts. ChemSusChem 2014, 7, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- Ardemani, L.; Cibin, G.; Dent, A.J.; Isaacs, M.A.; Kyriakou, G.; Lee, A.F.; Parlett, C.M.A.; Parry, S.A.; Wilson, K. Solid base catalysed 5-HMF oxidation to 2,5-FDCA over Au/hydrotalcites: fact or fiction? Chem. Sci. 2015, 6, 4940–4945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neaţu, F.; Marin, R.S.; Florea, M.; Petrea, M.; Pavel, O.D.; Pârvulescu, V.I. Selective oxidation of 5-hydroxymethyl furfural over non-precious metal heterogeneous catalysts. Appl. Catal. B 2016, 180, 751–757. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, F.; Sasaki, M.; Wang, F.; Jing, Z.; Goto, M. Selective conversion of glucose into lactic acid and acetic acid with copper oxide under hydrothermal conditions. AIChE J. 2013, 59, 2096–2104. [Google Scholar] [CrossRef]
- Yang, X.; Yang, L.; Fan, W.; Lin, H. Effect of redox properties of LaCoO3 perovskite catalyst on production of lactic acid from cellulosic biomass. Catal. Today 2016, 269, 56–64. [Google Scholar] [CrossRef]
- Rodenas, Y.; Mariscal, R.; Fierro, J.L.G.; Martín Alonso, D.; Dumesic, J.A.; López Granados, M. Improving the production of maleic acid from biomass: TS-1 catalysed aqueous phase oxidation of furfural in the presence of γ-valerolactone. Green Chem. 2018, 20, 2845–2856. [Google Scholar] [CrossRef]
- Deng, H.; Lin, L.; Liu, S. Catalysis of Cu-doped Co-based perovskite-type oxide in wet oxidation of lignin to produce aromatic aldehydes. Energy Fuels 2010, 24, 4797–4802. [Google Scholar] [CrossRef]
- Farrusseng, D. (Ed.) Metal-organic frameworks: applications from catalysis to gas storage; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Du, D.Y.; Qin, J.S.; Li, S.L.; Su, Z.M.; Lan, Y.Q. Recent advances in porous polyoxometalate-based metal–organic framework materials. Chem. Soc. Rev. 2014, 43, 4615–4632. [Google Scholar] [CrossRef] [PubMed]
- Kholdeeva, O.A. Liquid-phase selective oxidation catalysis with metal-organic frameworks. Catal. Today 2016, 278, 22–29. [Google Scholar] [CrossRef]
- Hill, C.L.; Kholdeeva, O.A. Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications; Clerici, M.G., Kholdeeva, O.A., Eds.; Wiley-VCH: Weinheim, Germany, 2013; pp. 263–319. [Google Scholar]
- Salomon, W.; Roch-Marchal, C.; Mialane, P.; Rouschemeyer, P.; Serre, C.; Haouas, M.; Taulelle, F.; Yang, S.; Ruhlmann, L.; Dolbecq, A. Immobilization of polyoxometalates in the Zr-based metal organic framework UiO-67. Chem. Commun. 2015, 51, 2972–2975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, J.K.; Ntainjua, E.; Carley, A.F.; Herzing, A.A.; Kiely, C.J.; Hutchings, G.J. Direct synthesis of H2O2 from H2 and O2 over gold, palladium and gold-palladium catalysts supported on acid pretreated TiO2. Angew. Chem. Int. Ed. 2009, 48, 8512–8515. [Google Scholar] [CrossRef] [PubMed]
- Selinsek, M.; Deschner, B.J.; Doronkin, D.E.; Sheppard, T.L.; Grunwaldt, J.D.; Dittmeyer, R. Revealing the structure and mechanism of palladium during direct synthesis of hydrogen peroxide in continuous flow using operando spectroscopy. ACS Catal. 2018, 8, 2546–2557. [Google Scholar] [CrossRef]
- Martina, K.; Manzoli, M.; Calcio Gaudino, E.; Cravotto, G. Eco-friendly physical activation methods for Susuki-Miyaura reactions. Catalysts 2017, 7, 98. [Google Scholar] [CrossRef]
- Sylla-Iyarreta Veitía, M.; Ferroud, C. New activation methods used in green chemistry for the synthesis of high added value molecules. Int. J. Energy Environ. Eng. 2015, 6, 37–46. [Google Scholar] [CrossRef]
- Behling, R.; Chatel, G.; Valange, S. Sonochemical oxidation of vanillyl alcohol to vanillin in the presence of a cobalt oxide catalyst under mild conditions. Ultrason. Sonochem. 2017, 36, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A. Green oxidation protocol: Selective conversions of alcohols and alkenes to aldehydes, ketones and epoxides by using a new multiwall carbon nanotube based hybrid nanocatalyst via ultrasound irradiation. Ultrason. Sonochem. 2018, 40, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Domini, C.E.; Álvarez, M.B.; Silbestri, G.F.; Cravotto, G.; Cintas, P. Merging metallic catalysts and sonication: A periodic table overview. Catalysts 2017, 7, 121. [Google Scholar] [CrossRef]
- Qu, C.; Kaneko, M.; Kashimura, K.; Tanaka, K.; Ozawa, S.; Watanabe, T. Direct production of vanillin from wood particles by copper oxide−peroxide reaction promoted by electric and magnetic fields of microwaves. ACS Sustain. Chem. Eng. 2017, 5, 11551–11557. [Google Scholar] [CrossRef]
- Horie, K.; Barón, M.; Fox, R.B.; He, J.; Hess, M.; Kahovec, J.; Kitayama, T.; Kubisa, P.; Maréchal, E.; Mormann, W.; et al. Definitions of terms relating to reactions of polymers and to functional polymeric materials (IUPAC Recommendations 2003). Pure Appl. Chem. 2004, 76, 889–906. [Google Scholar] [CrossRef] [Green Version]
- Porcheddu, A.; Colacino, E.; Cravotto, G.; Delogu, F.; De Luca, L. Mechanically induced oxidation of alcohols to aldehydes and ketones in ambient air: Revisiting TEMPO-assisted oxidations. Beilstein J. Org. Chem. 2017, 13, 2049–2055.00E9. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.; Baranton, S.; Coutanceau, C. Electrochemical valorisation of glycerol. ChemSusChem 2012, 5, 2106–2124. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wu, M.; Zhao, G. Electrocatalytic oxidation of cellulose to gluconate on carbon aerogel supported gold nanoparticles anode in alkaline medium. Catalysts 2016, 6, 5. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Luque, R. Heterogeneous photocatalytic nanomaterials: propects and challenges in selective transformation of biomass-derived compounds. Chem. Soc. Rev. 2014, 43, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Chatel, G.; Valange, S.; Behling, R.; Colmenares, J.C. A combined approach using sonochemistry and photocatalysis: How to apply sonophotocatalysis for biomass conversion? ChemCatChem 2017, 9, 2615–2621. [Google Scholar] [CrossRef]
- Hinman, J.J.; Suslick, K.S. Nanostructured materials synthesis using ultrasound. Top. Curr. Chem. (Z) 2017, 375, 12. [Google Scholar] [CrossRef] [PubMed]
- Mansoli, M.; Bonelli, B. Microwave, ultrasound, and mechanochemistry: unconventional tools that are used to obtain “smart” catalysts for CO2 hydrogenation. Catalysts 2018, 8, 262. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Rodriguez-Padron, D.; Puente-Santiago, A.R.; Luque, R. Mechanochemistry: toward sustainable design of advanced nanomaterials for electrochemical energy storage and catalytic applications. ACS Sustain. Chem. Eng. 2018, 6, 9530–9544. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Wang, B.; Ma, M.-G.; Lin, K.-L. The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials. Mater. Today Chem. 2016, 1–2, 63–83. [Google Scholar] [CrossRef]
- Magdziarz, A.; Colmenares, J.C. In situ coupling of ultrasound to electro- and photo-deposition methods for materials synthesis. Molecules 2017, 22, 216. [Google Scholar] [CrossRef] [PubMed]
- Dharmarathna, S.; King’ondu, C.K.; Pedrick, W.; Pahalagedara, L.; Suib, S.L. Direct sonochemical synthesis of manganese octahedral molecular sieve (OMS-2) nanomaterials using cosolvent systems, their characterization, and catalytic applications. Chem. Mater. 2012, 24, 705–712. [Google Scholar] [CrossRef]
- Amaniampong, P.N.; Trinh, Q.T.; Varghese, J.J.; Behling, R.; Valange, S.; Mushrif, S.H.; Jérôme, F. Unraveling the mechanism of the oxidation of glycerol to dicarboxylic acids over a sonochemically synthesized copper oxide catalyst. Green Chem. 2018, 20, 2730–2741. [Google Scholar] [CrossRef]
- Xu, C.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Sun, L.; Yun, H.; Li, J.; Lai, Y.K.; Lin, C.J. Sonoelectrochemical synthesis of highly photoelectrochemically active TiO2 nanotubes by incorporating CdS nanoparticles. Nanotechnology 2009, 20, 295601. [Google Scholar] [CrossRef] [PubMed]
- Magdziarz, A.; Colmenares, J.C.; Chernyayeva, O.; Kurzydlowski, K.; Grzonka, J. Iron-containing titania photocatalyst prepared by the sonophotodeposition method for the oxidation of benzyl alcohol. ChemCatChem 2016, 8, 536–539. [Google Scholar] [CrossRef]
- Bion, N.; Can, F.; Courtois, X.; Duprez, D. Transition metal oxides for combustion and depollution processes. In Heterogeneous Catalysis by Metal Oxides; Védrine, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 287–352. [Google Scholar]
- Xie, X.; Li, Y.; Liu, Z.-Q.; Haruta, M.; Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.P.; Carabineiro, S.A.C.; Tavares, P.B.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Oxidation of CO, ethanol and toluene over TiO2 supported noble metal catalysts. Appl. Catal. B 2010, 99, 198–205. [Google Scholar] [CrossRef]
- Scirè, S.; Liotta, L.F. Supported gold catalysts for the total oxidation of volatile organic compounds. Appl. Catal. B 2012, 125, 222–246. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Garcia, T.; Solsona, B.; Taylor, S.H. The catalytic oxidation of hydrocarbon volatile organic compounds. In Advanced Methods and Processes on Oxidation Catalysis: From Laboratory to Industry; Duprez, D., Cavani, F., Eds.; Imperial College Press: London, UK, 2014; pp. 51–90. [Google Scholar]
- Wang, F.; Dai, H.; Deng, J.; Bai, G.; Ji, K.; Liu, Y. Manganese oxides with rod-, wire-, tube-, and flower-like morphologies: highly effective catalysts for the removal of toluene. Environ. Sci. Technol. 2012, 46, 4034–4041. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Genty, E.; Barroo, C.; Cazier, F.; Poupin, C.; Siffert, S.; Thomas, D.; De Weireld, G.; Visart de Bocarmé, T.; Cousin, R. The CoAlCeO mixed oxide: An alternative to palladium-based catalysts for total oxidation of industrial VOCs. Catalysts 2018, 8, 64. [Google Scholar] [CrossRef]
- Burgos, N.; Paulis, M.; Mirari Antxustegi, M.; Montes, M. Deep oxidation of VOC mixtures with platinum supported on Al2O3/Al monoliths. Appl. Catal. B Environ. 2002, 38, 251–258. [Google Scholar] [CrossRef]
- Pena, M.A.; Fierro, J.L.G. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Gomathi, L.D. Review on modified TiO2 photocatalysis under UV/visiblelight: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2015, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Review on preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shagguan, W. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Rezaei, E.; Soltan, J. Low temperature oxidation of toluene by ozone over MnOx/γ-alumina and MnOx/MCM-41 catalysts. Chem. Eng. J. 2012, 198–199, 482–490. [Google Scholar] [CrossRef]
- Zhao, D.-Z.; Shi, C.; Li, X.-S.; Zhu, A.-M.; Jang, B.-W. Enhanced effect of water vapor on complete oxidation of formaldehyde in air with ozone over MnOx catalysts at room temperature. J. Hazard. Mater. 2012, 239–240, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Einaga, H.; Ogata, A. Benzene oxidation with ozone over supported manganese oxide catalysts: effect of catalyst support and reaction conditions. J. Hazard. Mater. 2009, 164, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Giraudon, J.-M.; De Geyter, N.; Morent, R.; Lamonier, J.-F. The design of MnOx based catalyst in post-plasma catalysis configuration for toluene abatement. Catalysts 2018, 8, 91. [Google Scholar] [CrossRef]
- Trinh, Q.H.; Mok, Y.S. Non-thermal plasma combined with cordierite-supported Mn and Fe based catalysts for the decomposition of diethylether. Catalysts 2015, 5, 800–814. [Google Scholar] [CrossRef]
- Sultana, S.; Vandenbroucke, A.M.; Leys, C.; De Geyter, N.; Morent, R. Abatement of VOCs with alternate adsorption and plasma-assisted regeneration: a review. Catalysts 2015, 5, 718–746. [Google Scholar] [CrossRef] [Green Version]
- Teramoto, Y.; Kim, H.-H.; Negishi, N.; Ogata, A. The role of ozone in the reaction mechanism of a bare zeolite-plasma hybrid system. Catalysts 2015, 5, 838–850. [Google Scholar] [CrossRef]
- Genty, E.; Brunet, J.; Poupin, C.; Casale, S.; Capelle, S.; Massiani, P.; Siffert, S.; Cousin, R. Co-Al mixed oxides prepared via LDH route using microwaves or ultrasound: application for catalytic toluene total oxidation. Catalysts 2015, 5, 851–867. [Google Scholar] [CrossRef]
- Arena, F.; Di Chio, R.; Gumina, B.; Spadaro, L.; Trunfio, G. Recent advances on wet air oxidation catalysts for treatment of industrial wastewaters. Inorg. Chim. Acta 2015, 431, 101–109. [Google Scholar] [CrossRef]
- Jing, G.; Luan, M.; Chen, T. Progress of catalytic wet air oxidation technology. Arabian J. Chem. 2016, 9, S1208–S1213. [Google Scholar] [CrossRef]
- Fu, J.; Kyzas, G.Z. Wet air oxidation for the decolorization of dye wastewater: An overview of the last two decades. Chin. J. Catal. 2014, 35, 1–7. [Google Scholar] [CrossRef]
- Divyapriya, G.; Nambi, I.M.; Senthilnathan, J. Nanocatalysts in Fenton based advanced oxidation process for water and wastewater treatment. J. Bionanosci. 2016, 10, 356–368. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Mahajan, V.K.; Sonwane, G.H. Effective degradation and mineralization of real textile effluent by sonolysis, photocatalysis, and sonophotocatalysis using ZnO nano catalyst. Nanochem. Res. 2016, 1, 258–263. [Google Scholar]
- Ziylan, A.; Ince, N.H. Catalytic ozonation of ibuprofen with ultrasound and Fe-based catalysts. Catal. Today 2015, 240, 2–8. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valange, S.; Védrine, J.C. General and Prospective Views on Oxidation Reactions in Heterogeneous Catalysis. Catalysts 2018, 8, 483. https://doi.org/10.3390/catal8100483
Valange S, Védrine JC. General and Prospective Views on Oxidation Reactions in Heterogeneous Catalysis. Catalysts. 2018; 8(10):483. https://doi.org/10.3390/catal8100483
Chicago/Turabian StyleValange, Sabine, and Jacques C. Védrine. 2018. "General and Prospective Views on Oxidation Reactions in Heterogeneous Catalysis" Catalysts 8, no. 10: 483. https://doi.org/10.3390/catal8100483